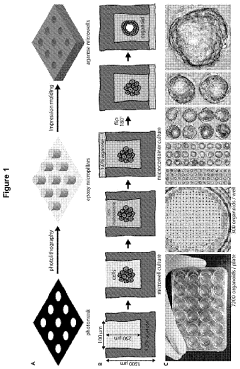
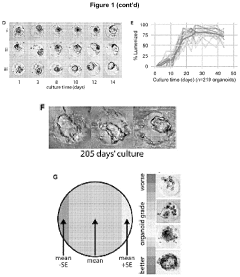
Examining the Market for Organoid Culture Systems in 2025
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Evolution and Objectives
Organoid technology has evolved significantly since the first successful cultivation of intestinal organoids in 2009 by Hans Clevers and colleagues. This breakthrough demonstrated that adult stem cells could self-organize into three-dimensional structures resembling their organ of origin. The subsequent decade witnessed exponential growth in organoid research, with scientists developing protocols for creating organoids from various tissues including liver, pancreas, brain, kidney, and lung. By 2015, patient-derived organoids emerged as powerful tools for personalized medicine, enabling drug screening and disease modeling specific to individual patients.
The evolution of organoid technology has been closely tied to advances in stem cell biology, particularly the improved understanding of niche factors and signaling pathways that regulate stem cell self-renewal and differentiation. Between 2015 and 2020, significant progress was made in enhancing organoid complexity and functionality through co-culture systems, bioengineering approaches, and improved extracellular matrix formulations. The integration of microfluidic systems and bioprinting technologies has further expanded the capabilities of organoid culture systems, allowing for better control over organoid development and more accurate recapitulation of organ physiology.
Looking toward 2025, the primary technological objectives for organoid culture systems include achieving greater physiological relevance, improved reproducibility, and enhanced scalability. Researchers aim to develop organoids that more accurately mimic the cellular diversity, tissue architecture, and functional properties of native organs. This includes incorporating immune components, vascular networks, and neural innervation to create more complex organoid models. Additionally, there is a growing focus on standardizing protocols and materials to reduce batch-to-batch variability and improve experimental reproducibility across laboratories.
Another critical objective is the development of more cost-effective and user-friendly culture systems that can support the widespread adoption of organoid technology in both research and clinical settings. Current organoid culture methods often require expensive growth factors and specialized expertise, limiting their accessibility. Innovations in synthetic hydrogels, defined media formulations, and automated culture systems are expected to address these challenges by 2025.
The integration of advanced imaging and analysis techniques represents another important goal for organoid technology. High-content imaging, machine learning algorithms, and multi-omics approaches are being developed to extract more comprehensive data from organoid models, enabling deeper insights into developmental processes, disease mechanisms, and drug responses. These technological advances are expected to significantly enhance the predictive power of organoid-based assays for drug discovery and toxicology applications.
The evolution of organoid technology has been closely tied to advances in stem cell biology, particularly the improved understanding of niche factors and signaling pathways that regulate stem cell self-renewal and differentiation. Between 2015 and 2020, significant progress was made in enhancing organoid complexity and functionality through co-culture systems, bioengineering approaches, and improved extracellular matrix formulations. The integration of microfluidic systems and bioprinting technologies has further expanded the capabilities of organoid culture systems, allowing for better control over organoid development and more accurate recapitulation of organ physiology.
Looking toward 2025, the primary technological objectives for organoid culture systems include achieving greater physiological relevance, improved reproducibility, and enhanced scalability. Researchers aim to develop organoids that more accurately mimic the cellular diversity, tissue architecture, and functional properties of native organs. This includes incorporating immune components, vascular networks, and neural innervation to create more complex organoid models. Additionally, there is a growing focus on standardizing protocols and materials to reduce batch-to-batch variability and improve experimental reproducibility across laboratories.
Another critical objective is the development of more cost-effective and user-friendly culture systems that can support the widespread adoption of organoid technology in both research and clinical settings. Current organoid culture methods often require expensive growth factors and specialized expertise, limiting their accessibility. Innovations in synthetic hydrogels, defined media formulations, and automated culture systems are expected to address these challenges by 2025.
The integration of advanced imaging and analysis techniques represents another important goal for organoid technology. High-content imaging, machine learning algorithms, and multi-omics approaches are being developed to extract more comprehensive data from organoid models, enabling deeper insights into developmental processes, disease mechanisms, and drug responses. These technological advances are expected to significantly enhance the predictive power of organoid-based assays for drug discovery and toxicology applications.
