How Comparative Studies Inform Organoid Culture Systems
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Evolution and Research Objectives
Organoid technology has evolved significantly since the first successful cultivation of intestinal organoids in 2009 by Hans Clevers and colleagues. This breakthrough demonstrated that adult stem cells could self-organize into three-dimensional structures resembling their tissue of origin when provided with appropriate growth factors and extracellular matrix components. Prior to this milestone, two-dimensional cell culture systems dominated biological research, limiting our understanding of complex tissue architecture and function.
The evolution of organoid technology has been characterized by progressive refinements in culture conditions, expanding from intestinal to multiple organ systems including brain, liver, kidney, and pancreas. Each advancement has required careful optimization of growth factors, extracellular matrix components, and physical parameters to recapitulate the native tissue microenvironment. Comparative studies across different culture systems have been instrumental in identifying critical factors that influence organoid development and maturation.
Recent technological innovations have further enhanced organoid systems through the integration of microfluidics, bioprinting, and advanced biomaterials. These developments have improved the reproducibility, scalability, and physiological relevance of organoid models. Particularly significant has been the incorporation of insights from developmental biology, which has informed the temporal sequence of signaling cues required for proper organogenesis in vitro.
The field has witnessed a paradigm shift from purely descriptive studies to mechanistic investigations that leverage comparative approaches. By systematically varying culture conditions and comparing outcomes across different systems, researchers have identified key parameters that govern organoid formation, cellular differentiation, and tissue-specific functions. These comparative studies have been particularly valuable in elucidating the role of mechanical forces, oxygen tension, and cell-cell interactions in organoid development.
The primary research objectives in the field now focus on several key areas. First, enhancing the maturation and functionality of organoids to more closely mimic adult tissues, as many current systems remain developmentally immature. Second, improving reproducibility and standardization across laboratories to facilitate comparative studies and clinical applications. Third, developing more complex multi-organ systems that can recapitulate tissue interactions and systemic responses.
Additionally, researchers aim to incorporate immune components and vascularization into organoid systems, addressing major limitations of current models. The integration of patient-derived cells into organoid cultures represents another frontier, with significant implications for personalized medicine and disease modeling. Comparative studies across these various approaches are essential for identifying optimal conditions for specific applications and advancing the field toward more physiologically relevant models.
The evolution of organoid technology has been characterized by progressive refinements in culture conditions, expanding from intestinal to multiple organ systems including brain, liver, kidney, and pancreas. Each advancement has required careful optimization of growth factors, extracellular matrix components, and physical parameters to recapitulate the native tissue microenvironment. Comparative studies across different culture systems have been instrumental in identifying critical factors that influence organoid development and maturation.
Recent technological innovations have further enhanced organoid systems through the integration of microfluidics, bioprinting, and advanced biomaterials. These developments have improved the reproducibility, scalability, and physiological relevance of organoid models. Particularly significant has been the incorporation of insights from developmental biology, which has informed the temporal sequence of signaling cues required for proper organogenesis in vitro.
The field has witnessed a paradigm shift from purely descriptive studies to mechanistic investigations that leverage comparative approaches. By systematically varying culture conditions and comparing outcomes across different systems, researchers have identified key parameters that govern organoid formation, cellular differentiation, and tissue-specific functions. These comparative studies have been particularly valuable in elucidating the role of mechanical forces, oxygen tension, and cell-cell interactions in organoid development.
The primary research objectives in the field now focus on several key areas. First, enhancing the maturation and functionality of organoids to more closely mimic adult tissues, as many current systems remain developmentally immature. Second, improving reproducibility and standardization across laboratories to facilitate comparative studies and clinical applications. Third, developing more complex multi-organ systems that can recapitulate tissue interactions and systemic responses.
Additionally, researchers aim to incorporate immune components and vascularization into organoid systems, addressing major limitations of current models. The integration of patient-derived cells into organoid cultures represents another frontier, with significant implications for personalized medicine and disease modeling. Comparative studies across these various approaches are essential for identifying optimal conditions for specific applications and advancing the field toward more physiologically relevant models.
