Comparison of Carbon Capture Sorbents Under Different Temperatures
OCT 21, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Capture Technology Background and Objectives
Carbon capture technology has evolved significantly over the past several decades, driven by growing concerns about climate change and greenhouse gas emissions. Initially developed in the 1970s for enhanced oil recovery applications, carbon capture has transformed into a critical climate mitigation strategy. The technology aims to prevent large quantities of CO2 from entering the atmosphere by capturing emissions from power plants and industrial facilities, with the ultimate goal of achieving carbon neutrality while maintaining economic growth.
The evolution of carbon capture sorbents represents a key technological advancement in this field. Early carbon capture systems primarily utilized liquid amine solutions, which, while effective, suffered from high energy penalties during regeneration. This led to the development of solid sorbents with improved thermal stability and reduced regeneration energy requirements. The comparison of these sorbents under different temperature conditions has become increasingly important as researchers seek to optimize capture efficiency across various industrial applications.
Current carbon capture technologies can be categorized into three main approaches: post-combustion, pre-combustion, and oxy-fuel combustion. Each approach presents unique challenges and opportunities regarding sorbent performance at different temperature ranges. Post-combustion capture, the most widely applicable method for existing infrastructure, typically operates at temperatures between 40-60°C, while pre-combustion capture may involve temperatures exceeding 300°C.
The technical objectives in sorbent development focus on several critical parameters: high CO2 selectivity, substantial adsorption capacity, rapid adsorption/desorption kinetics, and long-term stability under repeated temperature cycling. Additionally, researchers aim to develop sorbents that maintain performance across broader temperature ranges, reducing the need for costly temperature control systems in industrial settings.
Recent technological trends indicate growing interest in advanced materials such as metal-organic frameworks (MOFs), zeolites, and functionalized porous carbons, which demonstrate promising performance characteristics across various temperature conditions. The development of these next-generation sorbents represents a significant opportunity to reduce the energy penalty associated with carbon capture, currently estimated at 20-30% for conventional technologies.
Global research efforts are increasingly focused on understanding the fundamental mechanisms of CO2 adsorption and desorption under fluctuating temperature conditions, as industrial processes rarely maintain constant operating temperatures. This research direction aligns with the broader goal of developing carbon capture systems that are not only technically effective but also economically viable for widespread deployment across diverse industrial sectors.
The evolution of carbon capture sorbents represents a key technological advancement in this field. Early carbon capture systems primarily utilized liquid amine solutions, which, while effective, suffered from high energy penalties during regeneration. This led to the development of solid sorbents with improved thermal stability and reduced regeneration energy requirements. The comparison of these sorbents under different temperature conditions has become increasingly important as researchers seek to optimize capture efficiency across various industrial applications.
Current carbon capture technologies can be categorized into three main approaches: post-combustion, pre-combustion, and oxy-fuel combustion. Each approach presents unique challenges and opportunities regarding sorbent performance at different temperature ranges. Post-combustion capture, the most widely applicable method for existing infrastructure, typically operates at temperatures between 40-60°C, while pre-combustion capture may involve temperatures exceeding 300°C.
The technical objectives in sorbent development focus on several critical parameters: high CO2 selectivity, substantial adsorption capacity, rapid adsorption/desorption kinetics, and long-term stability under repeated temperature cycling. Additionally, researchers aim to develop sorbents that maintain performance across broader temperature ranges, reducing the need for costly temperature control systems in industrial settings.
Recent technological trends indicate growing interest in advanced materials such as metal-organic frameworks (MOFs), zeolites, and functionalized porous carbons, which demonstrate promising performance characteristics across various temperature conditions. The development of these next-generation sorbents represents a significant opportunity to reduce the energy penalty associated with carbon capture, currently estimated at 20-30% for conventional technologies.
Global research efforts are increasingly focused on understanding the fundamental mechanisms of CO2 adsorption and desorption under fluctuating temperature conditions, as industrial processes rarely maintain constant operating temperatures. This research direction aligns with the broader goal of developing carbon capture systems that are not only technically effective but also economically viable for widespread deployment across diverse industrial sectors.
Market Analysis for Temperature-Optimized Carbon Capture Solutions
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. Current market valuations place the carbon capture technology sector at approximately $2 billion, with projections indicating expansion to $7 billion by 2030. Temperature-optimized carbon capture solutions represent a rapidly growing segment within this market, as operational temperature ranges directly impact capture efficiency and economic viability.
Market demand for temperature-optimized sorbents is primarily concentrated in three key sectors: power generation, industrial manufacturing, and direct air capture initiatives. The power generation sector currently dominates market share at 45%, followed by industrial applications at 35%, with the remaining 20% distributed across emerging applications including direct air capture and mobile carbon capture systems.
Regional analysis reveals distinct market characteristics across different geographies. North America leads in market adoption, particularly in retrofitting existing power plants with temperature-flexible capture systems. The European market shows strong preference for low-temperature sorbents compatible with renewable energy integration, while Asia-Pacific demonstrates accelerating demand for high-temperature sorbents suitable for industrial applications, particularly in cement and steel manufacturing.
Customer requirements vary significantly across temperature ranges. Low-temperature applications (below 100°C) prioritize integration with existing infrastructure and minimal energy penalties. Mid-temperature applications (100-300°C) focus on operational flexibility and regeneration efficiency. High-temperature applications (above 300°C) emphasize thermal stability and resistance to degradation under extreme conditions.
