Market Trends Analysis in Organoid Culture Systems
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Evolution and Research Objectives
Organoid technology has evolved significantly since the first successful cultivation of intestinal organoids in 2009 by Hans Clevers and colleagues. This breakthrough demonstrated that adult stem cells could self-organize into three-dimensional structures resembling their tissue of origin. The evolution of organoid technology has been characterized by progressive improvements in culture methods, expansion of tissue types, and increasing complexity of the models.
Early organoid systems primarily focused on epithelial tissues, with relatively simple structures and limited functionality. The development of Matrigel as a supportive extracellular matrix was crucial in these early stages, providing the necessary physical and biochemical cues for stem cell differentiation and organization. Between 2010 and 2015, researchers expanded the repertoire of organoid types to include liver, pancreas, lung, and brain tissues, each requiring specific growth factors and culture conditions.
The period from 2015 to 2020 saw significant advancements in culture system sophistication, with the integration of multiple cell types to create more physiologically relevant models. This included the incorporation of immune cells, vascular elements, and stromal components, moving beyond purely epithelial systems. Concurrently, bioengineering approaches emerged to address limitations of Matrigel-based cultures, including the development of synthetic hydrogels with tunable properties and microfluidic systems for improved nutrient delivery.
Recent years have witnessed the convergence of organoid technology with other cutting-edge fields. Integration with gene editing tools like CRISPR-Cas9 has enabled precise genetic manipulation, while combination with bioprinting technologies has improved reproducibility and scalability. The application of artificial intelligence for image analysis and prediction of organoid development represents another frontier in the field.
The primary research objectives in organoid culture systems currently focus on several key areas. First, enhancing physiological relevance through improved vascularization, innervation, and immune system integration. Second, increasing reproducibility and standardization to address batch-to-batch variability in both biological and synthetic matrices. Third, scaling up production for high-throughput applications in drug screening and toxicology testing.
Additional objectives include developing specialized culture systems for patient-derived organoids to advance personalized medicine applications, particularly in cancer treatment. There is also significant interest in creating more complex multi-organ systems or "body-on-a-chip" platforms to study organ interactions and systemic responses. Finally, researchers aim to reduce costs and technical complexity to democratize access to organoid technology across research institutions and biotechnology companies globally.
Early organoid systems primarily focused on epithelial tissues, with relatively simple structures and limited functionality. The development of Matrigel as a supportive extracellular matrix was crucial in these early stages, providing the necessary physical and biochemical cues for stem cell differentiation and organization. Between 2010 and 2015, researchers expanded the repertoire of organoid types to include liver, pancreas, lung, and brain tissues, each requiring specific growth factors and culture conditions.
The period from 2015 to 2020 saw significant advancements in culture system sophistication, with the integration of multiple cell types to create more physiologically relevant models. This included the incorporation of immune cells, vascular elements, and stromal components, moving beyond purely epithelial systems. Concurrently, bioengineering approaches emerged to address limitations of Matrigel-based cultures, including the development of synthetic hydrogels with tunable properties and microfluidic systems for improved nutrient delivery.
Recent years have witnessed the convergence of organoid technology with other cutting-edge fields. Integration with gene editing tools like CRISPR-Cas9 has enabled precise genetic manipulation, while combination with bioprinting technologies has improved reproducibility and scalability. The application of artificial intelligence for image analysis and prediction of organoid development represents another frontier in the field.
The primary research objectives in organoid culture systems currently focus on several key areas. First, enhancing physiological relevance through improved vascularization, innervation, and immune system integration. Second, increasing reproducibility and standardization to address batch-to-batch variability in both biological and synthetic matrices. Third, scaling up production for high-throughput applications in drug screening and toxicology testing.
Additional objectives include developing specialized culture systems for patient-derived organoids to advance personalized medicine applications, particularly in cancer treatment. There is also significant interest in creating more complex multi-organ systems or "body-on-a-chip" platforms to study organ interactions and systemic responses. Finally, researchers aim to reduce costs and technical complexity to democratize access to organoid technology across research institutions and biotechnology companies globally.