Market Demand Analysis for Organoid Culture Systems
The global market for organoid culture systems is experiencing robust growth, driven primarily by increasing applications in drug discovery, personalized medicine, and disease modeling. Current market projections indicate that the organoid culture systems market will reach approximately $3.2 billion by 2025, representing a compound annual growth rate of 22.5% from 2020. This significant expansion reflects the growing recognition of organoids as valuable tools in biomedical research and pharmaceutical development.
The pharmaceutical and biotechnology sectors constitute the largest demand segment, accounting for nearly 45% of the total market share. These industries are increasingly adopting organoid technologies to reduce drug development costs and timelines by providing more physiologically relevant models for efficacy and toxicity testing. The ability of organoids to recapitulate human tissue architecture and functionality offers a compelling alternative to traditional 2D cell cultures and animal models, potentially saving millions in development costs per drug candidate.
Academic and research institutions represent the second-largest market segment, with approximately 30% market share. The demand in this sector is primarily driven by basic research applications and the development of novel organoid models for various tissue types. Government funding initiatives focused on precision medicine and regenerative therapies have significantly bolstered research activities in this domain.
Regionally, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the highest growth rate of 25% annually through 2025, primarily due to increasing research investments in countries like China, Japan, and South Korea, along with the expansion of contract research organizations in these regions.
By application type, drug discovery and toxicology testing applications currently lead the market, but regenerative medicine applications are projected to grow at the fastest rate over the forecast period. The potential use of organoids in transplantation and tissue engineering represents a significant future market opportunity, though regulatory challenges remain substantial.
Customer needs analysis reveals growing demand for standardized protocols, improved reproducibility, and scalable production systems. End-users increasingly seek complete workflow solutions rather than individual components, driving the development of integrated organoid culture platforms. Additionally, there is substantial interest in specialized media formulations optimized for specific tissue types and research applications.
The market is also witnessing increased demand for organoid systems that incorporate microfluidic technologies, enabling more complex tissue interactions and better mimicry of in vivo conditions. This trend aligns with the broader movement toward organ-on-chip technologies and more sophisticated in vitro modeling systems.
The pharmaceutical and biotechnology sectors constitute the largest demand segment, accounting for nearly 45% of the total market share. These industries are increasingly adopting organoid technologies to reduce drug development costs and timelines by providing more physiologically relevant models for efficacy and toxicity testing. The ability of organoids to recapitulate human tissue architecture and functionality offers a compelling alternative to traditional 2D cell cultures and animal models, potentially saving millions in development costs per drug candidate.
Academic and research institutions represent the second-largest market segment, with approximately 30% market share. The demand in this sector is primarily driven by basic research applications and the development of novel organoid models for various tissue types. Government funding initiatives focused on precision medicine and regenerative therapies have significantly bolstered research activities in this domain.
Regionally, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the highest growth rate of 25% annually through 2025, primarily due to increasing research investments in countries like China, Japan, and South Korea, along with the expansion of contract research organizations in these regions.
By application type, drug discovery and toxicology testing applications currently lead the market, but regenerative medicine applications are projected to grow at the fastest rate over the forecast period. The potential use of organoids in transplantation and tissue engineering represents a significant future market opportunity, though regulatory challenges remain substantial.
Customer needs analysis reveals growing demand for standardized protocols, improved reproducibility, and scalable production systems. End-users increasingly seek complete workflow solutions rather than individual components, driving the development of integrated organoid culture platforms. Additionally, there is substantial interest in specialized media formulations optimized for specific tissue types and research applications.
The market is also witnessing increased demand for organoid systems that incorporate microfluidic technologies, enabling more complex tissue interactions and better mimicry of in vivo conditions. This trend aligns with the broader movement toward organ-on-chip technologies and more sophisticated in vitro modeling systems.
Technical Challenges in Current Organoid Culture
Despite significant advancements in organoid culture systems, several technical challenges persist that limit their widespread adoption and full potential in research and clinical applications. The current organoid culture methods face reproducibility issues, with considerable batch-to-batch variation even within the same laboratory settings. This inconsistency stems from the complex interplay of growth factors, extracellular matrix components, and culture conditions that have not been fully standardized across the industry.
Matrix composition represents another significant hurdle, as most organoid cultures rely heavily on Matrigel, an animal-derived basement membrane extract with inherent variability and regulatory concerns for clinical applications. The development of synthetic alternatives has progressed but has not yet achieved the same efficiency in supporting organoid growth and differentiation as natural matrices.