Market research indicates a price sensitivity threshold of approximately $40-60 per ton of CO₂ captured for commercial viability. Solutions optimized for specific temperature ranges that can achieve this cost target while maintaining 90%+ capture efficiency are positioned to capture significant market share. Current pricing structures show a premium of 15-30% for temperature-optimized sorbents compared to conventional alternatives, though this gap is narrowing as production scales increase.
Emerging market opportunities include the development of hybrid capture systems capable of operating across variable temperature conditions, particularly valuable for industrial processes with fluctuating thermal profiles. Additionally, the market for specialized high-temperature sorbents for challenging industrial environments such as cement kilns and steel furnaces shows annual growth rates exceeding 25%, representing a high-value niche segment.
Customer adoption barriers primarily center on installation costs, operational complexity, and concerns regarding long-term sorbent stability across temperature fluctuations. Market research indicates that solutions demonstrating operational reliability for at least 5,000 hours without significant performance degradation achieve substantially higher market penetration rates.
Market demand for temperature-optimized sorbents is primarily concentrated in three key sectors: power generation, industrial manufacturing, and direct air capture initiatives. The power generation sector currently dominates market share at 45%, followed by industrial applications at 35%, with the remaining 20% distributed across emerging applications including direct air capture and mobile carbon capture systems.
Regional analysis reveals distinct market characteristics across different geographies. North America leads in market adoption, particularly in retrofitting existing power plants with temperature-flexible capture systems. The European market shows strong preference for low-temperature sorbents compatible with renewable energy integration, while Asia-Pacific demonstrates accelerating demand for high-temperature sorbents suitable for industrial applications, particularly in cement and steel manufacturing.
Customer requirements vary significantly across temperature ranges. Low-temperature applications (below 100°C) prioritize integration with existing infrastructure and minimal energy penalties. Mid-temperature applications (100-300°C) focus on operational flexibility and regeneration efficiency. High-temperature applications (above 300°C) emphasize thermal stability and resistance to degradation under extreme conditions.
Market research indicates a price sensitivity threshold of approximately $40-60 per ton of CO₂ captured for commercial viability. Solutions optimized for specific temperature ranges that can achieve this cost target while maintaining 90%+ capture efficiency are positioned to capture significant market share. Current pricing structures show a premium of 15-30% for temperature-optimized sorbents compared to conventional alternatives, though this gap is narrowing as production scales increase.
Emerging market opportunities include the development of hybrid capture systems capable of operating across variable temperature conditions, particularly valuable for industrial processes with fluctuating thermal profiles. Additionally, the market for specialized high-temperature sorbents for challenging industrial environments such as cement kilns and steel furnaces shows annual growth rates exceeding 25%, representing a high-value niche segment.
Customer adoption barriers primarily center on installation costs, operational complexity, and concerns regarding long-term sorbent stability across temperature fluctuations. Market research indicates that solutions demonstrating operational reliability for at least 5,000 hours without significant performance degradation achieve substantially higher market penetration rates.
Current Sorbent Technologies and Temperature Challenges
Carbon capture technologies currently employ various sorbent materials, each with specific temperature operating ranges that significantly impact their efficiency and applicability. Physical sorbents such as activated carbon and zeolites typically operate at lower temperatures (below 100°C) and rely on weak van der Waals forces for CO2 adsorption. While these materials offer low regeneration energy requirements, their capacity diminishes rapidly as temperature increases, limiting their industrial deployment in high-temperature flue gas environments.
Chemical sorbents, particularly amine-based materials like monoethanolamine (MEA) and diethanolamine (DEA), function optimally in the 40-60°C range for absorption but require temperatures of 100-120°C for regeneration. This temperature swing creates significant energy penalties in commercial carbon capture systems. Additionally, these sorbents face degradation challenges when exposed to temperatures exceeding 130°C, resulting in reduced operational lifespans and increased replacement costs.
Solid sorbents such as metal-organic frameworks (MOFs) and hydrotalcites demonstrate promising performance across wider temperature ranges (20-400°C depending on the specific material). However, their stability under repeated temperature cycling remains problematic, with many advanced MOFs showing structural collapse after multiple adsorption-desorption cycles at varying temperatures. This presents a critical challenge for their implementation in industrial settings where continuous operation is essential.
High-temperature sorbents, including lithium zirconate and calcium oxide, operate effectively between 450-700°C, making them suitable for pre-combustion capture or direct integration with industrial processes like cement production. These materials exhibit excellent theoretical capacities but suffer from sintering effects during temperature cycling, leading to progressive capacity loss. Recent developments in supported sorbent structures have shown improvements in maintaining surface area during thermal cycling, but long-term stability remains unresolved.
Temperature fluctuations in industrial environments pose additional challenges for all sorbent types. Most carbon capture systems are designed for steady-state operation within narrow temperature bands, but real-world conditions often include transient temperature profiles. This mismatch results in suboptimal performance, with capture efficiencies frequently falling 15-30% below laboratory benchmarks when implemented at scale.