Market Analysis for Organoid-Based Applications
The global organoid market is experiencing robust growth, valued at approximately $1.3 billion in 2022 and projected to reach $5.2 billion by 2030, representing a compound annual growth rate of 19.8%. This significant expansion is driven by increasing applications in drug discovery, personalized medicine, regenerative medicine, and disease modeling. Pharmaceutical and biotechnology companies are the largest market segment, accounting for over 40% of the total market share due to their substantial investments in drug development and toxicity testing using organoid platforms.
Regionally, North America dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China, Japan, and South Korea, is expected to witness the fastest growth rate due to increasing research funding and expanding biotechnology sectors. The United States remains the single largest national market, driven by substantial research funding from organizations like the NIH and strong commercial adoption.
By organoid type, intestinal organoids currently hold the largest market share (approximately 25%), followed by liver, brain, and kidney organoids. This distribution reflects both the relative technical maturity of different organoid systems and the prevalence of associated diseases. Cancer research applications represent the largest end-use segment, comprising nearly 35% of the market, as organoids provide valuable patient-derived tumor models for drug screening and personalized treatment approaches.
Key market drivers include the rising costs of drug development, with organoids offering potential cost reductions by improving predictive accuracy in preclinical stages. Additionally, the growing emphasis on reducing animal testing aligns with organoids' potential as alternative testing platforms. The increasing prevalence of chronic diseases and cancer has also accelerated demand for better disease models and personalized medicine approaches.
Market challenges include standardization issues across organoid culture systems, which comparative studies are helping to address by identifying optimal protocols and quality control parameters. High production costs and technical complexity remain barriers to wider adoption, particularly for smaller research institutions. Regulatory uncertainties regarding the validation of organoid-based testing for clinical and pharmaceutical applications also present challenges that industry stakeholders are actively working to resolve through collaborative efforts with regulatory bodies.
Emerging market opportunities include biobanking of patient-derived organoids, contract research services for organoid-based testing, and the integration of organoids with other technologies such as microfluidics and AI-based analysis platforms. The development of more complex multi-organ systems represents another high-growth potential segment as researchers advance toward more physiologically relevant models.
Regionally, North America dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China, Japan, and South Korea, is expected to witness the fastest growth rate due to increasing research funding and expanding biotechnology sectors. The United States remains the single largest national market, driven by substantial research funding from organizations like the NIH and strong commercial adoption.
By organoid type, intestinal organoids currently hold the largest market share (approximately 25%), followed by liver, brain, and kidney organoids. This distribution reflects both the relative technical maturity of different organoid systems and the prevalence of associated diseases. Cancer research applications represent the largest end-use segment, comprising nearly 35% of the market, as organoids provide valuable patient-derived tumor models for drug screening and personalized treatment approaches.
Key market drivers include the rising costs of drug development, with organoids offering potential cost reductions by improving predictive accuracy in preclinical stages. Additionally, the growing emphasis on reducing animal testing aligns with organoids' potential as alternative testing platforms. The increasing prevalence of chronic diseases and cancer has also accelerated demand for better disease models and personalized medicine approaches.
Market challenges include standardization issues across organoid culture systems, which comparative studies are helping to address by identifying optimal protocols and quality control parameters. High production costs and technical complexity remain barriers to wider adoption, particularly for smaller research institutions. Regulatory uncertainties regarding the validation of organoid-based testing for clinical and pharmaceutical applications also present challenges that industry stakeholders are actively working to resolve through collaborative efforts with regulatory bodies.
Emerging market opportunities include biobanking of patient-derived organoids, contract research services for organoid-based testing, and the integration of organoids with other technologies such as microfluidics and AI-based analysis platforms. The development of more complex multi-organ systems represents another high-growth potential segment as researchers advance toward more physiologically relevant models.
