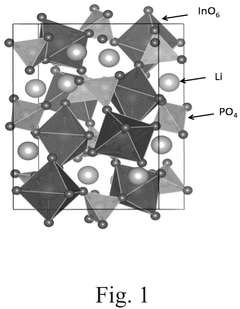
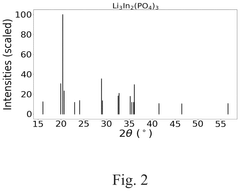
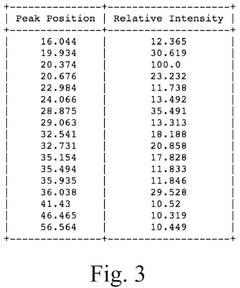
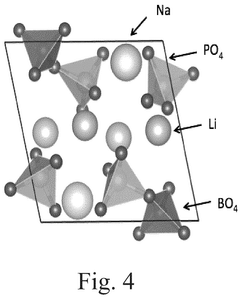
Compare Lithium Phosphate Entropy with Aluminum-Based Compounds
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Phosphate and Aluminum Compounds: Background and Objectives
The evolution of energy storage technologies has witnessed significant advancements over the past decades, with lithium-based compounds emerging as dominant materials in commercial battery applications. Lithium phosphate (LiFePO₄) has gained particular attention due to its thermal stability, long cycle life, and environmental compatibility compared to other lithium-ion battery cathode materials. Concurrently, aluminum-based compounds have been explored as potential alternatives, offering advantages in abundance, cost, and theoretical energy density.
The thermodynamic properties of these materials, particularly entropy characteristics, play a crucial role in determining their performance in energy storage applications. Entropy, as a fundamental thermodynamic parameter, influences phase stability, ion diffusion kinetics, and overall energy efficiency of battery systems. Understanding the entropic behavior of lithium phosphate in comparison with aluminum-based compounds provides valuable insights into their respective electrochemical behaviors under various operating conditions.
Historical development of lithium phosphate technology began in the 1990s as researchers sought safer alternatives to cobalt-based cathodes. The olivine structure of LiFePO₄ was identified as providing exceptional stability during charge-discharge cycles. Meanwhile, aluminum-based energy storage research has evolved from traditional aluminum-air batteries to more recent innovations in aluminum-ion technologies, with various compounds being investigated for electrode applications.
The technological trajectory indicates a growing interest in multi-valent ion systems, where aluminum's three-electron transfer capability presents theoretical advantages over lithium's single-electron chemistry. However, challenges related to ion mobility, electrolyte compatibility, and interfacial stability have limited practical implementations of aluminum-based energy storage systems compared to their lithium counterparts.
Current research trends focus on understanding and manipulating the entropy-related properties of both material systems to enhance performance metrics such as capacity retention, rate capability, and temperature tolerance. Computational modeling and advanced characterization techniques have enabled more precise quantification of entropic contributions to overall cell performance, revealing complex relationships between crystal structure, defect chemistry, and thermodynamic behavior.
This technical research aims to comprehensively compare the entropic properties of lithium phosphate and aluminum-based compounds, evaluate their implications for energy storage applications, and identify potential pathways for leveraging these thermodynamic characteristics to develop next-generation battery technologies with improved performance profiles and sustainability metrics.
The thermodynamic properties of these materials, particularly entropy characteristics, play a crucial role in determining their performance in energy storage applications. Entropy, as a fundamental thermodynamic parameter, influences phase stability, ion diffusion kinetics, and overall energy efficiency of battery systems. Understanding the entropic behavior of lithium phosphate in comparison with aluminum-based compounds provides valuable insights into their respective electrochemical behaviors under various operating conditions.
Historical development of lithium phosphate technology began in the 1990s as researchers sought safer alternatives to cobalt-based cathodes. The olivine structure of LiFePO₄ was identified as providing exceptional stability during charge-discharge cycles. Meanwhile, aluminum-based energy storage research has evolved from traditional aluminum-air batteries to more recent innovations in aluminum-ion technologies, with various compounds being investigated for electrode applications.
The technological trajectory indicates a growing interest in multi-valent ion systems, where aluminum's three-electron transfer capability presents theoretical advantages over lithium's single-electron chemistry. However, challenges related to ion mobility, electrolyte compatibility, and interfacial stability have limited practical implementations of aluminum-based energy storage systems compared to their lithium counterparts.