Moisture content interacts with temperature effects across all sorbent classes, creating complex performance landscapes. Water vapor competition for adsorption sites intensifies at lower temperatures for physical sorbents, while chemical sorbents may experience accelerated degradation at higher temperatures in the presence of steam. These interactions further complicate sorbent selection and system design for specific capture applications.
Chemical sorbents, particularly amine-based materials like monoethanolamine (MEA) and diethanolamine (DEA), function optimally in the 40-60°C range for absorption but require temperatures of 100-120°C for regeneration. This temperature swing creates significant energy penalties in commercial carbon capture systems. Additionally, these sorbents face degradation challenges when exposed to temperatures exceeding 130°C, resulting in reduced operational lifespans and increased replacement costs.
Solid sorbents such as metal-organic frameworks (MOFs) and hydrotalcites demonstrate promising performance across wider temperature ranges (20-400°C depending on the specific material). However, their stability under repeated temperature cycling remains problematic, with many advanced MOFs showing structural collapse after multiple adsorption-desorption cycles at varying temperatures. This presents a critical challenge for their implementation in industrial settings where continuous operation is essential.
High-temperature sorbents, including lithium zirconate and calcium oxide, operate effectively between 450-700°C, making them suitable for pre-combustion capture or direct integration with industrial processes like cement production. These materials exhibit excellent theoretical capacities but suffer from sintering effects during temperature cycling, leading to progressive capacity loss. Recent developments in supported sorbent structures have shown improvements in maintaining surface area during thermal cycling, but long-term stability remains unresolved.
Temperature fluctuations in industrial environments pose additional challenges for all sorbent types. Most carbon capture systems are designed for steady-state operation within narrow temperature bands, but real-world conditions often include transient temperature profiles. This mismatch results in suboptimal performance, with capture efficiencies frequently falling 15-30% below laboratory benchmarks when implemented at scale.
Moisture content interacts with temperature effects across all sorbent classes, creating complex performance landscapes. Water vapor competition for adsorption sites intensifies at lower temperatures for physical sorbents, while chemical sorbents may experience accelerated degradation at higher temperatures in the presence of steam. These interactions further complicate sorbent selection and system design for specific capture applications.
Comparative Analysis of Sorbent Performance Across Temperature Ranges
01 High-temperature carbon capture sorbents
Specialized sorbent materials designed to operate efficiently at elevated temperatures for carbon capture applications. These materials maintain structural integrity and adsorption capacity under high-temperature conditions, typically above 300°C. High-temperature operation can be advantageous in industrial settings where flue gases are hot, eliminating the need for cooling before carbon capture and thus improving overall energy efficiency of the capture process.- High-temperature carbon capture sorbents: Specialized sorbent materials designed to operate efficiently at elevated temperatures for carbon capture applications. These materials maintain structural integrity and adsorption capacity under high-temperature conditions, which is particularly important for industrial processes where flue gases are hot. High-temperature sorbents often incorporate metal oxides or ceramic supports that resist thermal degradation while maintaining CO2 selectivity.
- Temperature-swing adsorption systems: Carbon capture systems that utilize temperature variations to control the adsorption and desorption cycles of CO2 sorbents. These systems leverage the temperature-dependent behavior of sorbent materials, where CO2 is captured at one temperature range and released at another. The performance of these systems depends on the sorbent's thermal stability, heat transfer efficiency, and the temperature differential required for effective operation.
- Low-temperature carbon capture materials: Sorbent materials optimized for carbon capture at ambient or low temperatures, which can reduce energy requirements compared to high-temperature systems. These materials often include amine-functionalized adsorbents, metal-organic frameworks (MOFs), or specialized polymers that exhibit high CO2 selectivity and capacity at lower temperature ranges. Low-temperature operation can be advantageous for certain applications where waste heat is limited or where energy efficiency is paramount.
- Temperature-resistant composite sorbents: Advanced composite materials that combine multiple components to achieve enhanced temperature performance for carbon capture. These composites typically incorporate a high-surface-area support material with active capture components and stabilizing agents. The composite structure provides thermal stability while maintaining high CO2 adsorption capacity across varying temperature conditions, allowing for more flexible operation in industrial settings.
- Temperature-controlled regeneration techniques: Methods and systems focused on the temperature-controlled regeneration of carbon capture sorbents to maintain their performance over multiple cycles. These techniques involve precise temperature management during the desorption phase to efficiently release captured CO2 while minimizing energy consumption and preventing thermal degradation of the sorbent. Optimized regeneration temperatures are critical for maintaining sorbent longevity and overall system efficiency in carbon capture applications.
