How to Optimize Lithium Phosphate for Energy Density
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Phosphate Energy Density Background and Objectives
Lithium iron phosphate (LFP) batteries have emerged as a significant technology in the energy storage landscape since their commercial introduction in the late 1990s. The evolution of this technology has been marked by continuous improvements in performance, safety, and cost-effectiveness. Initially developed as an alternative to lithium cobalt oxide (LCO) batteries, LFP has gained prominence due to its inherent thermal stability and longer cycle life, despite its traditionally lower energy density compared to other lithium-ion chemistries.
The technological trajectory of LFP batteries has been characterized by incremental advancements in material science, electrode design, and manufacturing processes. Recent breakthroughs in nano-structuring and doping techniques have demonstrated potential for substantial energy density improvements, challenging the conventional limitations of this chemistry. The industry has witnessed a resurgence of interest in LFP technology, particularly in electric vehicles and grid storage applications, driven by supply chain considerations and sustainability concerns.
Current energy density limitations of LFP batteries (approximately 140-160 Wh/kg at the cell level) represent a significant barrier to wider adoption in applications where volumetric and gravimetric energy density are critical factors. This constraint has historically positioned LFP as a compromise solution, trading energy density for safety, longevity, and cost advantages.
The primary technical objective of optimizing lithium phosphate for energy density is to achieve a 30-50% improvement in specific energy without compromising the inherent safety advantages and cycle life that make LFP attractive. Secondary objectives include maintaining or reducing production costs, ensuring scalability of new solutions, and preserving the environmental benefits associated with cobalt-free chemistry.
Research trends indicate several promising pathways for energy density enhancement, including crystal structure modification, carbon coating optimization, particle size reduction, and novel electrolyte formulations. Additionally, cell-level innovations such as advanced packaging techniques and higher voltage operation are being explored to maximize volumetric efficiency.
The technological evolution is expected to continue along two parallel tracks: incremental improvements to existing LFP formulations and manufacturing processes, and more disruptive approaches involving hybrid cathode materials or completely novel phosphate-based compounds. Both approaches aim to position LFP as a competitive alternative to higher energy density chemistries like NMC (nickel manganese cobalt) and NCA (nickel cobalt aluminum) in applications where their use has been historically limited.
The technological trajectory of LFP batteries has been characterized by incremental advancements in material science, electrode design, and manufacturing processes. Recent breakthroughs in nano-structuring and doping techniques have demonstrated potential for substantial energy density improvements, challenging the conventional limitations of this chemistry. The industry has witnessed a resurgence of interest in LFP technology, particularly in electric vehicles and grid storage applications, driven by supply chain considerations and sustainability concerns.
Current energy density limitations of LFP batteries (approximately 140-160 Wh/kg at the cell level) represent a significant barrier to wider adoption in applications where volumetric and gravimetric energy density are critical factors. This constraint has historically positioned LFP as a compromise solution, trading energy density for safety, longevity, and cost advantages.
The primary technical objective of optimizing lithium phosphate for energy density is to achieve a 30-50% improvement in specific energy without compromising the inherent safety advantages and cycle life that make LFP attractive. Secondary objectives include maintaining or reducing production costs, ensuring scalability of new solutions, and preserving the environmental benefits associated with cobalt-free chemistry.
Research trends indicate several promising pathways for energy density enhancement, including crystal structure modification, carbon coating optimization, particle size reduction, and novel electrolyte formulations. Additionally, cell-level innovations such as advanced packaging techniques and higher voltage operation are being explored to maximize volumetric efficiency.
The technological evolution is expected to continue along two parallel tracks: incremental improvements to existing LFP formulations and manufacturing processes, and more disruptive approaches involving hybrid cathode materials or completely novel phosphate-based compounds. Both approaches aim to position LFP as a competitive alternative to higher energy density chemistries like NMC (nickel manganese cobalt) and NCA (nickel cobalt aluminum) in applications where their use has been historically limited.