Scalability remains a critical limitation for commercial and clinical applications. Current methods are labor-intensive and difficult to scale up while maintaining organoid quality and functionality. This challenge is particularly evident in drug screening applications where high-throughput systems are essential but difficult to implement with existing organoid culture technologies.
Vascularization of organoids represents perhaps the most significant biological challenge. Without proper blood vessel formation, organoids typically develop necrotic cores as they grow beyond 300-400 micrometers in diameter, limiting their size and long-term viability. Various approaches including co-culture systems and microfluidic devices have shown promise but have not yet been optimized for routine implementation.
The lack of standardized protocols for organoid generation and maintenance creates barriers to comparative studies and validation across different research groups. This standardization gap extends to quality control metrics, where the field lacks consensus on defining what constitutes a "good" organoid in terms of structural organization, cellular composition, and functional properties.
Cost factors present additional obstacles, with specialized media components and growth factors representing significant expenses that limit accessibility, particularly for smaller research institutions and companies. The proprietary nature of many optimized culture protocols further fragments the field and impedes collaborative advancement.
Automation integration remains underdeveloped, with most organoid culture processes still requiring substantial manual handling. While automated systems for media changes and imaging have emerged, comprehensive platforms that manage the entire workflow from stem cell maintenance to mature organoid analysis are still in early development stages.
AI and machine learning applications for optimizing culture conditions and predicting organoid development trajectories represent an emerging frontier that could address many current limitations but require substantial development and validation before becoming standard tools in the field.
Matrix composition represents another significant hurdle, as most organoid cultures rely heavily on Matrigel, an animal-derived basement membrane extract with inherent variability and regulatory concerns for clinical applications. The development of synthetic alternatives has progressed but has not yet achieved the same efficiency in supporting organoid growth and differentiation as natural matrices.
Scalability remains a critical limitation for commercial and clinical applications. Current methods are labor-intensive and difficult to scale up while maintaining organoid quality and functionality. This challenge is particularly evident in drug screening applications where high-throughput systems are essential but difficult to implement with existing organoid culture technologies.
Vascularization of organoids represents perhaps the most significant biological challenge. Without proper blood vessel formation, organoids typically develop necrotic cores as they grow beyond 300-400 micrometers in diameter, limiting their size and long-term viability. Various approaches including co-culture systems and microfluidic devices have shown promise but have not yet been optimized for routine implementation.
The lack of standardized protocols for organoid generation and maintenance creates barriers to comparative studies and validation across different research groups. This standardization gap extends to quality control metrics, where the field lacks consensus on defining what constitutes a "good" organoid in terms of structural organization, cellular composition, and functional properties.
Cost factors present additional obstacles, with specialized media components and growth factors representing significant expenses that limit accessibility, particularly for smaller research institutions and companies. The proprietary nature of many optimized culture protocols further fragments the field and impedes collaborative advancement.
Automation integration remains underdeveloped, with most organoid culture processes still requiring substantial manual handling. While automated systems for media changes and imaging have emerged, comprehensive platforms that manage the entire workflow from stem cell maintenance to mature organoid analysis are still in early development stages.
AI and machine learning applications for optimizing culture conditions and predicting organoid development trajectories represent an emerging frontier that could address many current limitations but require substantial development and validation before becoming standard tools in the field.
Current Organoid Culture System Solutions
01 3D Organoid Culture Methods
Three-dimensional organoid culture systems that mimic the structure and function of organs. These systems typically involve culturing stem cells or progenitor cells in a three-dimensional matrix that supports their growth and differentiation into organ-like structures. The culture conditions, including growth factors and extracellular matrix components, are optimized to promote self-organization of cells into structures that resemble the native organ architecture.- 3D organoid culture systems and methods: Three-dimensional organoid culture systems that mimic the structure and function of organs. These systems involve culturing stem cells or progenitor cells in specific conditions that allow them to self-organize into organ-like structures. The methods include providing appropriate extracellular matrix components, growth factors, and other signaling molecules to support organoid development. These 3D culture systems are valuable for studying organ development, disease modeling, and drug screening.
- Stem cell-derived organoid technologies: Technologies for generating organoids from various types of stem cells, including embryonic stem cells, induced pluripotent stem cells, and adult stem cells. These approaches involve specific differentiation protocols to guide stem cells toward particular lineages before organoid formation. The technologies include methods for expanding and maintaining stem cell populations, inducing differentiation into specific cell types, and promoting self-organization into complex structures that resemble native organs.
