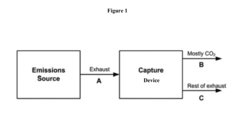
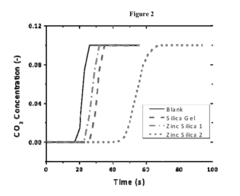
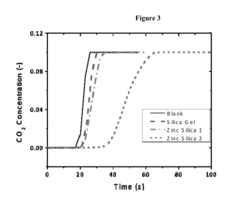
Comparison of Carbon Capture Sorbents for Energy Applications
OCT 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Capture Technology Evolution and Objectives
Carbon capture technology has evolved significantly over the past several decades, transitioning from theoretical concepts to practical applications in response to growing environmental concerns. The initial development phase in the 1970s focused primarily on basic separation principles, with limited commercial implementation due to cost constraints and lack of regulatory drivers. By the 1990s, increased awareness of climate change accelerated research efforts, leading to the first generation of commercial carbon capture systems primarily deployed in natural gas processing facilities.
The evolution accelerated in the early 2000s with the emergence of post-combustion, pre-combustion, and oxy-fuel combustion approaches. Post-combustion technologies, particularly amine-based absorption systems, gained prominence for their retrofit compatibility with existing power plants. Concurrently, solid sorbent materials began receiving attention as potentially more energy-efficient alternatives to liquid solvents.
The 2010s marked a significant shift toward advanced materials research, with substantial investment in developing novel sorbents with improved CO2 selectivity, capacity, and regeneration characteristics. Metal-organic frameworks (MOFs), zeolites, activated carbons, and functionalized polymers emerged as promising candidates, each offering unique advantages for specific capture scenarios. This period also witnessed increased focus on process integration and energy penalty reduction.
Current technological objectives center on addressing the critical challenges that have historically limited widespread carbon capture deployment. Primary among these is reducing the energy penalty associated with sorbent regeneration, which typically accounts for 70-80% of operational costs. Researchers aim to develop sorbents that can achieve capture efficiencies exceeding 90% while requiring regeneration energies below 2 GJ/tonne CO2, a significant improvement over first-generation systems requiring 3-4 GJ/tonne.
Additional objectives include enhancing sorbent durability to withstand thousands of adsorption-desorption cycles without significant performance degradation, improving tolerance to contaminants present in flue gases, and reducing material production costs to enable economic viability at scale. The development of multi-functional sorbents capable of capturing CO2 alongside other pollutants represents another frontier in research.
Looking forward, the field is increasingly focused on direct air capture applications, which demand sorbents with exceptional selectivity given the low atmospheric concentration of CO2. The ultimate goal remains developing carbon capture solutions that are economically competitive without subsidies, environmentally benign throughout their lifecycle, and capable of deployment at the gigaton scale necessary to meaningfully impact global carbon emissions.
The evolution accelerated in the early 2000s with the emergence of post-combustion, pre-combustion, and oxy-fuel combustion approaches. Post-combustion technologies, particularly amine-based absorption systems, gained prominence for their retrofit compatibility with existing power plants. Concurrently, solid sorbent materials began receiving attention as potentially more energy-efficient alternatives to liquid solvents.
The 2010s marked a significant shift toward advanced materials research, with substantial investment in developing novel sorbents with improved CO2 selectivity, capacity, and regeneration characteristics. Metal-organic frameworks (MOFs), zeolites, activated carbons, and functionalized polymers emerged as promising candidates, each offering unique advantages for specific capture scenarios. This period also witnessed increased focus on process integration and energy penalty reduction.
Current technological objectives center on addressing the critical challenges that have historically limited widespread carbon capture deployment. Primary among these is reducing the energy penalty associated with sorbent regeneration, which typically accounts for 70-80% of operational costs. Researchers aim to develop sorbents that can achieve capture efficiencies exceeding 90% while requiring regeneration energies below 2 GJ/tonne CO2, a significant improvement over first-generation systems requiring 3-4 GJ/tonne.
Additional objectives include enhancing sorbent durability to withstand thousands of adsorption-desorption cycles without significant performance degradation, improving tolerance to contaminants present in flue gases, and reducing material production costs to enable economic viability at scale. The development of multi-functional sorbents capable of capturing CO2 alongside other pollutants represents another frontier in research.