Current research trends focus on understanding and manipulating the entropy-related properties of both material systems to enhance performance metrics such as capacity retention, rate capability, and temperature tolerance. Computational modeling and advanced characterization techniques have enabled more precise quantification of entropic contributions to overall cell performance, revealing complex relationships between crystal structure, defect chemistry, and thermodynamic behavior.
This technical research aims to comprehensively compare the entropic properties of lithium phosphate and aluminum-based compounds, evaluate their implications for energy storage applications, and identify potential pathways for leveraging these thermodynamic characteristics to develop next-generation battery technologies with improved performance profiles and sustainability metrics.
Market Analysis for Energy Storage Materials
The energy storage materials market is experiencing unprecedented growth, driven by the global transition to renewable energy and electrification of transportation. Currently valued at approximately $54 billion in 2023, this market is projected to reach $163 billion by 2030, representing a compound annual growth rate of 17%. Lithium-based compounds, particularly lithium phosphate (LFP), have dominated the market due to their stability, safety profile, and increasingly competitive cost structure.
Within this landscape, lithium phosphate materials command roughly 30% of the lithium-ion battery market, with their share expanding rapidly in stationary storage applications and electric vehicles in the economy segment. The entropy characteristics of lithium phosphate compounds contribute significantly to their thermal stability advantages, a critical factor driving their adoption in applications where safety is paramount.
Aluminum-based compounds, while historically less prominent in commercial energy storage, are gaining attention as potential alternatives or complementary materials to lithium-based solutions. The market for aluminum-based energy storage materials remains nascent, estimated at less than $2 billion, but is experiencing accelerated research interest due to aluminum's abundance (the third most common element in Earth's crust) and potentially lower production costs.
Comparative entropy profiles between lithium phosphate and aluminum-based compounds reveal significant implications for market development. Materials with optimized entropy characteristics demonstrate superior cycle life and thermal management properties, directly impacting total cost of ownership calculations that increasingly favor lithium phosphate in certain applications despite higher initial costs.
Regional market dynamics show interesting patterns, with China dominating lithium phosphate production (approximately 75% of global capacity), while North America and Europe are investing heavily in both lithium phosphate and alternative technologies including aluminum-based solutions. This geographical diversification reflects strategic concerns about supply chain security and resource nationalism affecting material availability.
End-user segments demonstrate varying preferences, with utility-scale storage increasingly favoring lithium phosphate due to its entropy-related safety advantages, while consumer electronics maintain preference for higher energy density options. The electric vehicle segment shows a bifurcation, with economy models shifting toward lithium phosphate while premium vehicles maintain use of higher energy density chemistries.
Market forecasts indicate that materials with optimized entropy characteristics will continue gaining market share, with lithium phosphate expected to grow at 22% annually through 2030, outpacing the overall market. Aluminum-based compounds are projected to establish commercial viability in niche applications by 2025, potentially capturing 5-8% market share in specific segments by 2030 if current technical challenges related to their entropy characteristics can be overcome.
Within this landscape, lithium phosphate materials command roughly 30% of the lithium-ion battery market, with their share expanding rapidly in stationary storage applications and electric vehicles in the economy segment. The entropy characteristics of lithium phosphate compounds contribute significantly to their thermal stability advantages, a critical factor driving their adoption in applications where safety is paramount.
Aluminum-based compounds, while historically less prominent in commercial energy storage, are gaining attention as potential alternatives or complementary materials to lithium-based solutions. The market for aluminum-based energy storage materials remains nascent, estimated at less than $2 billion, but is experiencing accelerated research interest due to aluminum's abundance (the third most common element in Earth's crust) and potentially lower production costs.
Comparative entropy profiles between lithium phosphate and aluminum-based compounds reveal significant implications for market development. Materials with optimized entropy characteristics demonstrate superior cycle life and thermal management properties, directly impacting total cost of ownership calculations that increasingly favor lithium phosphate in certain applications despite higher initial costs.
Regional market dynamics show interesting patterns, with China dominating lithium phosphate production (approximately 75% of global capacity), while North America and Europe are investing heavily in both lithium phosphate and alternative technologies including aluminum-based solutions. This geographical diversification reflects strategic concerns about supply chain security and resource nationalism affecting material availability.