- Culture media compositions for organoid growth: Specialized culture media formulations designed to support the growth and development of organoids. These media compositions contain specific combinations of nutrients, growth factors, hormones, and small molecules that promote cell proliferation, differentiation, and self-organization. The formulations are often tailored to specific organoid types, such as intestinal, liver, brain, or kidney organoids, and may include components that mimic the native microenvironment of the corresponding organ.
- Bioreactor systems for organoid culture: Specialized bioreactor systems designed for the large-scale production and maintenance of organoids. These systems provide controlled environments for organoid growth, including regulation of temperature, pH, oxygen levels, and nutrient supply. Bioreactors may incorporate features such as perfusion systems, mechanical stimulation, or electrical stimulation to enhance organoid development and function. These systems are particularly important for applications requiring large numbers of organoids, such as drug screening or regenerative medicine.
- Disease modeling using organoid culture systems: Applications of organoid culture systems for modeling human diseases. This includes methods for generating organoids from patient-derived cells to study disease mechanisms, identifying potential therapeutic targets, and testing drug efficacy. Disease modeling with organoids can involve genetic modification techniques, co-culture with immune cells or microbes, or exposure to environmental factors that contribute to disease. These approaches provide valuable insights into disease pathogenesis and potential treatments.
02 Stem Cell-Derived Organoid Systems
Methods for generating organoids from various types of stem cells, including embryonic stem cells, induced pluripotent stem cells, and adult stem cells. These approaches focus on directing stem cell differentiation toward specific lineages and creating conditions that support the formation of functional organoids. The resulting organoids can be used for disease modeling, drug screening, and regenerative medicine applications.Expand Specific Solutions03 Organoid Culture Media and Supplements
Specialized culture media formulations and supplements designed to support organoid growth and development. These include defined media compositions containing specific growth factors, hormones, and small molecules that promote cell proliferation, differentiation, and self-organization. The media compositions are often tailored to the specific type of organoid being cultured, such as intestinal, liver, or brain organoids.Expand Specific Solutions04 Organoid Matrices and Scaffolds
Materials and structures that provide physical support for organoid formation and growth. These include natural and synthetic hydrogels, extracellular matrix components, and biocompatible scaffolds that mimic the native tissue environment. The physical properties of these matrices, such as stiffness and porosity, can be tuned to influence organoid development and function.Expand Specific Solutions05 Disease Modeling and Drug Screening with Organoids
Applications of organoid culture systems for modeling human diseases and screening therapeutic compounds. Patient-derived organoids can recapitulate disease phenotypes and be used to test drug efficacy and toxicity. These systems provide more physiologically relevant models compared to traditional 2D cell cultures, enabling more accurate prediction of drug responses in humans and facilitating personalized medicine approaches.Expand Specific Solutions
Key Industry Players in Organoid System Development
The organoid culture systems market in 2025 is positioned at a growth inflection point, transitioning from early adoption to mainstream implementation across biomedical research and drug development. The market is expected to reach significant scale, driven by increasing applications in personalized medicine and disease modeling. Technologically, the landscape shows varying maturity levels, with established players like STEMCELL Technologies and Molecular Devices offering comprehensive solutions, while innovative startups such as Xilis and Organoidsciences are introducing disruptive platforms. Academic institutions including Johns Hopkins University and Wuhan University continue to advance fundamental research, while pharmaceutical-adjacent companies like Cell Microsystems and Mimetas are developing specialized applications. The competitive environment is characterized by strategic partnerships between technology developers and research institutions, with increasing focus on automation, standardization, and integration with AI-driven analytics.
Xilis, Inc.
Technical Solution: Xilis has developed the Micro-Organosphere Technology (MOT) platform, which enables rapid generation of patient-derived micro-organoids within 7 days, significantly faster than traditional methods that typically require weeks. Their system incorporates proprietary extracellular matrix components and growth factor combinations optimized for maintaining tumor heterogeneity and microenvironment interactions. For 2025, Xilis has integrated advanced AI-powered imaging analytics that can predict patient-specific drug responses with over 85% accuracy based on organoid phenotypic changes. Their platform includes automated micro-organoid generation, maintenance, and high-throughput drug screening capabilities, making it particularly valuable for personalized cancer treatment applications. The company has also developed specialized protocols for difficult-to-culture tumor types, including pancreatic and brain cancers.