Market Demand Analysis for Organoid Culture Systems
The global organoid culture systems market has witnessed substantial growth in recent years, driven primarily by increasing applications in drug discovery, personalized medicine, and disease modeling. Current market valuations indicate that the organoid culture systems sector reached approximately 1.3 billion USD in 2022, with projections suggesting a compound annual growth rate (CAGR) of 22.5% through 2030. This remarkable growth trajectory reflects the expanding utility of organoid technologies across multiple biomedical research domains.
Pharmaceutical and biotechnology companies represent the largest consumer segment, accounting for nearly 45% of the total market demand. These organizations increasingly rely on organoid models to reduce drug development costs and timelines by providing more physiologically relevant screening platforms than traditional 2D cell cultures. The ability of organoids to recapitulate human tissue architecture and functionality has proven particularly valuable for toxicity testing and efficacy studies, potentially saving millions in development costs per compound.
Academic and research institutions constitute the second-largest market segment, contributing approximately 35% of the demand. This sector's growth is fueled by substantial research grants focused on organoid technology development and applications in basic science. The remaining market share is distributed among contract research organizations, healthcare providers, and other end-users exploring organoid applications in precision medicine initiatives.
Regionally, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing R&D investments in countries like China, Japan, and South Korea. These nations are rapidly developing their biomedical research infrastructure and implementing favorable regulatory frameworks to support advanced cell culture technologies.
The demand for specific organoid types varies by application area. Brain, liver, and intestinal organoids currently command the highest market share due to their established protocols and widespread applications. However, emerging demand for more complex multi-organ systems and tumor organoids is expected to reshape market dynamics over the next five years as these technologies mature.
Key market drivers include the growing emphasis on reducing animal testing, increasing prevalence of chronic diseases requiring better models, and the push toward personalized medicine approaches. Additionally, technological advancements in supporting technologies such as microfluidics, bioprinting, and artificial intelligence-driven analysis tools are expanding the capabilities and applications of organoid systems, further stimulating market growth.
Pharmaceutical and biotechnology companies represent the largest consumer segment, accounting for nearly 45% of the total market demand. These organizations increasingly rely on organoid models to reduce drug development costs and timelines by providing more physiologically relevant screening platforms than traditional 2D cell cultures. The ability of organoids to recapitulate human tissue architecture and functionality has proven particularly valuable for toxicity testing and efficacy studies, potentially saving millions in development costs per compound.
Academic and research institutions constitute the second-largest market segment, contributing approximately 35% of the demand. This sector's growth is fueled by substantial research grants focused on organoid technology development and applications in basic science. The remaining market share is distributed among contract research organizations, healthcare providers, and other end-users exploring organoid applications in precision medicine initiatives.
Regionally, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing R&D investments in countries like China, Japan, and South Korea. These nations are rapidly developing their biomedical research infrastructure and implementing favorable regulatory frameworks to support advanced cell culture technologies.
The demand for specific organoid types varies by application area. Brain, liver, and intestinal organoids currently command the highest market share due to their established protocols and widespread applications. However, emerging demand for more complex multi-organ systems and tumor organoids is expected to reshape market dynamics over the next five years as these technologies mature.
Key market drivers include the growing emphasis on reducing animal testing, increasing prevalence of chronic diseases requiring better models, and the push toward personalized medicine approaches. Additionally, technological advancements in supporting technologies such as microfluidics, bioprinting, and artificial intelligence-driven analysis tools are expanding the capabilities and applications of organoid systems, further stimulating market growth.
Current Challenges in Organoid Culture Technologies
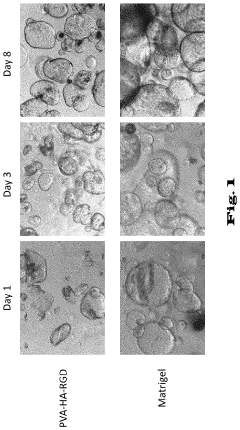
Despite significant advancements in organoid culture technologies, several critical challenges continue to impede broader adoption and application in both research and clinical settings. The complexity of extracellular matrix (ECM) components represents a fundamental obstacle, as current matrices like Matrigel suffer from batch-to-batch variability, undefined composition, and animal origin concerns that limit reproducibility and clinical translation. This variability directly impacts experimental consistency and hampers regulatory approval pathways.