Looking forward, the field is increasingly focused on direct air capture applications, which demand sorbents with exceptional selectivity given the low atmospheric concentration of CO2. The ultimate goal remains developing carbon capture solutions that are economically competitive without subsidies, environmentally benign throughout their lifecycle, and capable of deployment at the gigaton scale necessary to meaningfully impact global carbon emissions.
Market Analysis for Carbon Capture Solutions
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market size has reached approximately $7 billion, with projections indicating expansion to $20 billion by 2030, representing a compound annual growth rate of 16.3%. This growth trajectory is supported by substantial government investments, with the United States allocating $12 billion for carbon capture development through the Infrastructure Investment and Jobs Act, and the European Union committing €10 billion through various climate innovation funds.
The demand landscape for carbon capture solutions spans multiple sectors, with power generation, cement production, and steel manufacturing emerging as primary markets. Power generation currently accounts for 45% of the total addressable market, followed by industrial applications at 35% and direct air capture initiatives at 20%. Geographically, North America leads adoption with 40% market share, followed by Europe at 30%, and Asia-Pacific rapidly expanding at 25% with China making substantial investments in clean coal technologies.
Customer segmentation reveals three distinct buyer categories: large industrial emitters seeking compliance solutions, energy companies pursuing enhanced oil recovery applications, and forward-thinking corporations implementing carbon neutrality strategies. Price sensitivity varies significantly across these segments, with compliance-driven customers demonstrating greater willingness to pay premium prices for proven technologies with established performance metrics.
The competitive landscape features both established industrial gas companies and innovative startups. Traditional players like Air Liquide, Linde, and Mitsubishi Heavy Industries control approximately 60% of current market share, leveraging their extensive industrial infrastructure and customer relationships. However, venture-backed startups focusing on novel sorbent technologies have attracted over $2 billion in funding since 2020, indicating strong investor confidence in technological disruption potential.
Market barriers include high capital expenditure requirements, with typical industrial-scale installations costing between $400-800 million, and uncertain regulatory frameworks in emerging markets. Additionally, the absence of standardized carbon pricing mechanisms in many regions creates economic uncertainty for potential adopters. Despite these challenges, the implementation of carbon pricing in 46 countries has created tangible economic incentives for carbon capture deployment.
Future market growth will likely be driven by technological innovations reducing capture costs, currently averaging $58-120 per ton depending on the application. Particularly promising are advancements in solid sorbent technologies, which demonstrate potential for 30-40% cost reductions compared to conventional amine-based solutions. As these technologies mature and economies of scale develop, market penetration is expected to accelerate significantly across all industrial sectors.
The demand landscape for carbon capture solutions spans multiple sectors, with power generation, cement production, and steel manufacturing emerging as primary markets. Power generation currently accounts for 45% of the total addressable market, followed by industrial applications at 35% and direct air capture initiatives at 20%. Geographically, North America leads adoption with 40% market share, followed by Europe at 30%, and Asia-Pacific rapidly expanding at 25% with China making substantial investments in clean coal technologies.
Customer segmentation reveals three distinct buyer categories: large industrial emitters seeking compliance solutions, energy companies pursuing enhanced oil recovery applications, and forward-thinking corporations implementing carbon neutrality strategies. Price sensitivity varies significantly across these segments, with compliance-driven customers demonstrating greater willingness to pay premium prices for proven technologies with established performance metrics.
The competitive landscape features both established industrial gas companies and innovative startups. Traditional players like Air Liquide, Linde, and Mitsubishi Heavy Industries control approximately 60% of current market share, leveraging their extensive industrial infrastructure and customer relationships. However, venture-backed startups focusing on novel sorbent technologies have attracted over $2 billion in funding since 2020, indicating strong investor confidence in technological disruption potential.
Market barriers include high capital expenditure requirements, with typical industrial-scale installations costing between $400-800 million, and uncertain regulatory frameworks in emerging markets. Additionally, the absence of standardized carbon pricing mechanisms in many regions creates economic uncertainty for potential adopters. Despite these challenges, the implementation of carbon pricing in 46 countries has created tangible economic incentives for carbon capture deployment.
Future market growth will likely be driven by technological innovations reducing capture costs, currently averaging $58-120 per ton depending on the application. Particularly promising are advancements in solid sorbent technologies, which demonstrate potential for 30-40% cost reductions compared to conventional amine-based solutions. As these technologies mature and economies of scale develop, market penetration is expected to accelerate significantly across all industrial sectors.