Current Challenges in Organoid Culture Systems
Despite significant advancements in organoid technology, several critical challenges continue to impede progress in developing fully functional and physiologically relevant organoid culture systems. One primary obstacle remains the lack of standardization across protocols, making reproducibility difficult between laboratories. This variability stems from differences in starting cell populations, extracellular matrix compositions, and growth factor concentrations, resulting in heterogeneous organoid populations with inconsistent functionality.
The absence of vascularization represents another major limitation, as organoids typically lack blood vessels necessary for nutrient and oxygen delivery to inner cell layers. This constraint restricts organoid size and leads to necrotic cores in larger structures, ultimately limiting their long-term viability and functional maturation. Current approaches using microfluidic devices or co-culture systems show promise but have not yet achieved full integration of functional vasculature.
Immune system components are notably absent from most organoid models, representing a significant gap in recapitulating in vivo conditions. This absence limits the utility of organoids for studying immune-related diseases, infection responses, and immunotherapeutic applications. While some researchers have begun incorporating immune cells into organoid cultures, achieving proper immune cell diversity and functionality remains challenging.
Matrix dependency presents another obstacle, as most organoid cultures rely heavily on animal-derived matrices like Matrigel, which suffer from batch-to-batch variability and undefined composition. These inconsistencies introduce experimental variables that complicate data interpretation and clinical translation. Development of synthetic alternatives has progressed but has not yet fully replicated the complex biochemical and mechanical properties of natural matrices.
Scalability and cost-effectiveness concerns persist as barriers to widespread adoption of organoid technologies. Current protocols often require expensive growth factors and specialized equipment, limiting accessibility for many research institutions and hampering industrial applications. The labor-intensive nature of organoid culture further complicates high-throughput applications necessary for drug screening or toxicology testing.
Technical challenges in real-time monitoring and analysis of organoid development and function remain significant. Non-invasive methods for assessing organoid health, differentiation status, and functional outputs are limited, making it difficult to track developmental processes without disrupting the culture. Advanced imaging techniques and biosensor integration are emerging but require further refinement for routine implementation.
The absence of vascularization represents another major limitation, as organoids typically lack blood vessels necessary for nutrient and oxygen delivery to inner cell layers. This constraint restricts organoid size and leads to necrotic cores in larger structures, ultimately limiting their long-term viability and functional maturation. Current approaches using microfluidic devices or co-culture systems show promise but have not yet achieved full integration of functional vasculature.
Immune system components are notably absent from most organoid models, representing a significant gap in recapitulating in vivo conditions. This absence limits the utility of organoids for studying immune-related diseases, infection responses, and immunotherapeutic applications. While some researchers have begun incorporating immune cells into organoid cultures, achieving proper immune cell diversity and functionality remains challenging.
Matrix dependency presents another obstacle, as most organoid cultures rely heavily on animal-derived matrices like Matrigel, which suffer from batch-to-batch variability and undefined composition. These inconsistencies introduce experimental variables that complicate data interpretation and clinical translation. Development of synthetic alternatives has progressed but has not yet fully replicated the complex biochemical and mechanical properties of natural matrices.
Scalability and cost-effectiveness concerns persist as barriers to widespread adoption of organoid technologies. Current protocols often require expensive growth factors and specialized equipment, limiting accessibility for many research institutions and hampering industrial applications. The labor-intensive nature of organoid culture further complicates high-throughput applications necessary for drug screening or toxicology testing.
Technical challenges in real-time monitoring and analysis of organoid development and function remain significant. Non-invasive methods for assessing organoid health, differentiation status, and functional outputs are limited, making it difficult to track developmental processes without disrupting the culture. Advanced imaging techniques and biosensor integration are emerging but require further refinement for routine implementation.