Medium composition optimization remains problematic, with researchers struggling to balance the complex interplay of growth factors, small molecules, and nutrients required for specific organoid types. The lack of standardized protocols across different tissue types necessitates extensive trial-and-error approaches, increasing research costs and timelines while reducing cross-laboratory reproducibility.
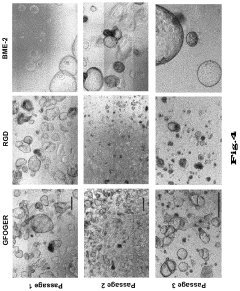
Scalability presents another significant hurdle, as current culture methods are labor-intensive and difficult to automate. The transition from laboratory-scale production to industrial applications faces technical barriers in maintaining consistent organoid quality during scale-up processes. This limitation particularly affects drug screening applications and potential therapeutic uses where large quantities of uniform organoids are required.
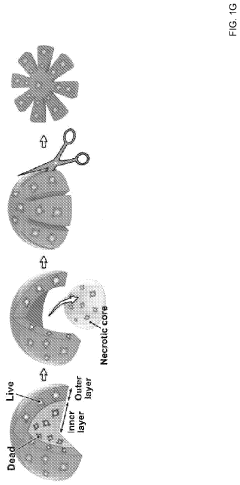
Vascularization deficiency in organoids restricts their size and functional maturity, as diffusion limitations create necrotic cores in larger structures. Without integrated vascular networks, organoids cannot fully recapitulate the complexity of native tissues, limiting their physiological relevance for disease modeling and drug testing applications.
Long-term culture stability remains elusive, with most organoid systems showing phenotypic drift, altered gene expression profiles, and eventual deterioration over extended culture periods. This temporal instability undermines applications requiring prolonged observation or chronic disease modeling.
Functional maturation challenges persist across multiple organoid types, which often remain developmentally immature compared to adult tissues. This immaturity manifests in incomplete cellular differentiation, limited functional capacity, and absence of key tissue-specific markers, reducing their predictive value for adult disease modeling and drug response studies.
Cost barriers significantly limit widespread adoption, with specialized media components and growth factors representing substantial recurring expenses. The high technical expertise required for successful organoid culture further restricts accessibility to specialized laboratories with significant resources and experience.
Medium composition optimization remains problematic, with researchers struggling to balance the complex interplay of growth factors, small molecules, and nutrients required for specific organoid types. The lack of standardized protocols across different tissue types necessitates extensive trial-and-error approaches, increasing research costs and timelines while reducing cross-laboratory reproducibility.
Scalability presents another significant hurdle, as current culture methods are labor-intensive and difficult to automate. The transition from laboratory-scale production to industrial applications faces technical barriers in maintaining consistent organoid quality during scale-up processes. This limitation particularly affects drug screening applications and potential therapeutic uses where large quantities of uniform organoids are required.
Vascularization deficiency in organoids restricts their size and functional maturity, as diffusion limitations create necrotic cores in larger structures. Without integrated vascular networks, organoids cannot fully recapitulate the complexity of native tissues, limiting their physiological relevance for disease modeling and drug testing applications.
Long-term culture stability remains elusive, with most organoid systems showing phenotypic drift, altered gene expression profiles, and eventual deterioration over extended culture periods. This temporal instability undermines applications requiring prolonged observation or chronic disease modeling.
Functional maturation challenges persist across multiple organoid types, which often remain developmentally immature compared to adult tissues. This immaturity manifests in incomplete cellular differentiation, limited functional capacity, and absence of key tissue-specific markers, reducing their predictive value for adult disease modeling and drug response studies.