End-user segments demonstrate varying preferences, with utility-scale storage increasingly favoring lithium phosphate due to its entropy-related safety advantages, while consumer electronics maintain preference for higher energy density options. The electric vehicle segment shows a bifurcation, with economy models shifting toward lithium phosphate while premium vehicles maintain use of higher energy density chemistries.
Market forecasts indicate that materials with optimized entropy characteristics will continue gaining market share, with lithium phosphate expected to grow at 22% annually through 2030, outpacing the overall market. Aluminum-based compounds are projected to establish commercial viability in niche applications by 2025, potentially capturing 5-8% market share in specific segments by 2030 if current technical challenges related to their entropy characteristics can be overcome.
Current Technical Challenges in Entropy Comparison
The comparison of entropy between lithium phosphate and aluminum-based compounds presents several significant technical challenges that researchers and industry professionals must overcome. The primary difficulty lies in the accurate measurement and calculation of entropy values for these complex materials, as entropy is highly sensitive to structural variations, impurities, and measurement conditions.
Experimental determination of entropy requires precise calorimetric measurements under carefully controlled conditions. For lithium phosphate compounds, their hygroscopic nature introduces complications as moisture absorption can significantly alter thermal properties and measured entropy values. Similarly, aluminum-based compounds often exhibit polymorphism, with different crystal structures possessing distinct entropy characteristics, making standardized comparisons problematic.
Computational approaches face their own set of challenges. Density Functional Theory (DFT) calculations, while powerful, struggle with accurately representing the electronic structure of transition metal compounds containing lithium or aluminum. The strong electron correlation effects in these materials often require advanced computational methods that are computationally intensive and time-consuming.
Temperature dependence adds another layer of complexity. Entropy values change significantly with temperature, and many applications require understanding these properties across wide temperature ranges. This necessitates multiple measurements or calculations at different temperature points, increasing the complexity of comprehensive comparisons.
Phase transitions represent a particular challenge, as both lithium phosphate and aluminum compounds can undergo structural transformations at certain temperatures or pressures. These transitions are accompanied by entropy changes that can be difficult to predict or measure accurately, especially when metastable phases are involved.
The influence of defects and dopants further complicates entropy comparisons. Real-world materials contain various imperfections that can dramatically alter entropic properties. Quantifying these effects requires sophisticated models that can account for the statistical distribution of defects and their impact on the overall thermodynamic properties.
Standardization issues persist across the field, with different research groups employing varied methodologies for entropy determination. This lack of standardized protocols makes direct comparison between studies challenging, often leading to discrepancies in reported values for seemingly identical materials.
Finally, the multiscale nature of entropy presents a fundamental challenge. Contributions to entropy come from various structural levels—from electronic and vibrational to configurational and magnetic sources—requiring integrated approaches that can bridge these different scales to provide comprehensive entropy comparisons between lithium phosphate and aluminum-based compounds.
Experimental determination of entropy requires precise calorimetric measurements under carefully controlled conditions. For lithium phosphate compounds, their hygroscopic nature introduces complications as moisture absorption can significantly alter thermal properties and measured entropy values. Similarly, aluminum-based compounds often exhibit polymorphism, with different crystal structures possessing distinct entropy characteristics, making standardized comparisons problematic.
Computational approaches face their own set of challenges. Density Functional Theory (DFT) calculations, while powerful, struggle with accurately representing the electronic structure of transition metal compounds containing lithium or aluminum. The strong electron correlation effects in these materials often require advanced computational methods that are computationally intensive and time-consuming.
Temperature dependence adds another layer of complexity. Entropy values change significantly with temperature, and many applications require understanding these properties across wide temperature ranges. This necessitates multiple measurements or calculations at different temperature points, increasing the complexity of comprehensive comparisons.
Phase transitions represent a particular challenge, as both lithium phosphate and aluminum compounds can undergo structural transformations at certain temperatures or pressures. These transitions are accompanied by entropy changes that can be difficult to predict or measure accurately, especially when metastable phases are involved.
The influence of defects and dopants further complicates entropy comparisons. Real-world materials contain various imperfections that can dramatically alter entropic properties. Quantifying these effects requires sophisticated models that can account for the statistical distribution of defects and their impact on the overall thermodynamic properties.