Established Comparative Methodologies in Organoid Research
01 3D Organoid Culture Techniques
Three-dimensional organoid culture systems that mimic the structure and function of native organs. These systems typically involve embedding stem cells or progenitor cells in extracellular matrix components to support their growth and differentiation into organ-like structures. The 3D environment allows for proper cell-cell interactions, spatial organization, and development of tissue-specific architecture, resulting in more physiologically relevant models compared to traditional 2D cultures.- 3D Organoid Culture Techniques: Three-dimensional organoid culture systems that mimic in vivo tissue architecture and function. These systems typically involve embedding stem cells or progenitor cells in extracellular matrix components to support self-organization into organ-like structures. The 3D environment allows for proper cell-cell interactions, differentiation, and development of tissue-specific functions that are not achievable in traditional 2D cultures.
- Stem Cell-Derived Organoid Systems: Methods for generating organoids from various types of stem cells, including embryonic stem cells, induced pluripotent stem cells, and adult stem cells. These approaches focus on directing stem cell differentiation toward specific lineages and promoting self-organization into functional organoids. The techniques often involve sequential exposure to growth factors and signaling molecules that recapitulate developmental processes.
- Specialized Media and Growth Factors for Organoid Culture: Formulations of culture media, supplements, and growth factors optimized for organoid development and maintenance. These specialized media compositions contain specific combinations of nutrients, hormones, and signaling molecules that support organoid growth while maintaining physiological relevance. The media formulations are often tissue-specific and designed to promote long-term culture stability.
- Organoid Applications in Disease Modeling and Drug Testing: Methods for using organoid culture systems to model human diseases and test therapeutic compounds. These approaches involve generating organoids from patient-derived cells or introducing disease-specific genetic modifications to create disease models. The resulting organoids can be used for drug screening, toxicity testing, and personalized medicine applications, providing more physiologically relevant results than traditional cell culture models.
- Bioengineering Approaches for Organoid Culture: Advanced bioengineering techniques to enhance organoid culture systems, including microfluidic devices, bioreactors, and scaffolding materials. These technologies provide improved control over the organoid microenvironment, nutrient delivery, and waste removal. Engineered systems can incorporate sensors for real-time monitoring and allow for higher-throughput applications in research and drug development.
02 Stem Cell-Derived Organoid Systems
Culture systems utilizing various types of stem cells, including embryonic, induced pluripotent, and adult stem cells, to generate organoids. These systems employ specific growth factors and signaling molecules to direct stem cell differentiation toward particular organ lineages. The resulting organoids can recapitulate key aspects of organ development, structure, and function, making them valuable tools for studying human development and disease.Expand Specific Solutions03 Specialized Media and Growth Factors for Organoid Culture
Formulations of culture media and growth factor combinations optimized for specific organoid types. These specialized media contain precise ratios of nutrients, hormones, and signaling molecules that support organoid formation, growth, and maintenance. Different organoid types (brain, liver, intestinal, etc.) require unique media compositions to properly recapitulate their native tissue environments and cellular functions.Expand Specific Solutions04 Organoid Culture Platforms and Devices
Specialized equipment, vessels, and microfluidic systems designed specifically for organoid culture. These platforms may incorporate features such as controlled perfusion, mechanical stimulation, or gradient generation to better mimic physiological conditions. Advanced systems may integrate biosensors for real-time monitoring of organoid function or allow for high-throughput screening applications in drug discovery and toxicology studies.Expand Specific Solutions05 Disease Modeling and Drug Testing with Organoids
Applications of organoid culture systems for modeling human diseases and testing therapeutic compounds. Patient-derived organoids can recapitulate disease phenotypes, enabling personalized medicine approaches. These systems allow for the study of disease mechanisms, identification of potential drug targets, and evaluation of drug efficacy and toxicity in a physiologically relevant context, potentially reducing reliance on animal models in preclinical research.Expand Specific Solutions
Leading Research Institutions and Biotech Companies
The organoid culture systems market is currently in a growth phase, characterized by increasing adoption across research and clinical applications. The global market size is expanding rapidly, driven by advancements in 3D cell culture technologies and growing applications in disease modeling and drug discovery. Technologically, the field shows varying maturity levels, with companies like Cincinnati Children's Hospital Medical Center and Memorial Sloan Kettering Cancer Center leading fundamental research, while commercial entities such as Cellesce Ltd. and Cell Microsystems are developing specialized platforms for organoid culture automation. Pharmaceutical giants including Takeda Pharmaceutical and JSR Corp. are investing in organoid technologies for drug development, while biotechnology firms like Chuangxin International Biotechnology and Shanghai Ruiyu Biotech are focusing on clinical applications. Academic institutions including Johns Hopkins University and Columbia University continue to drive innovation through comparative studies that inform improvements in organoid culture methodologies.