Cost barriers significantly limit widespread adoption, with specialized media components and growth factors representing substantial recurring expenses. The high technical expertise required for successful organoid culture further restricts accessibility to specialized laboratories with significant resources and experience.
Current Organoid Culture System Solutions
01 3D Organoid Culture Techniques
Three-dimensional organoid culture systems that mimic the structure and function of organs in vitro. These systems typically involve culturing stem cells or progenitor cells in specialized matrices that support self-organization into organ-like structures. The techniques often include the use of extracellular matrix components, growth factors, and specific culture conditions to promote organoid development and maintenance.- 3D Organoid Culture Techniques: Three-dimensional organoid culture systems that mimic the structure and function of organs in vitro. These systems typically involve culturing stem cells or progenitor cells in specialized matrices that support three-dimensional growth and differentiation. The resulting organoids can recapitulate key aspects of organ development, structure, and function, making them valuable tools for research and drug testing.
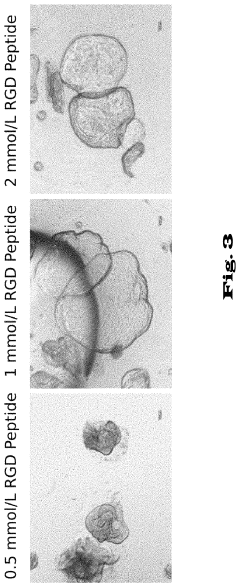
- Matrix Components for Organoid Culture: Specific extracellular matrix components and hydrogels that support organoid growth and development. These matrices provide structural support and biochemical cues necessary for proper cell organization and differentiation. Common matrix components include Matrigel, collagen, fibronectin, and synthetic hydrogels with tunable properties that can be optimized for different organoid types.
- Growth Factors and Signaling Molecules: Specific combinations of growth factors, cytokines, and signaling molecules that direct organoid formation and maturation. These bioactive compounds regulate stem cell proliferation, differentiation, and morphogenesis in organoid culture systems. Different organoid types require specific cocktails of factors that recapitulate the signaling environment of the corresponding organ during development.
- Disease Modeling with Organoids: Methods for using organoid culture systems to model human diseases and test potential therapeutics. Patient-derived organoids can recapitulate disease phenotypes, allowing for personalized medicine approaches. These systems are particularly valuable for studying genetic disorders, cancer, infectious diseases, and developmental abnormalities in a physiologically relevant context.
- Bioreactors and Automated Culture Systems: Specialized bioreactors and automated systems designed for large-scale, reproducible organoid culture. These technologies address challenges in scaling up organoid production while maintaining quality and consistency. Features include controlled nutrient delivery, waste removal, mechanical stimulation, and monitoring capabilities that enhance organoid development and maturation.
02 Stem Cell-Derived Organoid Systems
Methods for generating organoids from various types of stem cells, including embryonic stem cells, induced pluripotent stem cells, and adult stem cells. These approaches focus on directing stem cell differentiation toward specific lineages and promoting self-organization into functional organoid structures that recapitulate key aspects of organ development and physiology.Expand Specific Solutions03 Specialized Culture Media and Supplements
Formulations of culture media, growth factors, and supplements specifically designed for organoid culture systems. These specialized media compositions support the growth, differentiation, and maintenance of organoids by providing essential nutrients, signaling molecules, and environmental cues necessary for proper organoid development and function.Expand Specific Solutions04 Disease Modeling with Organoid Systems
Applications of organoid culture systems for modeling human diseases, including cancer, genetic disorders, and infectious diseases. These models enable the study of disease mechanisms, drug responses, and potential therapeutic interventions in a physiologically relevant context that better represents human pathophysiology compared to traditional 2D cell culture systems.Expand Specific Solutions05 Bioreactors and Advanced Culture Platforms
Specialized bioreactors and culture platforms designed for large-scale production, long-term maintenance, and high-throughput screening of organoids. These systems often incorporate automated feeding, monitoring, and environmental control features to optimize organoid growth conditions and enable more consistent and reproducible organoid culture outcomes.Expand Specific Solutions
Leading Companies and Research Institutions in Organoid Field
The organoid culture systems market is currently in a growth phase, characterized by increasing adoption across research and clinical applications. The market size is expanding rapidly, projected to reach significant value due to rising demand in drug discovery, personalized medicine, and disease modeling. Technologically, the field shows moderate maturity with established protocols but continues to evolve rapidly. Key players demonstrate varying levels of specialization: STEMCELL Technologies and Mimetas lead in providing standardized culture systems; Xilis and Organoidsciences focus on precision medicine applications; while academic institutions like Wuhan University and EPFL drive fundamental research innovations. Companies like Chuangxin International and Hefei Zhongke Presheng are emerging as significant regional players in Asia, indicating the global distribution of expertise in this developing field.