Current Sorbent Technologies and Limitations
Carbon capture technologies have evolved significantly over the past decades, with various sorbent materials being developed to address the growing need for CO2 reduction in energy applications. Currently, the most widely deployed sorbent technologies include amine-based solvents, solid adsorbents, membrane systems, and cryogenic separation methods. Each of these approaches presents distinct advantages and limitations that affect their commercial viability and environmental impact.
Amine-based solvents, particularly monoethanolamine (MEA), represent the most mature technology with extensive industrial implementation. These solvents offer high CO2 absorption capacity (typically 0.4-0.5 g CO2/g sorbent) and selectivity. However, they suffer from significant drawbacks including high regeneration energy requirements (3.5-4.2 GJ/ton CO2), equipment corrosion issues, and degradation through oxidation and thermal stress. The solvent loss rate (1.4-3.1 kg/ton CO2) also contributes to operational costs and environmental concerns.
Solid adsorbents such as zeolites, metal-organic frameworks (MOFs), and activated carbons have emerged as promising alternatives. Zeolites demonstrate good CO2 selectivity but show performance degradation in humid conditions and require substantial energy for regeneration. MOFs offer exceptional surface areas (up to 7000 m²/g) and tunable pore structures, enabling theoretical capacities exceeding 1.5 g CO2/g sorbent. However, their commercial deployment remains limited due to synthesis complexity, cost barriers, and stability concerns in industrial environments.
Calcium looping technology, utilizing limestone as a sorbent, presents a cost-effective option for high-temperature applications. While the raw material is abundant and inexpensive, the process suffers from rapid capacity degradation over multiple cycles, with performance typically declining by 30-40% after just 20 cycles due to sintering and sulfation reactions.
Membrane-based systems offer energy efficiency advantages and operational simplicity but face challenges in achieving both high permeability and selectivity simultaneously. Current polymeric membranes demonstrate a performance trade-off, with those exhibiting high CO2 permeability typically showing lower selectivity, limiting their practical application in mixed gas streams.
Emerging technologies include ionic liquids and phase-change solvents, which show promise in reducing regeneration energy requirements by 30-40% compared to conventional amines. However, these materials face challenges related to viscosity, cost, and long-term stability under industrial conditions.
A critical limitation across most current sorbent technologies is the energy penalty associated with regeneration, which typically reduces power plant efficiency by 7-12 percentage points. This energy requirement significantly impacts the economic viability of carbon capture implementation in energy applications, particularly for retrofitting existing facilities.
Amine-based solvents, particularly monoethanolamine (MEA), represent the most mature technology with extensive industrial implementation. These solvents offer high CO2 absorption capacity (typically 0.4-0.5 g CO2/g sorbent) and selectivity. However, they suffer from significant drawbacks including high regeneration energy requirements (3.5-4.2 GJ/ton CO2), equipment corrosion issues, and degradation through oxidation and thermal stress. The solvent loss rate (1.4-3.1 kg/ton CO2) also contributes to operational costs and environmental concerns.
Solid adsorbents such as zeolites, metal-organic frameworks (MOFs), and activated carbons have emerged as promising alternatives. Zeolites demonstrate good CO2 selectivity but show performance degradation in humid conditions and require substantial energy for regeneration. MOFs offer exceptional surface areas (up to 7000 m²/g) and tunable pore structures, enabling theoretical capacities exceeding 1.5 g CO2/g sorbent. However, their commercial deployment remains limited due to synthesis complexity, cost barriers, and stability concerns in industrial environments.
Calcium looping technology, utilizing limestone as a sorbent, presents a cost-effective option for high-temperature applications. While the raw material is abundant and inexpensive, the process suffers from rapid capacity degradation over multiple cycles, with performance typically declining by 30-40% after just 20 cycles due to sintering and sulfation reactions.
Membrane-based systems offer energy efficiency advantages and operational simplicity but face challenges in achieving both high permeability and selectivity simultaneously. Current polymeric membranes demonstrate a performance trade-off, with those exhibiting high CO2 permeability typically showing lower selectivity, limiting their practical application in mixed gas streams.
Emerging technologies include ionic liquids and phase-change solvents, which show promise in reducing regeneration energy requirements by 30-40% compared to conventional amines. However, these materials face challenges related to viscosity, cost, and long-term stability under industrial conditions.