02 Temperature-swing adsorption systems
Carbon capture systems that utilize temperature variations to control the adsorption and desorption cycles of CO2 on sorbent materials. These systems exploit the temperature-dependent nature of adsorption, where lower temperatures favor CO2 uptake while higher temperatures facilitate release. The performance of these systems depends on the sorbent's thermal stability, heat capacity, and the kinetics of adsorption/desorption at different temperature ranges, allowing for efficient carbon capture with reduced energy penalties.Expand Specific Solutions03 Low-temperature carbon capture sorbents
Sorbent materials optimized for carbon capture at ambient or low temperatures, typically below 100°C. These materials often include amine-functionalized adsorbents, metal-organic frameworks (MOFs), or specialized polymeric materials that can selectively capture CO2 at lower temperature ranges. Low-temperature operation can be beneficial for direct air capture applications or for post-combustion capture where flue gases have already been cooled.Expand Specific Solutions04 Temperature-resistant composite sorbents
Composite materials that combine multiple components to enhance temperature stability and carbon capture performance across wider temperature ranges. These composites often incorporate support materials that provide thermal stability along with active components that facilitate CO2 adsorption. The synergistic effects between components can lead to improved thermal conductivity, reduced degradation rates, and maintained adsorption capacity even after multiple temperature-swing cycles.Expand Specific Solutions05 Temperature impact on sorbent regeneration
Studies and technologies focused on how temperature affects the regeneration process of carbon capture sorbents. This includes optimization of regeneration temperatures to minimize energy consumption while ensuring complete desorption of captured CO2. The regeneration temperature significantly impacts the overall energy efficiency of carbon capture systems, sorbent lifetime, and the purity of the captured CO2 stream, making it a critical parameter in sorbent design and system operation.Expand Specific Solutions
Leading Organizations in Carbon Capture Sorbent Development
The carbon capture sorbent market is in a growth phase, characterized by increasing commercial deployments amid global decarbonization efforts. The market is projected to expand significantly, driven by stringent emissions regulations and net-zero commitments. Temperature performance of sorbents remains a critical technical challenge, with research institutions like MIT, Zhejiang University, and Korea Institute of Energy Research advancing fundamental understanding. Major industrial players including ExxonMobil, Climeworks, and Sinopec are developing proprietary sorbent technologies optimized for different temperature ranges. Chinese entities (Huaneng Clean Energy Research Institute, CHN ENERGY) are particularly active in high-temperature applications, while companies like Hitachi and Mitsubishi Gas Chemical focus on mid-temperature solutions. The technology is approaching commercial maturity for specific applications, though cost-effectiveness at scale remains a barrier.
Huaneng Clean Energy Research Institute
Technical Solution: Huaneng Clean Energy Research Institute has pioneered advanced carbon capture sorbent technologies optimized for various temperature ranges in coal-fired power plant applications. Their flagship development is a series of modified activated carbon and amine-functionalized mesoporous silica sorbents that operate effectively across low (25-60°C), medium (60-120°C), and high (>120°C) temperature regimes. The institute's proprietary polyethyleneimine (PEI)-impregnated mesoporous silica demonstrates CO2 adsorption capacities exceeding 4.5 mmol/g at 75°C, with excellent stability over 100+ adsorption-desorption cycles. For higher temperature applications, they've developed novel lithium-based ceramic sorbents that maintain performance at 500-700°C, ideal for pre-combustion capture scenarios. These technologies have been successfully demonstrated at Huaneng's Shanghai Shidongkou Power Plant, China's first carbon capture demonstration facility, which achieves 85-90% CO2 removal efficiency with significantly reduced energy penalties compared to conventional amine scrubbing.
Strengths: Comprehensive sorbent portfolio covering the entire temperature spectrum relevant to power generation; proven large-scale implementation in actual power plant conditions; lower regeneration energy requirements compared to liquid amine systems. Weaknesses: Some high-temperature sorbents show degradation in the presence of sulfur compounds; scaling challenges for certain specialized materials; higher initial capital costs compared to conventional technologies.
Climeworks AG
Technical Solution: Climeworks has developed a proprietary Direct Air Capture (DAC) technology using solid sorbent filters that can operate across various temperature ranges. Their approach employs amine-functionalized sorbents embedded in porous filter materials that selectively capture CO2 from ambient air. The process works in a temperature-swing adsorption cycle where ambient air passes through the collectors at lower temperatures (typically ambient conditions) for adsorption, followed by heating to 80-100°C for regeneration and CO2 release. This temperature swing allows for efficient CO2 capture with relatively low energy requirements compared to other DAC technologies. Climeworks has demonstrated the technology at commercial scale with their Orca plant in Iceland, which leverages geothermal energy for the heating phase and stores captured CO2 permanently through mineralization in basaltic rock formations.
Strengths: Operates effectively at ambient temperatures for adsorption, reducing energy costs; modular design allows for scalable deployment; proven commercial implementation with multiple plants operating globally. Weaknesses: Regeneration still requires significant thermal energy input; efficiency decreases in humid conditions; higher capital costs compared to some industrial point-source capture technologies.