STEMCELL Technologies Canada, Inc.
Technical Solution: STEMCELL Technologies has developed the IntestiCult™ and CerebralCult™ organoid growth media systems, specifically formulated to support the development of intestinal and brain organoids from stem cells. Their technology focuses on providing defined, serum-free media components that eliminate variability in organoid culture. The company has pioneered specialized extracellular matrix solutions like Matrigel alternatives that offer better batch-to-batch consistency. Their systems include optimized protocols for organoid establishment, expansion, and differentiation that have been validated across multiple stem cell sources. Recent market analysis indicates their organoid culture products have seen adoption in over 80 countries, with particularly strong growth in cancer research applications where patient-derived organoids are increasingly used for personalized medicine approaches. Their technologies support both basic research and translational applications, with documented use in drug screening platforms.
Strengths: Highly specialized media formulations for specific organoid types; excellent batch-to-batch consistency; comprehensive technical support. Weaknesses: Premium pricing compared to generic alternatives; some systems require proprietary components creating dependency; limited flexibility for novel organoid types not covered by their product line.
Organoidsciences Ltd.
Technical Solution: Organoidsciences has developed a comprehensive organoid biobank platform that combines patient-derived organoid (PDO) technology with advanced analytics for drug discovery and personalized medicine. Their system includes proprietary cryopreservation methods that maintain organoid viability and functionality after long-term storage, addressing a critical challenge in the field. The company has implemented standardized protocols for organoid derivation across multiple tissue types, ensuring consistency in their biobank collections. Their platform incorporates automated high-content imaging and machine learning algorithms to quantify drug responses and identify molecular signatures associated with treatment outcomes. Market analysis indicates they have established one of the largest commercial organoid biobanks, with over 1,000 patient-derived models spanning multiple cancer types and rare diseases. The company has developed specialized culture media formulations that enhance organoid establishment rates from difficult-to-culture tissues, achieving approximately 25% higher success rates than standard methods for certain tissue types.
Strengths: Comprehensive biobank resource eliminates need for in-house organoid development; standardized protocols ensure consistency; integrated analytics platform. Weaknesses: Dependency on their proprietary systems for optimal results; subscription-based access model can be costly for smaller research organizations; limited customization options for specialized research questions.
Key Patents and Innovations in Organoid Technology
Hydrogels for cultivating pancreatic organoids
PatentInactiveUS20190367869A1
Innovation
- A chemically defined hydrogel composition with a reduced shear modulus, composed of functionalized polymer molecules and linker molecules, and low-molecular peptides with cell adhesion motifs, which allows for the cultivation and expansion of pancreatic cells into stable and functional 3D organoids without natural extracellular matrix proteins.
Engineering of organoid culture for enhanced organogenesis in a dish
PatentPendingUS20240026261A1
Innovation
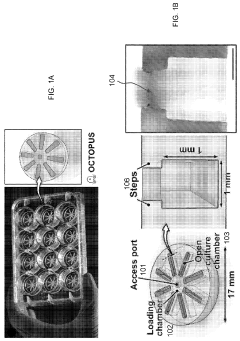
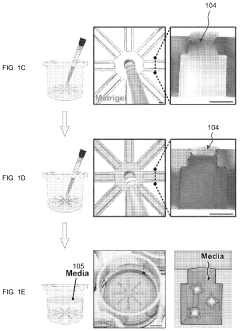
- The OCTOPUS device provides a 3D culture system with radially arranged culture chambers that reduce diffusion limitations by allowing unrestricted access to nutrients and oxygen, enabling continuous culture of organoids for extended periods without passaging, using a simple and scalable design compatible with standard cell culture plates.