Market Analysis for High-Density Energy Storage Solutions
The global energy storage market is experiencing unprecedented growth, driven by the increasing adoption of renewable energy sources and the electrification of transportation. The market for high-density energy storage solutions is projected to reach $546 billion by 2035, with a compound annual growth rate of 19.7% from 2023 to 2035. Lithium phosphate-based batteries, particularly lithium iron phosphate (LFP), have emerged as a significant segment within this market due to their safety profile and cost advantages.
Consumer demand for electric vehicles with longer ranges and portable electronics with extended battery life is creating substantial market pull for higher energy density solutions. While traditional lithium-ion batteries with nickel-manganese-cobalt (NMC) cathodes currently dominate the high-energy-density segment, the market is increasingly receptive to advanced lithium phosphate formulations that can close this energy density gap while maintaining safety advantages.
The stationary energy storage sector represents another major market opportunity for optimized lithium phosphate batteries. Grid-scale storage installations grew by 134% in 2022, with projections indicating continued robust expansion as renewable energy integration accelerates. Lithium phosphate chemistries captured 48% of this market in 2022, up from 33% in 2020, highlighting the growing preference for these formulations in large-scale applications.
Regional market analysis reveals varying adoption patterns. China leads manufacturing capacity for lithium phosphate batteries, controlling approximately 75% of global production. However, recent policy initiatives in North America and Europe aim to establish regional supply chains, with planned investments exceeding $25 billion through 2030 for advanced battery manufacturing facilities.
Commercial sensitivity to raw material costs is driving market interest in lithium phosphate solutions. The phosphate supply chain faces fewer geopolitical constraints compared to cobalt and nickel, offering potential price stability advantages. Market forecasts suggest that optimized lithium phosphate batteries could achieve price parity with lead-acid batteries in stationary applications by 2025, potentially unlocking a $12 billion market segment currently dominated by legacy technologies.
Customer segmentation analysis indicates three primary market targets for high-density lithium phosphate solutions: mid-range electric vehicle manufacturers seeking cost-performance balance, grid-scale energy storage developers prioritizing safety and longevity, and industrial equipment manufacturers requiring robust power solutions. Each segment presents distinct requirements and growth trajectories, with the EV segment showing the highest compound annual growth rate at 24.3% through 2030.
Consumer demand for electric vehicles with longer ranges and portable electronics with extended battery life is creating substantial market pull for higher energy density solutions. While traditional lithium-ion batteries with nickel-manganese-cobalt (NMC) cathodes currently dominate the high-energy-density segment, the market is increasingly receptive to advanced lithium phosphate formulations that can close this energy density gap while maintaining safety advantages.
The stationary energy storage sector represents another major market opportunity for optimized lithium phosphate batteries. Grid-scale storage installations grew by 134% in 2022, with projections indicating continued robust expansion as renewable energy integration accelerates. Lithium phosphate chemistries captured 48% of this market in 2022, up from 33% in 2020, highlighting the growing preference for these formulations in large-scale applications.
Regional market analysis reveals varying adoption patterns. China leads manufacturing capacity for lithium phosphate batteries, controlling approximately 75% of global production. However, recent policy initiatives in North America and Europe aim to establish regional supply chains, with planned investments exceeding $25 billion through 2030 for advanced battery manufacturing facilities.
Commercial sensitivity to raw material costs is driving market interest in lithium phosphate solutions. The phosphate supply chain faces fewer geopolitical constraints compared to cobalt and nickel, offering potential price stability advantages. Market forecasts suggest that optimized lithium phosphate batteries could achieve price parity with lead-acid batteries in stationary applications by 2025, potentially unlocking a $12 billion market segment currently dominated by legacy technologies.
Customer segmentation analysis indicates three primary market targets for high-density lithium phosphate solutions: mid-range electric vehicle manufacturers seeking cost-performance balance, grid-scale energy storage developers prioritizing safety and longevity, and industrial equipment manufacturers requiring robust power solutions. Each segment presents distinct requirements and growth trajectories, with the EV segment showing the highest compound annual growth rate at 24.3% through 2030.