The Johns Hopkins University
Technical Solution: Johns Hopkins University has developed advanced organoid culture systems informed by comparative studies across multiple tissue types. Their approach integrates microfluidic technology with 3D matrix systems to create more physiologically relevant organoid environments. Their researchers have pioneered methods that compare traditional 2D cultures with 3D organoid systems, identifying critical factors for maintaining stemness and differentiation. Johns Hopkins has particularly excelled in brain organoid development, where they've implemented comparative genomic and transcriptomic analyses to validate organoid models against primary tissue samples. Their platform incorporates real-time monitoring of organoid development using advanced imaging techniques, allowing for dynamic assessment of morphological and functional changes. This comparative approach has led to refinements in media composition and extracellular matrix formulations that better recapitulate in vivo conditions.
Strengths: Strong integration of multi-omics data to validate organoid models against primary tissues; excellent translational research infrastructure connecting basic science to clinical applications. Weaknesses: Their systems often require specialized equipment and expertise, potentially limiting widespread adoption; some of their more advanced platforms have higher costs associated with implementation.
Memorial Sloan Kettering Cancer Center
Technical Solution: Memorial Sloan Kettering has developed innovative organoid culture systems specifically focused on cancer modeling. Their approach utilizes comparative studies between patient-derived tumor samples and corresponding organoids to validate model fidelity. MSK researchers have established protocols for patient-derived organoids (PDOs) that maintain the genetic and phenotypic characteristics of original tumors, enabling personalized medicine applications. Their comparative studies have informed the development of specialized media formulations that support the growth of difficult-to-culture tumor types. MSK has pioneered co-culture systems that incorporate immune cells and stromal components alongside tumor organoids, creating more comprehensive models of the tumor microenvironment. Their comparative analyses between organoid drug responses and clinical outcomes have validated these models as predictive tools for therapeutic selection. Additionally, they've developed high-throughput screening platforms specifically optimized for organoid-based drug discovery.
Strengths: Exceptional expertise in cancer-specific organoid models with direct clinical applications; robust biobanking infrastructure supporting large-scale comparative studies. Weaknesses: Primary focus on cancer may limit applicability to other disease models; their systems often require patient samples which introduces variability and logistical challenges.
Key Scientific Breakthroughs in Comparative Organoid Studies
Methods for organoids production
PatentWO2021225794A1
Innovation
- A method involving microcontainers with hydrogel walls and lids that allow cells to self-organize without exogenous extracellular matrix, using a culturing medium with biological colloids to achieve a higher density than the hydrogel lid, enabling the formation of organoids that exhibit contractility and physiological differentiation.
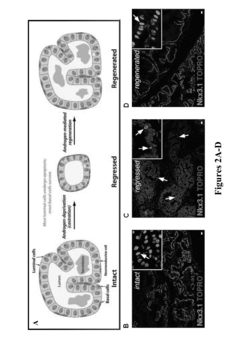
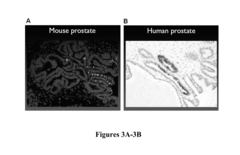
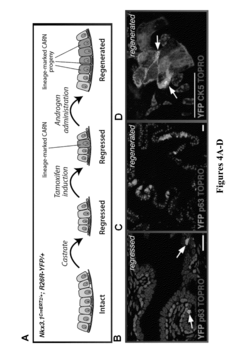
Method for culture of human and mouse prostate organoids and uses thereof
PatentInactiveUS20150329829A1
Innovation
- A method involving the culture of prostate organoids using a medium comprising basal hepatocyte medium, Matrigel, FBS, and a ROCK inhibitor, which allows dissociated prostate epithelial cells to form organoids that display luminal or basal differentiation and maintain the transformed phenotype of prostate tumors, enabling the growth of both normal and cancerous prostate tissue.