Regulatory Framework for Organoid-Based Applications
The regulatory landscape for organoid-based applications is evolving rapidly as these three-dimensional cellular models gain prominence in research and clinical settings. Currently, organoid technologies exist in a regulatory gray area, with frameworks primarily adapted from existing regulations for cell therapies, tissue engineering, and in vitro diagnostic devices rather than being specifically designed for organoid applications.
In the United States, the FDA has begun addressing organoid technologies through its regulatory pathways for biological products, medical devices, or combination products depending on the intended use. Organoids used for drug screening may fall under in vitro diagnostic device regulations, while those intended for transplantation would likely be regulated as biological products or advanced therapy medicinal products (ATMPs).
The European Medicines Agency (EMA) has established more comprehensive frameworks for advanced therapies that can encompass certain organoid applications. The Advanced Therapy Medicinal Products Regulation (Regulation EC No 1394/2007) provides guidance for cell-based products, though specific provisions for organoid technologies remain limited.
Ethical considerations significantly influence regulatory approaches, particularly regarding the use of human-derived materials in organoid development. Informed consent protocols for tissue donation have been expanded in many jurisdictions to specifically address the creation of organoids, especially for cerebral organoids that raise unique ethical questions about consciousness and neural activity.
Data privacy regulations such as GDPR in Europe and HIPAA in the US impact organoid research when patient-derived materials are used, requiring robust protocols for data protection and anonymization. These requirements become increasingly complex when organoids are used for personalized medicine applications where patient identifiability is inherent to the technology's utility.
Standardization remains a critical regulatory challenge, with international bodies like ISO and ICH working to develop consensus standards for organoid characterization, quality control, and manufacturing processes. The lack of standardized protocols currently impedes regulatory harmonization across different markets and applications.
Looking forward, regulatory agencies are increasingly adopting adaptive licensing approaches that allow for progressive authorization as evidence accumulates, particularly relevant for novel technologies like organoids. This reflects recognition of both the transformative potential of organoid technologies and the need for flexible yet robust oversight to ensure safety and efficacy while enabling innovation in this rapidly evolving field.
In the United States, the FDA has begun addressing organoid technologies through its regulatory pathways for biological products, medical devices, or combination products depending on the intended use. Organoids used for drug screening may fall under in vitro diagnostic device regulations, while those intended for transplantation would likely be regulated as biological products or advanced therapy medicinal products (ATMPs).
The European Medicines Agency (EMA) has established more comprehensive frameworks for advanced therapies that can encompass certain organoid applications. The Advanced Therapy Medicinal Products Regulation (Regulation EC No 1394/2007) provides guidance for cell-based products, though specific provisions for organoid technologies remain limited.
Ethical considerations significantly influence regulatory approaches, particularly regarding the use of human-derived materials in organoid development. Informed consent protocols for tissue donation have been expanded in many jurisdictions to specifically address the creation of organoids, especially for cerebral organoids that raise unique ethical questions about consciousness and neural activity.
Data privacy regulations such as GDPR in Europe and HIPAA in the US impact organoid research when patient-derived materials are used, requiring robust protocols for data protection and anonymization. These requirements become increasingly complex when organoids are used for personalized medicine applications where patient identifiability is inherent to the technology's utility.
Standardization remains a critical regulatory challenge, with international bodies like ISO and ICH working to develop consensus standards for organoid characterization, quality control, and manufacturing processes. The lack of standardized protocols currently impedes regulatory harmonization across different markets and applications.