A critical limitation across most current sorbent technologies is the energy penalty associated with regeneration, which typically reduces power plant efficiency by 7-12 percentage points. This energy requirement significantly impacts the economic viability of carbon capture implementation in energy applications, particularly for retrofitting existing facilities.
Comparative Analysis of Current Sorbent Materials
01 Metal-organic frameworks (MOFs) for carbon capture
Metal-organic frameworks are advanced porous materials with high surface area that can effectively capture carbon dioxide. These crystalline structures consist of metal ions coordinated with organic ligands, creating a framework with tunable pore sizes ideal for selective CO2 adsorption. MOFs can be designed with specific functional groups to enhance CO2 binding affinity and selectivity, making them promising sorbents for carbon capture applications.- Metal-organic frameworks (MOFs) for carbon capture: Metal-organic frameworks are advanced porous materials with high surface area that can be engineered for selective CO2 adsorption. These crystalline structures consist of metal ions coordinated to organic ligands, creating a framework with tunable pore sizes and functionalities. MOFs demonstrate exceptional CO2 capture capacity under various conditions and can be modified with specific functional groups to enhance selectivity and adsorption efficiency.
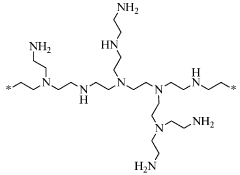
- Amine-functionalized sorbents: Amine-functionalized materials represent a significant class of carbon capture sorbents that operate through chemical adsorption mechanisms. These sorbents contain primary, secondary, or tertiary amine groups that react with CO2 to form carbamates or bicarbonates. Common supports include silica, polymers, and porous carbon materials that are impregnated or grafted with amines to create high-capacity CO2 capture materials with good selectivity even at low CO2 concentrations.
- Zeolite and molecular sieve-based carbon capture: Zeolites and molecular sieves are aluminosilicate materials with well-defined pore structures that enable molecular sieving of CO2 from gas mixtures. These materials offer thermal stability and can be tailored through ion exchange and framework composition modifications to enhance CO2 selectivity. Their crystalline structure provides uniform pore sizes that can be optimized for carbon dioxide adsorption while excluding other gases based on molecular dimensions.
- Regenerable solid sorbents with enhanced cycling stability: Advanced regenerable sorbents focus on maintaining performance over multiple adsorption-desorption cycles, which is crucial for practical carbon capture applications. These materials incorporate stabilizing components and structural reinforcements to prevent degradation during temperature or pressure swing regeneration processes. Innovations include composite structures, core-shell architectures, and support materials that minimize sorbent attrition and maintain CO2 capture capacity over thousands of cycles.
- Biomass-derived and sustainable carbon capture materials: Environmentally friendly carbon capture sorbents derived from biomass and sustainable sources offer a green approach to CO2 sequestration. These materials include activated carbons from agricultural waste, chitosan-based adsorbents, and biochar modified with functional groups. The production processes typically require less energy than conventional sorbents, and the materials can be engineered to have high surface areas and tailored pore structures while maintaining biodegradability or recyclability.
02 Amine-functionalized sorbents
Amine-functionalized materials represent a significant class of carbon capture sorbents that utilize chemical adsorption mechanisms. These sorbents contain primary, secondary, or tertiary amine groups that react with CO2 to form carbamates or bicarbonates. The amine functionality can be incorporated into various support materials including silica, polymers, and porous carbons. These materials typically offer high CO2 selectivity and capacity under various operating conditions, including low CO2 partial pressures.Expand Specific Solutions03 Zeolite and molecular sieve-based carbon capture
Zeolites and molecular sieves are aluminosilicate materials with well-defined microporous structures that can selectively adsorb carbon dioxide. These materials utilize physical adsorption mechanisms based on molecular sieving effects and electrostatic interactions. Their crystalline structure provides uniform pore sizes that can be tailored for optimal CO2 capture. The high thermal stability of zeolites makes them suitable for temperature swing adsorption processes in industrial carbon capture applications.Expand Specific Solutions04 Regenerable carbon capture sorbents
Regenerable carbon capture sorbents are designed to undergo multiple adsorption-desorption cycles without significant loss of capacity. These materials can be regenerated through temperature swing, pressure swing, or vacuum swing processes to release captured CO2 and prepare the sorbent for reuse. Key considerations include energy requirements for regeneration, mechanical stability during cycling, and resistance to contaminants. Advanced regenerable sorbents incorporate phase-change materials or utilize novel desorption mechanisms to reduce energy penalties.Expand Specific Solutions05 Composite and hybrid carbon capture materials
Composite and hybrid carbon capture materials combine multiple components to achieve enhanced performance characteristics. These materials typically integrate different functional elements such as polymers with inorganic supports, or combine multiple capture mechanisms within a single material. Examples include polymer-inorganic composites, mixed matrix materials, and hierarchical porous structures. The synergistic effects between components can lead to improved adsorption capacity, selectivity, kinetics, and mechanical properties compared to single-component sorbents.Expand Specific Solutions
Leading Organizations in Carbon Capture Industry
Carbon capture technology is currently in a transitional phase from early commercialization to wider adoption, with the global market expected to reach $7-10 billion by 2030. The competitive landscape features established energy players alongside emerging specialists. Chinese institutions like Huaneng Clean Energy Research Institute and State Grid Corp. lead in large-scale implementation, while academic institutions such as Zhejiang University and University of Kentucky Research Foundation drive fundamental research. Energy majors including Schlumberger and Chevron are integrating carbon capture into their sustainability strategies. Technical maturity varies significantly across sorbent types, with traditional amine-based technologies being most mature, while novel materials developed by companies like BASF and PQ Silicas show promising efficiency improvements but require further scale-up validation.