Current Limitations and Technical Challenges in LFP Technology
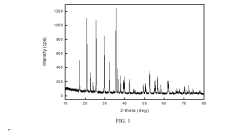
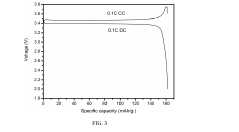
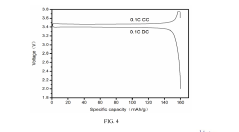
Lithium iron phosphate (LFP) batteries face significant energy density limitations compared to other lithium-ion chemistries. The theoretical specific capacity of LFP cathode material is approximately 170 mAh/g, substantially lower than nickel-rich cathodes like NMC811 (220 mAh/g) or NCA (200 mAh/g). This inherent limitation stems from LFP's olivine crystal structure and the single-electron redox reaction of Fe²⁺/Fe³⁺, which restricts the amount of lithium that can be stored per unit mass.
The volumetric energy density challenge is particularly acute for LFP batteries. With a relatively low tap density of 1.0-1.5 g/cm³ (compared to 2.0-2.5 g/cm³ for layered oxide cathodes), LFP cells typically achieve only 350-400 Wh/L, whereas high-nickel alternatives can reach 600-750 Wh/L. This creates significant constraints for applications where space is limited, such as electric vehicles and portable electronics.
Another technical hurdle is LFP's poor electronic conductivity (approximately 10⁻⁹ S/cm), which necessitates carbon coating and particle size reduction. While these modifications improve performance, they add manufacturing complexity and reduce the active material content in electrodes, further limiting energy density. The carbon coating typically constitutes 2-5% of the cathode weight but occupies valuable volume without contributing to energy storage.
LFP also exhibits relatively low lithium-ion diffusion coefficients (10⁻¹⁴-10⁻¹⁶ cm²/s), particularly along certain crystallographic directions. This anisotropic diffusion behavior creates challenges for fast charging capabilities and high-rate performance, especially at lower temperatures. The poor low-temperature performance is exacerbated by the relatively high activation energy for lithium-ion transport in the olivine structure.
The flat voltage profile of LFP (around 3.2-3.4V vs. Li/Li⁺) is significantly lower than other cathode materials like NMC (3.7-3.8V) or LCO (3.9V). This lower operating voltage directly impacts energy density, as energy is the product of capacity and voltage. The thermodynamic stability that makes LFP safe also limits its voltage window for energy storage.
Manufacturing challenges further complicate energy density optimization. LFP requires precise control of stoichiometry and phase purity during synthesis. Impurities and defects can block lithium diffusion pathways and reduce usable capacity. Additionally, the moisture sensitivity of LFP precursors necessitates stringent environmental controls during production, adding cost and complexity.
Recent advancements in cell design, such as cell-to-pack and cell-to-chassis technologies, have partially compensated for LFP's energy density limitations by improving pack-level energy density. However, these approaches do not address the fundamental material-level constraints of LFP chemistry.
The volumetric energy density challenge is particularly acute for LFP batteries. With a relatively low tap density of 1.0-1.5 g/cm³ (compared to 2.0-2.5 g/cm³ for layered oxide cathodes), LFP cells typically achieve only 350-400 Wh/L, whereas high-nickel alternatives can reach 600-750 Wh/L. This creates significant constraints for applications where space is limited, such as electric vehicles and portable electronics.
Another technical hurdle is LFP's poor electronic conductivity (approximately 10⁻⁹ S/cm), which necessitates carbon coating and particle size reduction. While these modifications improve performance, they add manufacturing complexity and reduce the active material content in electrodes, further limiting energy density. The carbon coating typically constitutes 2-5% of the cathode weight but occupies valuable volume without contributing to energy storage.
LFP also exhibits relatively low lithium-ion diffusion coefficients (10⁻¹⁴-10⁻¹⁶ cm²/s), particularly along certain crystallographic directions. This anisotropic diffusion behavior creates challenges for fast charging capabilities and high-rate performance, especially at lower temperatures. The poor low-temperature performance is exacerbated by the relatively high activation energy for lithium-ion transport in the olivine structure.
The flat voltage profile of LFP (around 3.2-3.4V vs. Li/Li⁺) is significantly lower than other cathode materials like NMC (3.7-3.8V) or LCO (3.9V). This lower operating voltage directly impacts energy density, as energy is the product of capacity and voltage. The thermodynamic stability that makes LFP safe also limits its voltage window for energy storage.