Key Innovations in Temperature-Resistant Carbon Capture Materials
Engineered process of manufacturing calcium aluminate carbonates for medium-high temperature co2 capture
PatentInactiveUS20140072501A1
Innovation
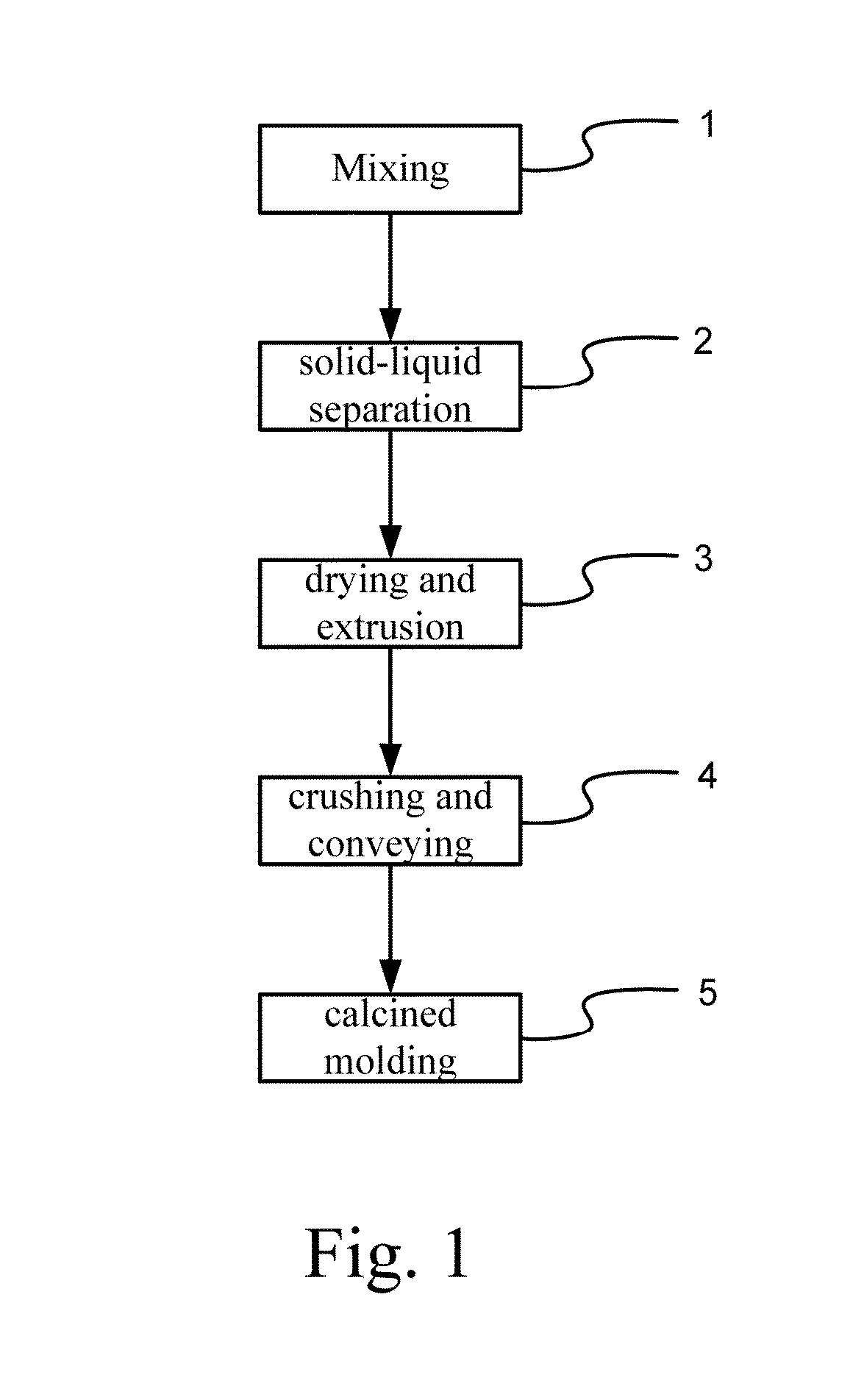
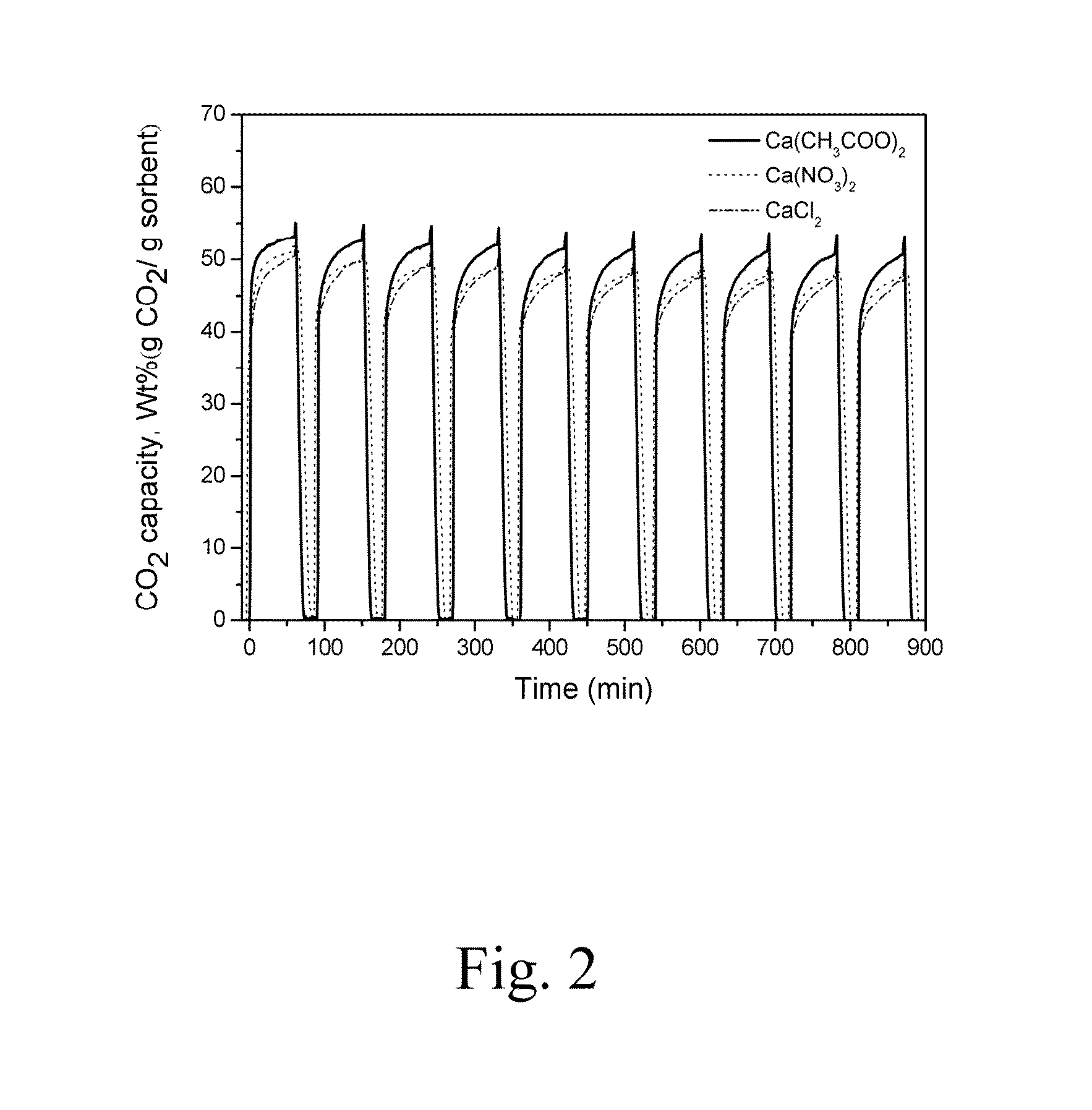
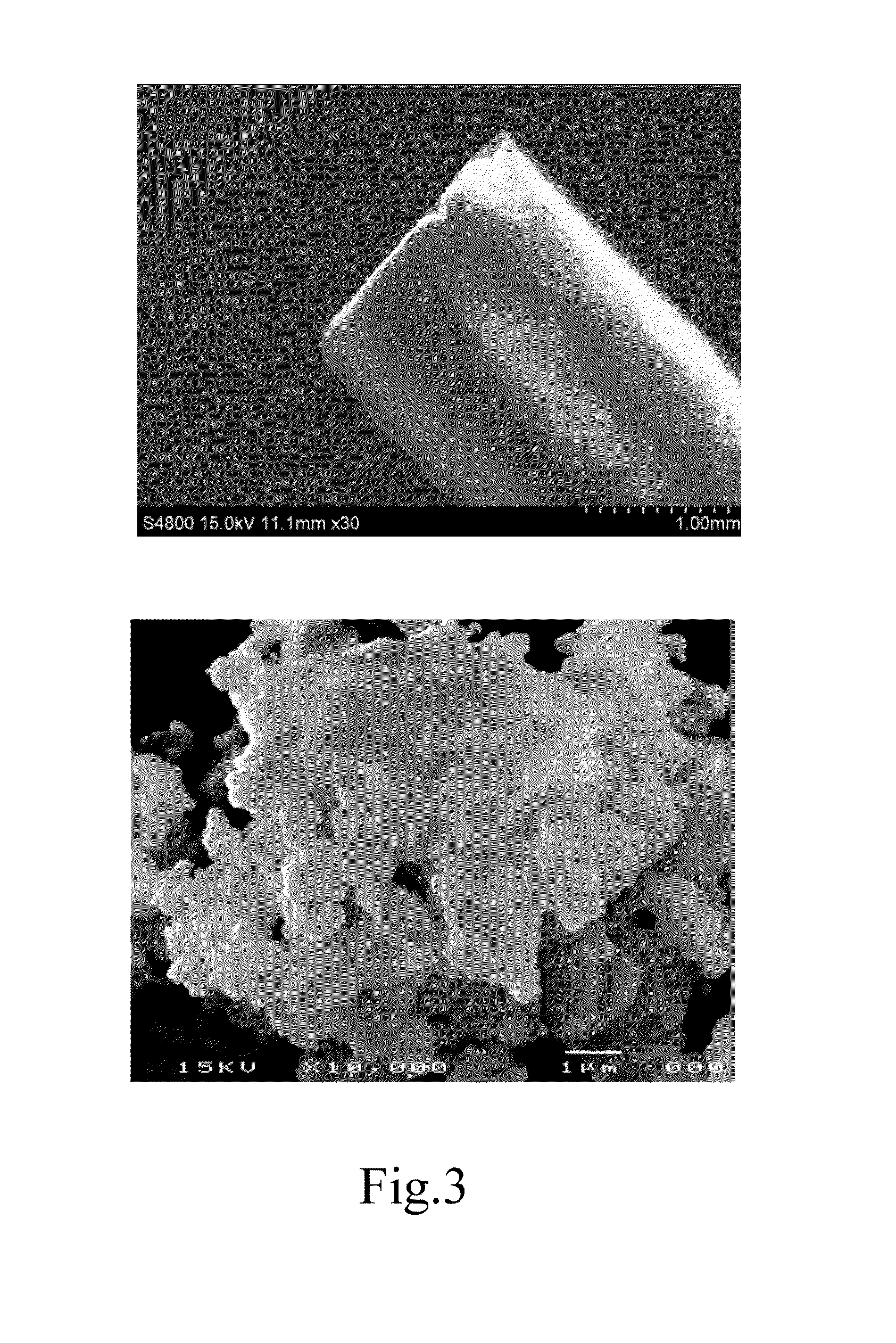
- A co-precipitation method is used to manufacture calcium aluminum carbonate (Ca—Al—CO3) as a carbon capturing agent, involving mixing calcium and aluminum nitrate with sodium carbonate and sodium hydroxide, followed by solid-liquid separation, drying, crushing, and calcination to produce a nano-layered composite sorbent with high porosity, suitable for medium-high temperature CO2 capture.