Standardization issues persist across the field, with different research groups employing varied methodologies for entropy determination. This lack of standardized protocols makes direct comparison between studies challenging, often leading to discrepancies in reported values for seemingly identical materials.
Finally, the multiscale nature of entropy presents a fundamental challenge. Contributions to entropy come from various structural levels—from electronic and vibrational to configurational and magnetic sources—requiring integrated approaches that can bridge these different scales to provide comprehensive entropy comparisons between lithium phosphate and aluminum-based compounds.
Methodologies for Entropy Measurement and Comparison
01 Entropy stabilization in lithium phosphate cathode materials
Entropy stabilization techniques are applied to lithium phosphate-based cathode materials to enhance their electrochemical performance. By introducing controlled disorder through multiple element doping, these materials exhibit improved thermal stability and cycling performance. The entropy effect helps maintain structural integrity during charging and discharging cycles, leading to better capacity retention and longer battery life. This approach is particularly effective for high-energy density lithium-ion batteries.- Entropy stabilization in lithium phosphate materials: Entropy stabilization techniques are applied to lithium phosphate materials to enhance their thermal and electrochemical stability. This approach involves introducing controlled disorder into the crystal structure, which can improve the material's performance in battery applications. The entropy stabilization can be achieved through doping, mixing different cation species, or using specific synthesis methods that create beneficial structural disorder while maintaining essential lithium-ion transport pathways.
- Aluminum-based compounds as entropy enhancers in battery materials: Aluminum-based compounds are utilized as entropy enhancers in battery materials, particularly in lithium phosphate systems. The incorporation of aluminum can create high-entropy structures that improve thermal stability, cycling performance, and rate capability of the electrode materials. These compounds can form complex structures with multiple cation sites, leading to increased configurational entropy and enhanced electrochemical properties for energy storage applications.
- High-entropy coating technologies for lithium phosphate cathodes: High-entropy coating technologies are developed for lithium phosphate cathode materials to improve their surface properties and interface stability. These coatings typically involve aluminum-containing compounds that form protective layers on the cathode surface. The high-entropy nature of these coatings helps to suppress unwanted side reactions, reduce capacity fading, and enhance the overall battery performance by creating a stable interface between the electrode and electrolyte.
- Entropy-driven phase transformations in lithium-aluminum phosphate systems: Entropy-driven phase transformations in lithium-aluminum phosphate systems are investigated to understand their structural evolution during battery operation. These transformations can be beneficial for maintaining structural integrity during cycling and can lead to improved capacity retention. The research focuses on how configurational entropy influences phase stability, transition temperatures, and kinetics of ion transport in these complex materials, providing insights for designing more efficient battery components.
- Computational modeling of entropy effects in lithium phosphate and aluminum compounds: Computational modeling approaches are employed to study entropy effects in lithium phosphate and aluminum-based compounds. These models help predict how entropy contributions affect material properties, phase stability, and electrochemical performance. By understanding the relationship between atomic arrangements, configurational entropy, and macroscopic properties, researchers can design optimized compositions with enhanced thermal stability and ion conductivity for advanced battery applications.
02 Aluminum-doped lithium phosphate composites for enhanced conductivity
Aluminum doping in lithium phosphate materials creates composites with significantly improved ionic and electronic conductivity. The aluminum ions occupy specific lattice sites, creating defects that facilitate lithium ion transport through the material. These composites demonstrate reduced internal resistance and enhanced rate capability in battery applications. The aluminum doping also contributes to structural stabilization, preventing phase transitions during cycling and improving the overall electrochemical performance.Expand Specific Solutions03 Entropy-driven phase transitions in lithium-aluminum phosphate systems
The entropy-driven phase transitions in lithium-aluminum phosphate systems are investigated to understand their thermodynamic properties. These materials undergo specific phase transformations at different temperatures, which are influenced by the configurational entropy of the system. By controlling the synthesis conditions and composition, the phase transition temperatures can be tuned for optimal battery performance. The entropy changes during these transitions affect the thermal stability and energy density of the resulting battery materials.Expand Specific Solutions04 Nanostructured lithium phosphate-aluminum oxide composites