Manufacturing challenges further complicate energy density optimization. LFP requires precise control of stoichiometry and phase purity during synthesis. Impurities and defects can block lithium diffusion pathways and reduce usable capacity. Additionally, the moisture sensitivity of LFP precursors necessitates stringent environmental controls during production, adding cost and complexity.
Recent advancements in cell design, such as cell-to-pack and cell-to-chassis technologies, have partially compensated for LFP's energy density limitations by improving pack-level energy density. However, these approaches do not address the fundamental material-level constraints of LFP chemistry.
Current Optimization Approaches for LFP Energy Density
01 Composition modifications to enhance energy density
Various compositional modifications can be made to lithium phosphate materials to enhance energy density. These include doping with elements like manganese, nickel, or cobalt, creating composite structures with carbon materials, and optimizing the lithium-to-phosphate ratio. These modifications can improve the electrochemical performance and increase the overall energy storage capacity of lithium phosphate-based batteries.- Lithium iron phosphate cathode material composition: Lithium iron phosphate (LiFePO4) is a key cathode material for lithium-ion batteries with specific energy density characteristics. Various compositions and doping strategies are employed to enhance its energy density performance. Modifications include adding conductive coatings, incorporating carbon materials, and doping with elements like manganese, nickel, or cobalt to improve electronic conductivity and energy storage capacity.
- Nano-structuring techniques for lithium phosphate materials: Nano-structuring techniques are applied to lithium phosphate materials to increase energy density. These include developing nano-sized particles, creating porous structures, and implementing hierarchical architectures. Such nano-engineering approaches reduce lithium ion diffusion paths, increase active surface area, and enhance electron transport, resulting in improved energy density and rate capability of lithium phosphate-based battery systems.
- Composite electrode structures with lithium phosphate: Composite electrode structures combine lithium phosphate materials with other components to enhance energy density. These composites typically incorporate conductive additives like carbon nanotubes, graphene, or conductive polymers. Some designs feature multi-layered structures or gradient compositions that optimize both energy density and power performance by balancing ionic and electronic conductivity throughout the electrode.
- Battery system design for lithium phosphate energy optimization: Battery system designs specifically optimized for lithium phosphate chemistry focus on maximizing energy density at the pack level. These designs include thermal management systems, cell arrangement strategies, and electronic control systems that allow lithium phosphate batteries to operate at optimal conditions. Advanced battery management systems monitor and control individual cells to ensure maximum energy utilization while maintaining safety and longevity.
- Manufacturing processes affecting lithium phosphate energy density: Manufacturing processes significantly impact the energy density of lithium phosphate materials. Advanced synthesis methods like hydrothermal processing, sol-gel techniques, and solid-state reactions with precise temperature control can produce materials with optimized crystal structure and particle morphology. Post-synthesis treatments including controlled annealing, carbon coating processes, and surface modification techniques further enhance the energy storage capabilities of lithium phosphate materials.
02 Nanostructured lithium phosphate materials
Developing nanostructured lithium phosphate materials can significantly improve energy density. Nano-sizing particles increases the surface area and shortens lithium ion diffusion paths, leading to enhanced charge/discharge rates and higher energy density. Various synthesis methods including hydrothermal, sol-gel, and microwave-assisted techniques can be used to create these nanostructured materials with controlled morphology and improved electrochemical properties.Expand Specific Solutions03 Carbon coating and conductive additives
Carbon coating of lithium phosphate particles and incorporation of conductive additives can significantly enhance the energy density of lithium phosphate batteries. The carbon coating improves electronic conductivity, while conductive additives create efficient electron transport networks. These approaches address the inherent low conductivity of lithium phosphate materials, resulting in improved rate capability and higher energy density.Expand Specific Solutions04 Advanced electrode design and manufacturing
Advanced electrode design and manufacturing techniques can optimize the energy density of lithium phosphate batteries. These include high-density electrode preparation methods, optimized electrode thickness, porosity control, and improved current collector designs. By maximizing the active material loading while maintaining good ion transport properties, these approaches can significantly enhance the volumetric and gravimetric energy density of lithium phosphate batteries.Expand Specific Solutions05 Electrolyte optimization for lithium phosphate systems
Optimizing electrolyte formulations specifically for lithium phosphate battery systems can enhance energy density. This includes developing electrolytes with improved ionic conductivity, wider electrochemical stability windows, and better compatibility with lithium phosphate materials. Additives that form stable solid electrolyte interphase layers and reduce interfacial resistance can also contribute to higher energy density by improving cycling efficiency and reducing capacity fade.Expand Specific Solutions
Key Industry Players and Competitive Landscape Analysis
The lithium phosphate energy density optimization market is in a growth phase, with increasing demand driven by electric vehicle and energy storage applications. The market is expected to expand significantly as companies strive to overcome the energy density limitations of LFP batteries compared to other chemistries. Key players like CATL, BYD, and LG Energy Solution are leading technological advancements, with significant investments in R&D. Chinese manufacturers dominate the landscape, while global chemical companies like BASF and Johnson Matthey provide specialized materials. Research institutions such as CEA and Nankai University contribute fundamental innovations. The technology is approaching commercial maturity with several companies already implementing enhanced lithium phosphate formulations in production batteries.