Co2 capture sorbents with low regeneration temperature and high desorption rates
PatentPendingUS20240009613A1
Innovation
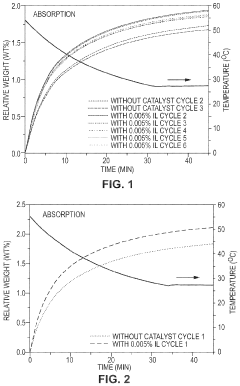
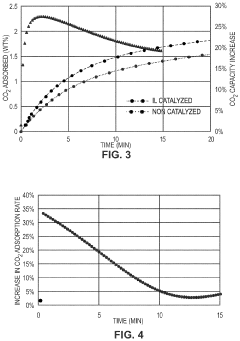
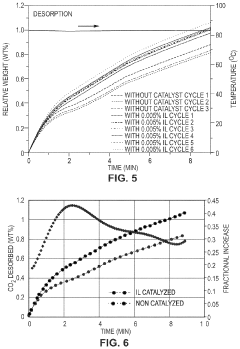
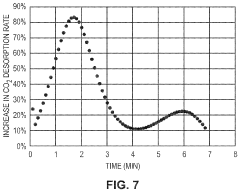
- Development of CO2 capture sorbents comprising a solid support with CO2-sorbing amine and ionic liquid, which enhances CO2 sorption and desorption characteristics, allowing for regeneration at lower temperatures and maintaining high selectivity and capacity through catalytic action.
Environmental Impact Assessment of Different Sorbent Technologies
The environmental impact of carbon capture sorbent technologies extends far beyond their primary function of CO2 sequestration. Different sorbent materials exhibit varying ecological footprints throughout their lifecycle, from production to disposal or regeneration. Physical sorbents such as activated carbon and zeolites generally require energy-intensive manufacturing processes, contributing to initial carbon emissions that must be offset through operational efficiency. However, their regeneration typically demands less energy compared to chemical sorbents, resulting in lower operational emissions over multiple capture cycles.
Chemical sorbents, particularly amine-based solutions, present significant environmental challenges including potential atmospheric release of volatile compounds and degradation products that may contribute to air pollution. The production of these sorbents often involves petrochemical processes with associated environmental impacts. Temperature sensitivity further complicates their environmental profile, as higher operating temperatures increase energy consumption and corresponding emissions from power generation.
Metal-organic frameworks (MOFs) and novel hybrid sorbents represent promising alternatives with potentially reduced environmental impacts. Their highly selective nature minimizes the capture of non-target gases, reducing purification requirements and associated energy consumption. However, the synthesis of these advanced materials often involves rare elements and complex chemical processes, raising concerns about resource depletion and manufacturing emissions.
Water consumption represents another critical environmental consideration, particularly for aqueous sorbent systems. Cooling requirements vary significantly with operating temperature, with high-temperature sorbents typically demanding more substantial water resources for temperature management. This aspect becomes increasingly important in water-stressed regions where carbon capture facilities may compete with agricultural and municipal water needs.
Land use impacts also differ among sorbent technologies. Systems requiring extensive regeneration infrastructure or larger absorption columns due to efficiency limitations at certain temperatures may have larger physical footprints. This expanded land requirement can contribute to habitat fragmentation or displacement of alternative land uses.
Waste management challenges vary significantly across sorbent types. Chemical sorbents often generate hazardous waste streams requiring specialized disposal, while physical sorbents may be more amenable to recycling or repurposing at end-of-life. Temperature-related degradation accelerates waste generation rates for certain sorbents, creating additional environmental management challenges and potential contamination risks if improperly handled.
Comprehensive lifecycle assessment studies indicate that the environmental superiority of any particular sorbent technology depends heavily on the specific implementation context, including energy source, temperature regime, and local environmental sensitivities. This underscores the importance of site-specific environmental impact assessments when selecting appropriate carbon capture sorbent technologies.
Chemical sorbents, particularly amine-based solutions, present significant environmental challenges including potential atmospheric release of volatile compounds and degradation products that may contribute to air pollution. The production of these sorbents often involves petrochemical processes with associated environmental impacts. Temperature sensitivity further complicates their environmental profile, as higher operating temperatures increase energy consumption and corresponding emissions from power generation.
Metal-organic frameworks (MOFs) and novel hybrid sorbents represent promising alternatives with potentially reduced environmental impacts. Their highly selective nature minimizes the capture of non-target gases, reducing purification requirements and associated energy consumption. However, the synthesis of these advanced materials often involves rare elements and complex chemical processes, raising concerns about resource depletion and manufacturing emissions.