Huaneng Clean Energy Research Institute
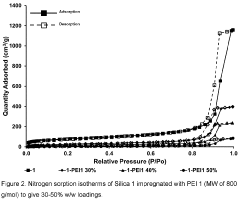
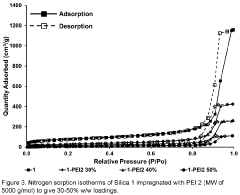
Technical Solution: Huaneng Clean Energy Research Institute has developed advanced amine-based carbon capture sorbents specifically optimized for coal-fired power plants. Their proprietary technology utilizes a modified polyethyleneimine (PEI) impregnated on mesoporous silica support, achieving CO2 capture capacities of 3.2-4.5 mmol/g under typical flue gas conditions. The institute has successfully demonstrated this technology at their Shidongkou Power Plant, where they achieved over 90% CO2 capture efficiency with significantly reduced energy penalties compared to conventional amine scrubbing. Their latest generation sorbents incorporate heat-resistant additives that extend operational lifetime by preventing thermal degradation during the regeneration cycle, allowing for stable performance over 1000+ adsorption-desorption cycles.
Strengths: High CO2 selectivity in the presence of other flue gas components; lower regeneration energy requirements (2.1-2.5 GJ/ton CO2) compared to liquid amine systems; demonstrated at commercial scale. Weaknesses: Still requires significant thermal energy for regeneration; potential for amine leaching during extended operation; performance degradation in the presence of SOx and NOx contaminants.
Korea Research Institute of Chemical Technology
Technical Solution: The Korea Research Institute of Chemical Technology (KRICT) has developed innovative hybrid carbon capture sorbents combining the advantages of physical and chemical adsorption mechanisms. Their patented technology utilizes hierarchically structured composites with microporous carbon cores and mesoporous silica shells functionalized with optimized amine groups. These materials demonstrate CO2 capacities of 4.8-5.5 mmol/g under simulated flue gas conditions with exceptional stability over 500+ temperature swing cycles. KRICT's sorbents feature rapid adsorption kinetics, achieving 80% of equilibrium capacity within 5 minutes, and can be regenerated at relatively low temperatures (80-100°C) compared to conventional amine-based systems. The institute has successfully scaled production to multi-kilogram quantities and demonstrated the technology in a 1 ton CO2/day pilot facility integrated with a power plant, achieving consistent capture efficiencies above 90% with regeneration energy requirements of approximately 2.3 GJ/ton CO2.
Strengths: Excellent balance of adsorption capacity, kinetics, and cycling stability; lower regeneration temperature requirements; scalable synthesis methodology; resistance to water vapor. Weaknesses: Complex multi-step manufacturing process increases production costs; potential for performance degradation in the presence of acid gas contaminants; requires precise control of operating conditions.
Key Innovations in Sorbent Technology
Sorbents for carbon dioxide capture
PatentWO2024133317A1
Innovation
- A sorbent comprising polyalkyleneimine or alkoxylated polyalkyleneimine loaded onto an inorganic solid support with a water content greater than 2 wt%, allowing for aqueous solvent use and reducing the need for organic solvents, which enhances CO2 absorption rates and oxidative stability.