Standardization Efforts in Organoid Research
The field of organoid research has experienced rapid growth, yet the lack of standardized protocols has led to significant variability in experimental outcomes. Recognizing this challenge, several international initiatives have emerged to establish common standards for organoid culture systems. The Human Cell Atlas project, launched in 2016, represents one of the earliest coordinated efforts to map all human cells, including those derived from organoids, using standardized methodologies.
In 2018, the International Society for Stem Cell Research (ISSCR) published guidelines specifically addressing organoid research, providing recommendations for culture conditions, characterization methods, and reporting standards. These guidelines emphasize the importance of detailed documentation of culture media components, growth factors, and environmental conditions to ensure reproducibility across laboratories.
The NIH-funded Human BioMolecular Atlas Program (HuBMAP) has also contributed significantly to standardization efforts by developing common protocols for organoid generation and analysis. Their framework includes standardized methods for tissue procurement, cell isolation, organoid culture, and multi-omics characterization.
European initiatives like the Horizon 2020-funded ORGANO-STRAT consortium have focused on creating reference materials and quality control metrics for organoid cultures. Their work has established benchmark organoid lines that serve as controls for validating new protocols and technologies across different research groups.
Commercial entities have also played a crucial role in standardization. Companies such as STEMCELL Technologies and Corning have developed defined media formulations and growth matrices that reduce batch-to-batch variability, although proprietary concerns sometimes limit full disclosure of components.
Recent collaborative efforts have focused on establishing minimum information standards for reporting organoid experiments. The Minimum Information about Organoid Experiments (MI-OE) initiative, launched in 2021, provides a framework for comprehensive documentation of experimental conditions, enabling more effective comparison across studies.
Despite these advances, challenges remain in harmonizing protocols across different organ systems and disease models. The balance between standardization and flexibility remains a key consideration, as overly rigid protocols may limit innovation while excessive variability undermines reproducibility. Future standardization efforts will likely focus on developing organ-specific guidelines while maintaining core principles applicable across all organoid systems.
In 2018, the International Society for Stem Cell Research (ISSCR) published guidelines specifically addressing organoid research, providing recommendations for culture conditions, characterization methods, and reporting standards. These guidelines emphasize the importance of detailed documentation of culture media components, growth factors, and environmental conditions to ensure reproducibility across laboratories.
The NIH-funded Human BioMolecular Atlas Program (HuBMAP) has also contributed significantly to standardization efforts by developing common protocols for organoid generation and analysis. Their framework includes standardized methods for tissue procurement, cell isolation, organoid culture, and multi-omics characterization.
European initiatives like the Horizon 2020-funded ORGANO-STRAT consortium have focused on creating reference materials and quality control metrics for organoid cultures. Their work has established benchmark organoid lines that serve as controls for validating new protocols and technologies across different research groups.
Commercial entities have also played a crucial role in standardization. Companies such as STEMCELL Technologies and Corning have developed defined media formulations and growth matrices that reduce batch-to-batch variability, although proprietary concerns sometimes limit full disclosure of components.
Recent collaborative efforts have focused on establishing minimum information standards for reporting organoid experiments. The Minimum Information about Organoid Experiments (MI-OE) initiative, launched in 2021, provides a framework for comprehensive documentation of experimental conditions, enabling more effective comparison across studies.
Despite these advances, challenges remain in harmonizing protocols across different organ systems and disease models. The balance between standardization and flexibility remains a key consideration, as overly rigid protocols may limit innovation while excessive variability undermines reproducibility. Future standardization efforts will likely focus on developing organ-specific guidelines while maintaining core principles applicable across all organoid systems.