Water consumption represents another critical environmental consideration, particularly for aqueous sorbent systems. Cooling requirements vary significantly with operating temperature, with high-temperature sorbents typically demanding more substantial water resources for temperature management. This aspect becomes increasingly important in water-stressed regions where carbon capture facilities may compete with agricultural and municipal water needs.
Land use impacts also differ among sorbent technologies. Systems requiring extensive regeneration infrastructure or larger absorption columns due to efficiency limitations at certain temperatures may have larger physical footprints. This expanded land requirement can contribute to habitat fragmentation or displacement of alternative land uses.
Waste management challenges vary significantly across sorbent types. Chemical sorbents often generate hazardous waste streams requiring specialized disposal, while physical sorbents may be more amenable to recycling or repurposing at end-of-life. Temperature-related degradation accelerates waste generation rates for certain sorbents, creating additional environmental management challenges and potential contamination risks if improperly handled.
Comprehensive lifecycle assessment studies indicate that the environmental superiority of any particular sorbent technology depends heavily on the specific implementation context, including energy source, temperature regime, and local environmental sensitivities. This underscores the importance of site-specific environmental impact assessments when selecting appropriate carbon capture sorbent technologies.
Economic Viability of Temperature-Optimized Carbon Capture Systems
The economic viability of temperature-optimized carbon capture systems represents a critical factor in the widespread adoption of carbon capture technologies. Current analyses indicate that temperature optimization can significantly reduce operational costs by 15-30% compared to non-optimized systems, primarily through energy efficiency improvements and extended sorbent lifecycles.
Temperature-optimized systems demonstrate varying cost-effectiveness across different industrial applications. In power generation, systems operating at 60-80°C show the most favorable economic profiles, with capital expenditure recovery periods of 5-7 years when carbon pricing exceeds $40/ton. For cement and steel manufacturing, where exhaust temperatures are naturally higher, systems optimized for 120-150°C operation reduce implementation costs by eliminating extensive cooling requirements.
The economic equation is further influenced by sorbent selection. Amine-based sorbents, while effective at lower temperatures (40-70°C), incur higher regeneration costs and suffer from thermal degradation, resulting in replacement cycles of 1-2 years. In contrast, metal-organic frameworks (MOFs) and certain zeolites optimized for mid-range temperatures (70-100°C) demonstrate superior longevity, with replacement intervals extending to 3-5 years, significantly reducing operational expenditures.
Energy consumption represents the largest operational cost factor in carbon capture systems. Temperature-optimized systems can reduce energy penalties by 20-35% compared to first-generation technologies. Systems utilizing waste heat recovery show particularly promising economics, with some industrial implementations achieving net energy penalties below 0.2 GJ/ton CO₂ captured, approaching the theoretical minimum energy requirements.
Market analysis suggests that temperature-optimized carbon capture technologies could reach cost parity with carbon taxation in major markets by 2026-2028, assuming carbon prices continue their projected trajectory of 8-12% annual increases. This economic inflection point would trigger widespread adoption across energy-intensive industries, potentially creating a $30-40 billion market for optimized sorbent technologies by 2030.
The scalability of temperature-optimized systems presents both economic challenges and opportunities. While initial deployment costs remain high ($60-80 million for utility-scale implementations), economies of scale are expected to reduce costs by 40-50% over the next decade as manufacturing capabilities expand and technological learning accelerates.
Temperature-optimized systems demonstrate varying cost-effectiveness across different industrial applications. In power generation, systems operating at 60-80°C show the most favorable economic profiles, with capital expenditure recovery periods of 5-7 years when carbon pricing exceeds $40/ton. For cement and steel manufacturing, where exhaust temperatures are naturally higher, systems optimized for 120-150°C operation reduce implementation costs by eliminating extensive cooling requirements.
The economic equation is further influenced by sorbent selection. Amine-based sorbents, while effective at lower temperatures (40-70°C), incur higher regeneration costs and suffer from thermal degradation, resulting in replacement cycles of 1-2 years. In contrast, metal-organic frameworks (MOFs) and certain zeolites optimized for mid-range temperatures (70-100°C) demonstrate superior longevity, with replacement intervals extending to 3-5 years, significantly reducing operational expenditures.
Energy consumption represents the largest operational cost factor in carbon capture systems. Temperature-optimized systems can reduce energy penalties by 20-35% compared to first-generation technologies. Systems utilizing waste heat recovery show particularly promising economics, with some industrial implementations achieving net energy penalties below 0.2 GJ/ton CO₂ captured, approaching the theoretical minimum energy requirements.
Market analysis suggests that temperature-optimized carbon capture technologies could reach cost parity with carbon taxation in major markets by 2026-2028, assuming carbon prices continue their projected trajectory of 8-12% annual increases. This economic inflection point would trigger widespread adoption across energy-intensive industries, potentially creating a $30-40 billion market for optimized sorbent technologies by 2030.
The scalability of temperature-optimized systems presents both economic challenges and opportunities. While initial deployment costs remain high ($60-80 million for utility-scale implementations), economies of scale are expected to reduce costs by 40-50% over the next decade as manufacturing capabilities expand and technological learning accelerates.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!