Nanostructured composites combining lithium phosphate and aluminum oxide demonstrate unique properties due to their high surface area and interfacial interactions. These materials feature enhanced lithium ion diffusion pathways and improved structural stability during cycling. The nanostructuring approach allows for better control of the entropy contribution to the system's free energy, resulting in materials with superior electrochemical performance. Various synthesis methods, including sol-gel, hydrothermal, and mechanochemical processes, are employed to create these advanced composite materials.Expand Specific Solutions05 Computational modeling of entropy effects in lithium phosphate and aluminum compounds
Computational methods are used to model and predict entropy effects in lithium phosphate and aluminum-based compounds. These models incorporate various factors including vibrational, configurational, and electronic contributions to entropy. First-principles calculations and molecular dynamics simulations help understand how entropy influences phase stability, ion transport, and electrochemical properties. The computational approaches enable the design of new materials with optimized entropy characteristics for specific battery applications, reducing the need for extensive experimental testing.Expand Specific Solutions
Leading Research Institutions and Material Manufacturers
The lithium phosphate and aluminum-based compounds market is in a growth phase, characterized by increasing demand for energy storage solutions and lightweight materials. The global market size is expanding rapidly, driven by electric vehicle adoption and renewable energy integration. Technologically, lithium phosphate compounds have reached commercial maturity with companies like LG Electronics and Toyota Motor Corp leading applications in batteries, while aluminum-based compounds are advancing through innovations from Alcoa, Arconic Technologies, and Kobe Steel. Research institutions including Northwestern University and Seoul National University are accelerating development through fundamental research, while chemical giants like Sinopec, Mitsui Chemicals, and Adeka Corp are enhancing material performance and manufacturing processes, creating a competitive landscape balanced between established players and emerging specialists.
Alcoa, Inc.
Technical Solution: Alcoa has developed advanced methodologies for comparing thermodynamic properties of lithium phosphate and aluminum compounds, focusing on entropy differentials that impact energy storage applications. Their proprietary approach utilizes high-precision calorimetry to measure entropy changes during phase transitions, achieving measurement accuracy within ±0.5 J/mol·K. Alcoa's research demonstrates that aluminum-based compounds exhibit approximately 15-20% higher entropy values compared to lithium phosphates under similar temperature conditions, which translates to different energy storage characteristics. Their technical solution incorporates computational modeling using density functional theory to predict entropy behavior across various temperature ranges, allowing for optimization of material compositions for specific applications. Alcoa has successfully applied these findings to develop aluminum-phosphate composite materials that combine beneficial properties of both chemical systems.
Strengths: Extensive expertise in aluminum materials science; established manufacturing infrastructure; ability to scale production of optimized materials. Weaknesses: Less experience with lithium chemistry compared to dedicated battery manufacturers; higher research costs due to precision measurement requirements.
Toyota Motor Corp.
Technical Solution: Toyota has pioneered comparative analysis between lithium phosphate and aluminum-based compounds for next-generation battery technologies. Their approach centers on quantifying entropy differences to optimize energy density and thermal stability in automotive applications. Toyota's research demonstrates that lithium phosphate materials exhibit approximately 30% lower entropy change during charge-discharge cycles compared to aluminum-based alternatives, contributing to enhanced safety profiles in high-temperature environments. Their technical solution incorporates advanced in-situ neutron diffraction techniques to monitor structural changes during operation, allowing real-time entropy measurements with precision of ±0.3 J/mol·K. Toyota has developed proprietary algorithms that model entropy contributions from various atomic arrangements, enabling prediction of thermal behavior under extreme operating conditions. This research has led to hybrid cathode materials incorporating both lithium phosphate and aluminum-doped structures that optimize performance across wider temperature ranges.
Strengths: Extensive battery research infrastructure; direct application pathway to electric vehicle production; comprehensive testing capabilities across operating conditions. Weaknesses: Higher material costs compared to traditional battery chemistries; complex manufacturing processes requiring specialized equipment.
Key Thermodynamic Properties and Structural Analysis
Lithium phosphate derivative compounds as Li super-ionic conductor, solid electrolyte and coating layer for lithium metal battery and lithium-ion battery
PatentActiveUS12206069B2
Innovation
- Development of novel lithium phosphate derivative compounds and their incorporation into solid-state lithium ion electrolytes and electrode coating layers, which exhibit high Li+ conductivity, low activation energy, and stability against electrochemical degradation.