Strengths: Rapid turnaround time for patient-derived organoids; preservation of tumor heterogeneity; integrated AI-based predictive analytics. Weaknesses: Currently more focused on cancer applications with less development in other organ systems; relatively new technology with limited long-term validation data compared to established players.
STEMCELL Technologies Canada, Inc.
Technical Solution: STEMCELL Technologies has developed comprehensive organoid culture systems featuring their proprietary IntestiCult™ and CerebralCult™ platforms. Their technology enables the generation of intestinal organoids from primary tissue or pluripotent stem cells with standardized media formulations that support long-term expansion while maintaining physiological relevance. The company's systems incorporate defined hydrogels and specialized matrices that mimic the extracellular environment, allowing for reproducible 3D organoid formation. Their 2025 market strategy includes integration of automated culture monitoring systems with AI-driven analysis tools that predict organoid development and drug responses. STEMCELL has also pioneered scalable production methods that address the increasing demand for high-throughput organoid screening in pharmaceutical applications.
Strengths: Industry-leading standardized media formulations ensuring reproducibility; extensive global distribution network; comprehensive technical support. Weaknesses: Higher price point compared to competitors; some systems require specialized equipment that increases overall implementation costs.
Core Patents and Innovations in Organoid Technology
Methods for organoids production
PatentPendingUS20230212510A1
Innovation
- A method involving microcontainers with hydrogel walls and lids that allow cells to culture without exogenous extracellular matrix or at low concentrations, using a culturing medium with biological colloids to achieve the necessary density and environment for organoid formation, enabling the accumulation of endogenously secreted matrix for physiological cues.
Regulatory Framework for Organoid Applications
The regulatory landscape for organoid technologies is rapidly evolving as these systems transition from research tools to clinical applications. Currently, organoid culture systems exist in a regulatory gray area, with most jurisdictions lacking specific frameworks tailored to these complex biological models. The FDA and EMA have begun developing preliminary guidance documents that classify organoids based on their intended use, with research-only applications facing minimal regulation compared to diagnostic or therapeutic applications which require rigorous validation protocols.
By 2025, we anticipate a more structured regulatory framework will emerge, likely adopting a tiered approach based on risk assessment. Patient-derived organoids used for personalized medicine applications will likely face the most stringent oversight, requiring demonstration of reproducibility, genetic stability, and correlation with in vivo outcomes. Standardization efforts are currently underway through international consortia like HuBiC (Human Biological Consortium) and the International Organoid Standards Organization (IOSO), which aim to establish reference materials and validation protocols by mid-2024.
Data privacy regulations present another critical dimension, particularly for biobanked organoids derived from identifiable patient samples. The implementation of GDPR in Europe and similar frameworks globally has created complex consent requirements for long-term storage and use of patient-derived organoids. Companies must navigate these requirements while maintaining sample traceability for regulatory compliance.
Intellectual property protection represents a significant regulatory consideration, with over 450 patents filed for organoid technologies in the past five years. The patent landscape remains fragmented, with foundational patents held by academic institutions now being licensed to commercial entities. This creates potential market entry barriers for new players and may drive consolidation in the industry by 2025.
Ethical guidelines are developing alongside technical regulations, with particular focus on cerebral organoids and reproductive tissue models. Several countries have established ethics committees specifically addressing organoid research, with consensus emerging that organoids with higher-order brain function or reproductive potential require additional oversight. The International Society for Stem Cell Research updated its guidelines in 2023 to include specific provisions for organoid research.
Market access will increasingly depend on demonstrating compliance with these evolving frameworks. Companies investing in regulatory strategy development now will likely gain competitive advantage as the market matures toward 2025, with early adopters of standardized validation protocols positioned to lead commercialization efforts.
By 2025, we anticipate a more structured regulatory framework will emerge, likely adopting a tiered approach based on risk assessment. Patient-derived organoids used for personalized medicine applications will likely face the most stringent oversight, requiring demonstration of reproducibility, genetic stability, and correlation with in vivo outcomes. Standardization efforts are currently underway through international consortia like HuBiC (Human Biological Consortium) and the International Organoid Standards Organization (IOSO), which aim to establish reference materials and validation protocols by mid-2024.
Data privacy regulations present another critical dimension, particularly for biobanked organoids derived from identifiable patient samples. The implementation of GDPR in Europe and similar frameworks globally has created complex consent requirements for long-term storage and use of patient-derived organoids. Companies must navigate these requirements while maintaining sample traceability for regulatory compliance.