Sorbents for carbon dioxide capture
PatentActiveUS20140286844A1
Innovation
- A mesoporous structure with functionalizing moieties, including zinc atoms or amines, is used for CO2 sorption, employing chemisorption and physisorption mechanisms to enhance CO2 capture selectivity and capacity, while maintaining low diffusional resistance and energy efficiency.
Environmental Impact Assessment
The environmental impact of carbon capture sorbents extends far beyond their primary function of CO2 removal. When evaluating different sorbent materials for energy applications, a comprehensive life cycle assessment (LCA) becomes essential to understand their true environmental footprint.
Physical amine-based sorbents, while effective at capturing CO2, often require significant energy for regeneration, leading to increased fossil fuel consumption if not powered by renewable sources. The production of these materials involves chemical processes that may release volatile organic compounds and contribute to air quality degradation. Additionally, the disposal of spent amine sorbents presents challenges due to potential leaching of harmful compounds into soil and water systems.
Metal-organic frameworks (MOFs) offer improved capture efficiency but raise concerns regarding the mining and processing of metal components. The extraction of zirconium, aluminum, and other metals used in MOF synthesis can lead to habitat destruction, water pollution, and energy-intensive refining processes. However, their longer operational lifespan compared to traditional sorbents may offset some of these initial environmental costs over time.
Zeolites and activated carbons present a more favorable environmental profile during production, especially when derived from waste biomass or sustainable sources. Their regeneration typically requires less energy than amine-based alternatives, reducing operational carbon footprints. Nevertheless, the alkaline conditions often needed for zeolite synthesis can generate wastewater requiring treatment before discharge.
Water consumption represents another critical environmental consideration across all sorbent types. Wet scrubbing systems using liquid amines demand substantial water resources, while solid sorbents generally require less. This difference becomes particularly significant in water-stressed regions where carbon capture facilities might operate.
Land use impacts vary considerably among sorbent technologies. Large-scale carbon capture installations using traditional sorbents require extensive infrastructure, while advanced materials with higher capacity can reduce spatial requirements. This aspect becomes particularly relevant when considering deployment in densely populated areas or sensitive ecosystems.
The environmental justice dimension must also be considered, as the manufacturing facilities for these sorbents and their deployment in carbon capture systems often affect communities unequally. Ensuring equitable distribution of environmental burdens and benefits remains a challenge that requires careful planning and stakeholder engagement throughout the sorbent lifecycle.
Physical amine-based sorbents, while effective at capturing CO2, often require significant energy for regeneration, leading to increased fossil fuel consumption if not powered by renewable sources. The production of these materials involves chemical processes that may release volatile organic compounds and contribute to air quality degradation. Additionally, the disposal of spent amine sorbents presents challenges due to potential leaching of harmful compounds into soil and water systems.
Metal-organic frameworks (MOFs) offer improved capture efficiency but raise concerns regarding the mining and processing of metal components. The extraction of zirconium, aluminum, and other metals used in MOF synthesis can lead to habitat destruction, water pollution, and energy-intensive refining processes. However, their longer operational lifespan compared to traditional sorbents may offset some of these initial environmental costs over time.
Zeolites and activated carbons present a more favorable environmental profile during production, especially when derived from waste biomass or sustainable sources. Their regeneration typically requires less energy than amine-based alternatives, reducing operational carbon footprints. Nevertheless, the alkaline conditions often needed for zeolite synthesis can generate wastewater requiring treatment before discharge.
Water consumption represents another critical environmental consideration across all sorbent types. Wet scrubbing systems using liquid amines demand substantial water resources, while solid sorbents generally require less. This difference becomes particularly significant in water-stressed regions where carbon capture facilities might operate.
Land use impacts vary considerably among sorbent technologies. Large-scale carbon capture installations using traditional sorbents require extensive infrastructure, while advanced materials with higher capacity can reduce spatial requirements. This aspect becomes particularly relevant when considering deployment in densely populated areas or sensitive ecosystems.
The environmental justice dimension must also be considered, as the manufacturing facilities for these sorbents and their deployment in carbon capture systems often affect communities unequally. Ensuring equitable distribution of environmental burdens and benefits remains a challenge that requires careful planning and stakeholder engagement throughout the sorbent lifecycle.