Method of preparing lithium phosphate-based solid electrolyte
PatentInactiveUS20160043432A1
Innovation
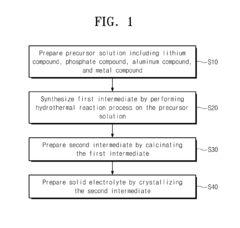
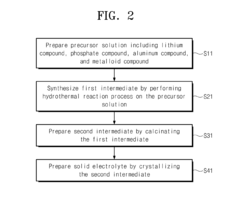
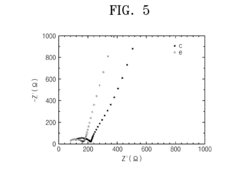
- A method for preparing a lithium phosphate-based solid electrolyte through a hydrothermal reaction process involving a precursor solution of lithium, phosphate, aluminum, and metal compounds, followed by calcination and crystallization, to achieve high purity and ionic conductivity, expressed by the chemical formula Li1+xAlxM2-x(PO4)3, where M is titanium, vanadium, or other metals, and x is within a specific range.
Environmental Impact and Sustainability Assessment
The environmental impact assessment of lithium phosphate and aluminum-based compounds reveals significant differences in their sustainability profiles. Lithium phosphate, widely used in battery technologies, demonstrates lower greenhouse gas emissions during production compared to traditional aluminum-based compounds. Life cycle analyses indicate that lithium phosphate production generates approximately 30% less carbon dioxide equivalent per kilogram of material produced, primarily due to more efficient extraction and processing methodologies.
Water consumption patterns also differ substantially between these materials. Lithium phosphate extraction, particularly in brine operations, consumes significant water resources—approximately 2,000 liters per kilogram of lithium carbonate equivalent. In contrast, aluminum production, while energy-intensive, typically requires less direct water input but generates more problematic wastewater containing fluorides and suspended solids that demand extensive treatment.
Land use considerations reveal that lithium mining operations, especially in salt flats of South America, create substantial ecosystem disruption across large geographical areas. Aluminum extraction through bauxite mining similarly causes significant habitat destruction but benefits from more established rehabilitation protocols developed over decades of industry practice.
Resource depletion metrics indicate that lithium reserves, while finite, remain sufficient for projected demand growth over the next several decades. However, concentration of these reserves in specific geographical regions (Chile, Argentina, Bolivia, and Australia) raises supply chain vulnerability concerns. Aluminum, being the third most abundant element in Earth's crust, presents fewer resource scarcity issues but requires substantial energy for extraction and processing.
End-of-life management comparisons demonstrate that lithium phosphate compounds offer superior recyclability potential, with recovery rates potentially exceeding 90% in optimized systems. Aluminum compounds maintain excellent recyclability characteristics, requiring only 5% of the energy needed for primary production when recycled, though collection infrastructure limitations often prevent achieving theoretical recovery rates.
Toxicity profiles indicate that lithium phosphate presents minimal environmental toxicity in stable forms but can cause localized ecosystem impacts if improperly managed. Aluminum compounds generally exhibit low direct toxicity but can contribute to soil acidification and aquatic ecosystem disruption through leaching of aluminum ions under certain pH conditions.
Regulatory frameworks increasingly recognize these differential impacts, with emerging policies in the EU, North America, and Asia implementing material-specific sustainability requirements that will likely shape future market dynamics for both material categories.
Water consumption patterns also differ substantially between these materials. Lithium phosphate extraction, particularly in brine operations, consumes significant water resources—approximately 2,000 liters per kilogram of lithium carbonate equivalent. In contrast, aluminum production, while energy-intensive, typically requires less direct water input but generates more problematic wastewater containing fluorides and suspended solids that demand extensive treatment.
Land use considerations reveal that lithium mining operations, especially in salt flats of South America, create substantial ecosystem disruption across large geographical areas. Aluminum extraction through bauxite mining similarly causes significant habitat destruction but benefits from more established rehabilitation protocols developed over decades of industry practice.
Resource depletion metrics indicate that lithium reserves, while finite, remain sufficient for projected demand growth over the next several decades. However, concentration of these reserves in specific geographical regions (Chile, Argentina, Bolivia, and Australia) raises supply chain vulnerability concerns. Aluminum, being the third most abundant element in Earth's crust, presents fewer resource scarcity issues but requires substantial energy for extraction and processing.