BYD Co., Ltd.
Technical Solution: BYD has pioneered the Blade Battery technology, which represents a significant advancement in LFP battery energy density optimization. The Blade Battery utilizes cell-to-pack (CTP) integration that eliminates traditional module structures, increasing volume utilization by approximately 50% compared to conventional LFP batteries[1]. BYD's approach involves redesigning the form factor of LFP cells into thinner, longer "blade" shapes that can be arranged more efficiently, achieving energy densities of 140-150 Wh/kg at the pack level[2]. Additionally, BYD employs advanced doping techniques with elements such as manganese and zinc to modify the crystal structure of LFP cathodes, enhancing ionic conductivity and capacity[3]. Their manufacturing process includes precise control of particle morphology and size distribution, creating hierarchical structures that optimize lithium-ion diffusion pathways while maintaining structural stability during cycling.
Strengths: Superior safety profile with virtually zero risk of thermal runaway; excellent cycle life (over 3000 cycles); cost-effective manufacturing; high volumetric efficiency through CTP design. Weaknesses: Still lower energy density compared to NCM/NCA chemistries; temperature sensitivity affecting performance in cold conditions; relatively slower charging capabilities at low temperatures.
BASF Corp.
Technical Solution: BASF has developed advanced materials engineering approaches to optimize LFP energy density through their "Cathode Active Materials" program. Their strategy centers on precise control of LFP particle synthesis, employing hydrothermal and solid-state reaction methods that yield particles with optimized morphology and size distribution (typically 50-150nm)[1]. BASF's approach includes sophisticated carbon coating techniques that create uniform conductive networks around LFP particles, significantly enhancing electronic conductivity without compromising the material's inherent safety advantages. Their process incorporates precise doping with elements such as manganese, cobalt and niobium to modify the olivine structure, creating controlled defects that enhance lithium-ion mobility within the crystal lattice[2]. BASF has pioneered advanced surface modification techniques that stabilize the electrode-electrolyte interface, reducing parasitic reactions and improving cycling stability. Additionally, they've developed specialized conductive additives and binders that improve the mechanical stability and electronic conductivity of LFP electrodes, allowing for higher material loading and improved energy density[3].
Strengths: Extensive materials science expertise; vertical integration from raw materials to finished cathode materials; strong intellectual property portfolio in battery materials; global manufacturing footprint enabling supply chain advantages. Weaknesses: As a materials supplier rather than cell manufacturer, dependent on implementation by battery producers; optimization benefits may be partially offset during cell manufacturing processes; requires close collaboration with cell manufacturers to realize full benefits.
Critical Patents and Research in LFP Density Enhancement
Positive electrode for rechargeable lithium battery and rechargeable lithium battery including same
PatentPendingUS20250105286A1
Innovation
- A positive electrode is developed using a dry process that incorporates a mixture of large-particle and small-particle lithium iron phosphate particles with a polytetrafluoroethylene binder, optimizing the weight ratio and particle size to enhance loading levels and energy density, while maintaining adherence to the current collector.