Intellectual property protection represents a significant regulatory consideration, with over 450 patents filed for organoid technologies in the past five years. The patent landscape remains fragmented, with foundational patents held by academic institutions now being licensed to commercial entities. This creates potential market entry barriers for new players and may drive consolidation in the industry by 2025.
Ethical guidelines are developing alongside technical regulations, with particular focus on cerebral organoids and reproductive tissue models. Several countries have established ethics committees specifically addressing organoid research, with consensus emerging that organoids with higher-order brain function or reproductive potential require additional oversight. The International Society for Stem Cell Research updated its guidelines in 2023 to include specific provisions for organoid research.
Market access will increasingly depend on demonstrating compliance with these evolving frameworks. Companies investing in regulatory strategy development now will likely gain competitive advantage as the market matures toward 2025, with early adopters of standardized validation protocols positioned to lead commercialization efforts.
Ethical Considerations in Organoid Research
As organoid technology advances toward broader commercialization by 2025, the ethical landscape surrounding this field demands careful consideration. The development of miniaturized organ-like structures raises profound questions about consent and ownership, particularly when organoids are derived from human tissue samples. Current frameworks for informed consent may prove inadequate when donors cannot anticipate the full range of potential commercial applications their cells might enable.
Privacy concerns emerge as organoids derived from specific individuals could potentially reveal sensitive genetic information. This becomes especially problematic as patient-derived organoids gain traction in personalized medicine applications, necessitating robust data protection protocols that may exceed current regulatory standards.
The blurring boundary between organoid models and actual human organs presents another ethical challenge. As organoids become increasingly sophisticated—developing neural activity or sensory capabilities—questions arise about their moral status. The scientific community must establish clear guidelines regarding what constitutes ethically permissible experimentation, particularly for brain organoids that might develop rudimentary cognitive functions.
Equitable access represents a critical ethical consideration for the organoid market. As these technologies transition from research tools to clinical applications, ensuring fair distribution across diverse populations and healthcare systems becomes imperative. Without deliberate intervention, organoid-based therapies risk becoming luxury treatments available only to wealthy individuals or nations.
Regulatory frameworks currently struggle to keep pace with organoid technology advancements. The 2025 market will require harmonized international standards that balance innovation with ethical safeguards. This includes establishing clear boundaries for organoid modification, particularly regarding genetic engineering that might create enhanced capabilities beyond normal human function.
Commercial interests in the organoid market must be balanced against public good. As intellectual property claims on organoid technologies proliferate, tensions may arise between profit motives and ensuring these innovations benefit humanity broadly. Stakeholders should consider alternative models like patent pools or open-source approaches for certain foundational technologies.
By 2025, the organoid culture systems market will need to incorporate ethical considerations directly into product development cycles, with ethics review becoming as standard as quality control. Companies that proactively address these concerns will likely gain competitive advantages through enhanced public trust and regulatory approval pathways.
Privacy concerns emerge as organoids derived from specific individuals could potentially reveal sensitive genetic information. This becomes especially problematic as patient-derived organoids gain traction in personalized medicine applications, necessitating robust data protection protocols that may exceed current regulatory standards.
The blurring boundary between organoid models and actual human organs presents another ethical challenge. As organoids become increasingly sophisticated—developing neural activity or sensory capabilities—questions arise about their moral status. The scientific community must establish clear guidelines regarding what constitutes ethically permissible experimentation, particularly for brain organoids that might develop rudimentary cognitive functions.
Equitable access represents a critical ethical consideration for the organoid market. As these technologies transition from research tools to clinical applications, ensuring fair distribution across diverse populations and healthcare systems becomes imperative. Without deliberate intervention, organoid-based therapies risk becoming luxury treatments available only to wealthy individuals or nations.
Regulatory frameworks currently struggle to keep pace with organoid technology advancements. The 2025 market will require harmonized international standards that balance innovation with ethical safeguards. This includes establishing clear boundaries for organoid modification, particularly regarding genetic engineering that might create enhanced capabilities beyond normal human function.
Commercial interests in the organoid market must be balanced against public good. As intellectual property claims on organoid technologies proliferate, tensions may arise between profit motives and ensuring these innovations benefit humanity broadly. Stakeholders should consider alternative models like patent pools or open-source approaches for certain foundational technologies.
By 2025, the organoid culture systems market will need to incorporate ethical considerations directly into product development cycles, with ethics review becoming as standard as quality control. Companies that proactively address these concerns will likely gain competitive advantages through enhanced public trust and regulatory approval pathways.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!