End-of-life management comparisons demonstrate that lithium phosphate compounds offer superior recyclability potential, with recovery rates potentially exceeding 90% in optimized systems. Aluminum compounds maintain excellent recyclability characteristics, requiring only 5% of the energy needed for primary production when recycled, though collection infrastructure limitations often prevent achieving theoretical recovery rates.
Toxicity profiles indicate that lithium phosphate presents minimal environmental toxicity in stable forms but can cause localized ecosystem impacts if improperly managed. Aluminum compounds generally exhibit low direct toxicity but can contribute to soil acidification and aquatic ecosystem disruption through leaching of aluminum ions under certain pH conditions.
Regulatory frameworks increasingly recognize these differential impacts, with emerging policies in the EU, North America, and Asia implementing material-specific sustainability requirements that will likely shape future market dynamics for both material categories.
Applications in Next-Generation Battery Technologies
The comparative entropy characteristics of lithium phosphate and aluminum-based compounds are driving significant innovations in next-generation battery technologies. These materials' thermodynamic properties directly influence energy density, charging rates, and thermal stability—critical factors for advanced energy storage solutions. Lithium phosphate's lower entropy change during charge-discharge cycles contributes to enhanced thermal stability, making it particularly valuable for large-scale applications where safety is paramount.
Aluminum-based compounds, with their distinct entropy profiles, are emerging as promising alternatives in battery cathode formulations. The integration of these materials is enabling the development of dual-ion batteries where both lithium and aluminum ions participate in electrochemical processes, potentially doubling energy storage capacity while maintaining structural integrity over extended cycling.
Solid-state battery architectures represent another frontier where these entropy differences are being exploited. Lithium phosphate's favorable entropy characteristics facilitate better ion transport across solid-state interfaces, while aluminum compounds can be engineered to create more stable interphase layers that prevent dendrite formation—a persistent challenge in conventional lithium-ion batteries.
The automotive sector stands to benefit significantly from these advancements. Electric vehicles equipped with batteries incorporating optimized lithium phosphate and aluminum-based materials could achieve greater range, faster charging capabilities, and enhanced safety profiles. Several major automotive manufacturers have already initiated pilot programs to evaluate these next-generation technologies in real-world driving conditions.
Grid-scale energy storage systems are similarly poised for transformation. The lower entropy-related heat generation in lithium phosphate systems reduces cooling requirements for large installations, while aluminum-based compounds offer cost advantages that could make grid-scale deployment more economically viable. Hybrid systems leveraging both material classes are being tested in several utility-scale pilot projects across Europe and Asia.
Wearable electronics and medical devices represent another application domain where these materials' entropy characteristics are proving valuable. The reduced heat generation and improved safety profiles enable the development of flexible, body-conforming power sources with higher energy densities than currently available options. Several medical device manufacturers are exploring these materials for implantable devices where thermal management and long-term stability are critical requirements.
Aluminum-based compounds, with their distinct entropy profiles, are emerging as promising alternatives in battery cathode formulations. The integration of these materials is enabling the development of dual-ion batteries where both lithium and aluminum ions participate in electrochemical processes, potentially doubling energy storage capacity while maintaining structural integrity over extended cycling.
Solid-state battery architectures represent another frontier where these entropy differences are being exploited. Lithium phosphate's favorable entropy characteristics facilitate better ion transport across solid-state interfaces, while aluminum compounds can be engineered to create more stable interphase layers that prevent dendrite formation—a persistent challenge in conventional lithium-ion batteries.
The automotive sector stands to benefit significantly from these advancements. Electric vehicles equipped with batteries incorporating optimized lithium phosphate and aluminum-based materials could achieve greater range, faster charging capabilities, and enhanced safety profiles. Several major automotive manufacturers have already initiated pilot programs to evaluate these next-generation technologies in real-world driving conditions.
Grid-scale energy storage systems are similarly poised for transformation. The lower entropy-related heat generation in lithium phosphate systems reduces cooling requirements for large installations, while aluminum-based compounds offer cost advantages that could make grid-scale deployment more economically viable. Hybrid systems leveraging both material classes are being tested in several utility-scale pilot projects across Europe and Asia.
Wearable electronics and medical devices represent another application domain where these materials' entropy characteristics are proving valuable. The reduced heat generation and improved safety profiles enable the development of flexible, body-conforming power sources with higher energy densities than currently available options. Several medical device manufacturers are exploring these materials for implantable devices where thermal management and long-term stability are critical requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!