Lithium iron phosphate positive electrode material having a high tap density, method for preparing the same and application thereof
PatentUndeterminedIN202224075998A
Innovation
- A method involving grinding and sintering a mixed solution of an iron source, lithium source, carbon source, and ion doping agent, using anhydrous ferric phosphate with controlled tap density and morphology, to produce lithium iron phosphate with a high tap density and spherical particle structure, compatible with ternary materials for enhanced performance.
Environmental Impact and Sustainability Considerations
The optimization of lithium phosphate for energy density must be evaluated not only for its technical merits but also for its environmental impact and sustainability profile. Lithium iron phosphate (LFP) batteries inherently possess several environmental advantages compared to other lithium-ion chemistries, particularly those containing cobalt and nickel. The raw materials for LFP production—lithium, iron, and phosphorus—are more abundant and geographically distributed, reducing supply chain vulnerabilities and associated environmental impacts from concentrated mining operations.
Manufacturing processes for optimized lithium phosphate materials generally require lower energy inputs compared to other cathode materials, resulting in reduced carbon footprints during production. Recent life cycle assessments indicate that LFP batteries can have up to 30% lower greenhouse gas emissions during manufacturing compared to nickel-manganese-cobalt (NMC) alternatives. This advantage becomes particularly significant when considering large-scale energy storage applications.
Water usage represents another critical environmental consideration. Traditional lithium extraction methods can consume substantial water resources, particularly in water-stressed regions. Advanced optimization techniques for lithium phosphate should incorporate water-efficient processing methods, such as dry coating technologies and closed-loop water recycling systems, which can reduce water consumption by up to 60% compared to conventional manufacturing processes.
The recyclability of lithium phosphate batteries presents both challenges and opportunities. While LFP batteries contain fewer toxic materials than other lithium-ion chemistries, their lower material value has historically made recycling less economically attractive. However, emerging direct recycling technologies specifically designed for LFP chemistry can recover up to 90% of cathode materials while consuming significantly less energy than pyrometallurgical processes used for other battery types.
Safety considerations also contribute to the sustainability profile of optimized lithium phosphate batteries. Their inherent thermal stability reduces the risk of thermal runaway events, potentially extending service life and reducing waste generation from premature battery failures. This enhanced safety profile also minimizes the environmental risks associated with battery fires or other catastrophic failures during use or transportation.
Long-term sustainability strategies for lithium phosphate optimization should incorporate principles of circular economy. This includes designing for disassembly, standardizing battery formats to facilitate recycling, and developing second-life applications that can extend the useful life of these energy storage systems before final recycling. Such approaches could potentially double the effective carbon footprint reduction of these battery systems over their complete life cycle.
Manufacturing processes for optimized lithium phosphate materials generally require lower energy inputs compared to other cathode materials, resulting in reduced carbon footprints during production. Recent life cycle assessments indicate that LFP batteries can have up to 30% lower greenhouse gas emissions during manufacturing compared to nickel-manganese-cobalt (NMC) alternatives. This advantage becomes particularly significant when considering large-scale energy storage applications.
Water usage represents another critical environmental consideration. Traditional lithium extraction methods can consume substantial water resources, particularly in water-stressed regions. Advanced optimization techniques for lithium phosphate should incorporate water-efficient processing methods, such as dry coating technologies and closed-loop water recycling systems, which can reduce water consumption by up to 60% compared to conventional manufacturing processes.
The recyclability of lithium phosphate batteries presents both challenges and opportunities. While LFP batteries contain fewer toxic materials than other lithium-ion chemistries, their lower material value has historically made recycling less economically attractive. However, emerging direct recycling technologies specifically designed for LFP chemistry can recover up to 90% of cathode materials while consuming significantly less energy than pyrometallurgical processes used for other battery types.
Safety considerations also contribute to the sustainability profile of optimized lithium phosphate batteries. Their inherent thermal stability reduces the risk of thermal runaway events, potentially extending service life and reducing waste generation from premature battery failures. This enhanced safety profile also minimizes the environmental risks associated with battery fires or other catastrophic failures during use or transportation.