Ethical and Regulatory Considerations for Organoid Technology
The advancement of organoid technology raises significant ethical and regulatory considerations that must be addressed as comparative studies continue to inform culture system development. Patient consent for tissue donation represents a foundational ethical concern, particularly when organoids are derived from clinical samples. The informed consent process must clearly communicate how tissues will be used in research, potential commercialization pathways, and the possibility of generating genetic information that could impact donors and their families.
Privacy considerations become increasingly complex as organoid biobanks expand and patient-derived models are shared across research institutions. Comparative studies involving multiple tissue sources require robust data protection frameworks to prevent unauthorized access to sensitive genetic information while still enabling scientific collaboration. The development of standardized anonymization protocols specifically designed for organoid research remains an urgent priority.
Regulatory frameworks for organoid technology vary significantly across jurisdictions, creating challenges for international collaborative research. While some regions have established clear guidelines for human tissue research, many lack specific provisions addressing the unique characteristics of organoids as self-organizing structures with developmental potential. Comparative studies that inform culture system improvements must navigate these regulatory disparities, particularly when exchanging materials between institutions under different governance structures.
The potential development of increasingly complex organoids, especially cerebral organoids with rudimentary neural activity, raises profound questions about moral status and ethical boundaries. As comparative studies enhance culture systems to produce more physiologically accurate models, researchers must establish clear limitations regarding organoid complexity and functionality. This includes developing consensus guidelines on appropriate experimental endpoints and restrictions on certain types of organoid-animal chimera research.
Commercialization and intellectual property considerations present additional challenges as organoid technologies move toward clinical applications. Patent landscapes surrounding culture methods, growth factors, and organoid-derived therapies are becoming increasingly complex. Equitable access to these technologies, particularly in resource-limited settings, requires thoughtful approaches to licensing and technology transfer that balance innovation incentives with public health needs.
Stakeholder engagement represents a critical component of ethical governance for organoid research. Incorporating diverse perspectives—including patients, ethicists, regulatory experts, and the broader public—into policy development can help ensure that comparative studies and resulting culture system improvements align with societal values and priorities. Transparent communication about both the potential benefits and limitations of organoid technology is essential for maintaining public trust in this rapidly evolving field.
Privacy considerations become increasingly complex as organoid biobanks expand and patient-derived models are shared across research institutions. Comparative studies involving multiple tissue sources require robust data protection frameworks to prevent unauthorized access to sensitive genetic information while still enabling scientific collaboration. The development of standardized anonymization protocols specifically designed for organoid research remains an urgent priority.
Regulatory frameworks for organoid technology vary significantly across jurisdictions, creating challenges for international collaborative research. While some regions have established clear guidelines for human tissue research, many lack specific provisions addressing the unique characteristics of organoids as self-organizing structures with developmental potential. Comparative studies that inform culture system improvements must navigate these regulatory disparities, particularly when exchanging materials between institutions under different governance structures.
The potential development of increasingly complex organoids, especially cerebral organoids with rudimentary neural activity, raises profound questions about moral status and ethical boundaries. As comparative studies enhance culture systems to produce more physiologically accurate models, researchers must establish clear limitations regarding organoid complexity and functionality. This includes developing consensus guidelines on appropriate experimental endpoints and restrictions on certain types of organoid-animal chimera research.
Commercialization and intellectual property considerations present additional challenges as organoid technologies move toward clinical applications. Patent landscapes surrounding culture methods, growth factors, and organoid-derived therapies are becoming increasingly complex. Equitable access to these technologies, particularly in resource-limited settings, requires thoughtful approaches to licensing and technology transfer that balance innovation incentives with public health needs.
Stakeholder engagement represents a critical component of ethical governance for organoid research. Incorporating diverse perspectives—including patients, ethicists, regulatory experts, and the broader public—into policy development can help ensure that comparative studies and resulting culture system improvements align with societal values and priorities. Transparent communication about both the potential benefits and limitations of organoid technology is essential for maintaining public trust in this rapidly evolving field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!