Cost-Efficiency Analysis of Sorbent Technologies
The economic viability of carbon capture technologies hinges significantly on the cost-efficiency of sorbent materials. Amine-based sorbents, while effective in CO2 capture, often incur high regeneration costs due to their substantial energy requirements, typically ranging from 2.5-3.5 GJ/ton CO2. This energy penalty translates directly to operational expenses, making the overall process less economically attractive despite high capture rates of 85-95%.
Metal-organic frameworks (MOFs) present a more balanced cost profile. Although their synthesis costs remain relatively high at $100-500/kg, their exceptional CO2 selectivity and lower regeneration energy (1.8-2.4 GJ/ton CO2) offer better long-term economics. The trade-off between initial investment and operational savings becomes favorable for MOFs in scenarios with extended operational lifespans, particularly in large-scale industrial applications.
Zeolites demonstrate compelling cost-efficiency metrics with moderate production costs ($20-100/kg) and reasonable regeneration energy requirements (2.0-2.8 GJ/ton CO2). Their established manufacturing processes and widespread availability contribute to their economic viability, though their performance in humid conditions necessitates additional process considerations that may impact overall costs.
Activated carbons emerge as particularly cost-effective solutions, with production costs as low as $5-50/kg and regeneration energy requirements of 1.6-2.2 GJ/ton CO2. Their production from waste biomass further enhances their economic profile through circular economy benefits, though their lower selectivity may require larger equipment dimensions, affecting capital expenditure.
Recent techno-economic analyses indicate that the total cost of carbon capture using advanced sorbents ranges from $40-100 per ton of CO2, with sorbent costs contributing 15-30% of this total. Notably, the economic equation is shifting as manufacturing scales increase and novel synthesis routes emerge, particularly for MOFs and specialized polymer-based sorbents.
Lifecycle cost assessment reveals that while initial sorbent costs are important, regeneration energy requirements and sorbent longevity (measured in capture-regeneration cycles) often dominate the long-term economic equation. Materials demonstrating stability over 1,000+ cycles, such as certain zeolites and advanced MOFs, present superior lifetime value despite potentially higher acquisition costs.
The economic landscape is further complicated by regional energy pricing variations, with regeneration costs fluctuating by 30-50% between different global markets. This variability creates distinct optimal sorbent selections based on geographic deployment context, suggesting that a regionally tailored approach to sorbent selection maximizes cost-efficiency in carbon capture implementations.
Metal-organic frameworks (MOFs) present a more balanced cost profile. Although their synthesis costs remain relatively high at $100-500/kg, their exceptional CO2 selectivity and lower regeneration energy (1.8-2.4 GJ/ton CO2) offer better long-term economics. The trade-off between initial investment and operational savings becomes favorable for MOFs in scenarios with extended operational lifespans, particularly in large-scale industrial applications.
Zeolites demonstrate compelling cost-efficiency metrics with moderate production costs ($20-100/kg) and reasonable regeneration energy requirements (2.0-2.8 GJ/ton CO2). Their established manufacturing processes and widespread availability contribute to their economic viability, though their performance in humid conditions necessitates additional process considerations that may impact overall costs.
Activated carbons emerge as particularly cost-effective solutions, with production costs as low as $5-50/kg and regeneration energy requirements of 1.6-2.2 GJ/ton CO2. Their production from waste biomass further enhances their economic profile through circular economy benefits, though their lower selectivity may require larger equipment dimensions, affecting capital expenditure.
Recent techno-economic analyses indicate that the total cost of carbon capture using advanced sorbents ranges from $40-100 per ton of CO2, with sorbent costs contributing 15-30% of this total. Notably, the economic equation is shifting as manufacturing scales increase and novel synthesis routes emerge, particularly for MOFs and specialized polymer-based sorbents.
Lifecycle cost assessment reveals that while initial sorbent costs are important, regeneration energy requirements and sorbent longevity (measured in capture-regeneration cycles) often dominate the long-term economic equation. Materials demonstrating stability over 1,000+ cycles, such as certain zeolites and advanced MOFs, present superior lifetime value despite potentially higher acquisition costs.
The economic landscape is further complicated by regional energy pricing variations, with regeneration costs fluctuating by 30-50% between different global markets. This variability creates distinct optimal sorbent selections based on geographic deployment context, suggesting that a regionally tailored approach to sorbent selection maximizes cost-efficiency in carbon capture implementations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!