Looking forward, regulatory agencies are increasingly adopting adaptive licensing approaches that allow for progressive authorization as evidence accumulates, particularly relevant for novel technologies like organoids. This reflects recognition of both the transformative potential of organoid technologies and the need for flexible yet robust oversight to ensure safety and efficacy while enabling innovation in this rapidly evolving field.
Ethical Considerations in Organoid Research and Commercialization
The ethical landscape surrounding organoid research and commercialization presents complex challenges that require careful consideration as the market expands. Patient consent and ownership of biological materials remain central concerns, particularly regarding the derivation of organoids from patient tissues. Current frameworks often inadequately address whether patients retain rights to organoids developed from their cells, especially when these models lead to commercial products or intellectual property.
Privacy considerations have become increasingly significant as organoid biobanks grow and patient-derived models proliferate. The potential for organoids to reveal sensitive genetic information about donors necessitates robust data protection protocols that balance scientific advancement with individual privacy rights. This tension becomes particularly acute when considering the long-term storage and distribution of organoid lines across international boundaries with varying regulatory standards.
The commercialization pathway for organoid technologies introduces additional ethical dimensions. As private companies develop proprietary culture systems and organoid-derived products, questions of equitable access emerge. Premium pricing structures for organoid-based drug screening services may create healthcare disparities, with advanced personalized medicine approaches becoming available only to privileged populations or well-resourced healthcare systems.
Animal welfare considerations have evolved alongside organoid technology. While organoids offer alternatives to traditional animal testing, potentially reducing animal use in research, they simultaneously create new ethical questions regarding the development of increasingly complex neural organoids that may possess rudimentary cognitive functions. The scientific community continues to debate appropriate boundaries for cerebral organoid development and the potential need for specialized oversight mechanisms.
Regulatory frameworks for organoid research vary significantly across global markets, creating inconsistent ethical standards. The European Union has implemented more comprehensive guidelines addressing consent and commercialization compared to less structured approaches in emerging markets. This regulatory fragmentation presents challenges for multinational research collaborations and commercial development pipelines, potentially impeding innovation while also creating opportunities for regulatory arbitrage.
Looking forward, stakeholder engagement will be crucial in developing ethical frameworks that can adapt to rapidly evolving organoid technologies. Inclusive dialogue involving patients, researchers, ethicists, industry representatives, and regulatory bodies will be essential to establish consensus standards that protect individual rights while enabling scientific progress and commercial development in this promising field.
Privacy considerations have become increasingly significant as organoid biobanks grow and patient-derived models proliferate. The potential for organoids to reveal sensitive genetic information about donors necessitates robust data protection protocols that balance scientific advancement with individual privacy rights. This tension becomes particularly acute when considering the long-term storage and distribution of organoid lines across international boundaries with varying regulatory standards.
The commercialization pathway for organoid technologies introduces additional ethical dimensions. As private companies develop proprietary culture systems and organoid-derived products, questions of equitable access emerge. Premium pricing structures for organoid-based drug screening services may create healthcare disparities, with advanced personalized medicine approaches becoming available only to privileged populations or well-resourced healthcare systems.
Animal welfare considerations have evolved alongside organoid technology. While organoids offer alternatives to traditional animal testing, potentially reducing animal use in research, they simultaneously create new ethical questions regarding the development of increasingly complex neural organoids that may possess rudimentary cognitive functions. The scientific community continues to debate appropriate boundaries for cerebral organoid development and the potential need for specialized oversight mechanisms.
Regulatory frameworks for organoid research vary significantly across global markets, creating inconsistent ethical standards. The European Union has implemented more comprehensive guidelines addressing consent and commercialization compared to less structured approaches in emerging markets. This regulatory fragmentation presents challenges for multinational research collaborations and commercial development pipelines, potentially impeding innovation while also creating opportunities for regulatory arbitrage.
Looking forward, stakeholder engagement will be crucial in developing ethical frameworks that can adapt to rapidly evolving organoid technologies. Inclusive dialogue involving patients, researchers, ethicists, industry representatives, and regulatory bodies will be essential to establish consensus standards that protect individual rights while enabling scientific progress and commercial development in this promising field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!