Long-term sustainability strategies for lithium phosphate optimization should incorporate principles of circular economy. This includes designing for disassembly, standardizing battery formats to facilitate recycling, and developing second-life applications that can extend the useful life of these energy storage systems before final recycling. Such approaches could potentially double the effective carbon footprint reduction of these battery systems over their complete life cycle.
Manufacturing Scalability and Cost Analysis
The scalability of lithium phosphate (LFP) manufacturing processes represents a critical factor in its widespread adoption for energy storage applications. Current production methods for high-energy-density LFP materials involve several key processes including solid-state reactions, hydrothermal synthesis, sol-gel methods, and spray pyrolysis. Each method presents distinct advantages and challenges when scaled to industrial production levels.
Solid-state reaction methods, while relatively straightforward to scale, often require high-temperature processing (600-800°C) that contributes significantly to production costs. Energy consumption during these thermal treatments accounts for approximately 30-40% of manufacturing expenses. However, recent innovations in microwave-assisted solid-state synthesis have demonstrated potential energy savings of 25-35% while maintaining comparable material quality.
Hydrothermal synthesis routes offer better control over particle morphology and size distribution, which directly impacts energy density optimization. The capital expenditure for industrial-scale hydrothermal reactors remains 40-60% higher than conventional equipment, though operational costs over a 5-year period typically offset this initial investment through improved product consistency and reduced waste generation.
From a cost perspective, raw material selection significantly influences economic viability. Traditional LFP formulations rely on high-purity lithium carbonate, which represents 35-45% of material costs. Alternative lithium sources such as lithium hydroxide can reduce costs by 8-12% but may require process modifications. Phosphate sources contribute another 20-25% of material expenses, with opportunities for cost reduction through recycled phosphate streams from adjacent industries.
Labor and overhead costs vary significantly by manufacturing region, with fully automated facilities in developed economies operating at 15-20% higher capital costs but 30-40% lower labor expenses compared to semi-automated facilities. The economies of scale are particularly pronounced for LFP production, with facilities exceeding 5,000 metric tons annual capacity achieving 22-28% lower unit costs than smaller operations.
Environmental compliance and waste management represent increasingly significant cost factors. Water recycling systems for hydrothermal processes typically add 5-8% to capital costs but reduce operational expenses by 12-15% over facility lifetime. Energy recovery systems during calcination stages can further improve cost structures by 7-10% while reducing carbon footprint.
For optimized high-energy-density LFP formulations, specialized coating and doping processes add 15-25% to production costs but can yield energy density improvements of 10-20%, presenting favorable cost-performance trade-offs for premium applications where volumetric efficiency is prioritized over absolute cost minimization.
Solid-state reaction methods, while relatively straightforward to scale, often require high-temperature processing (600-800°C) that contributes significantly to production costs. Energy consumption during these thermal treatments accounts for approximately 30-40% of manufacturing expenses. However, recent innovations in microwave-assisted solid-state synthesis have demonstrated potential energy savings of 25-35% while maintaining comparable material quality.
Hydrothermal synthesis routes offer better control over particle morphology and size distribution, which directly impacts energy density optimization. The capital expenditure for industrial-scale hydrothermal reactors remains 40-60% higher than conventional equipment, though operational costs over a 5-year period typically offset this initial investment through improved product consistency and reduced waste generation.
From a cost perspective, raw material selection significantly influences economic viability. Traditional LFP formulations rely on high-purity lithium carbonate, which represents 35-45% of material costs. Alternative lithium sources such as lithium hydroxide can reduce costs by 8-12% but may require process modifications. Phosphate sources contribute another 20-25% of material expenses, with opportunities for cost reduction through recycled phosphate streams from adjacent industries.
Labor and overhead costs vary significantly by manufacturing region, with fully automated facilities in developed economies operating at 15-20% higher capital costs but 30-40% lower labor expenses compared to semi-automated facilities. The economies of scale are particularly pronounced for LFP production, with facilities exceeding 5,000 metric tons annual capacity achieving 22-28% lower unit costs than smaller operations.
Environmental compliance and waste management represent increasingly significant cost factors. Water recycling systems for hydrothermal processes typically add 5-8% to capital costs but reduce operational expenses by 12-15% over facility lifetime. Energy recovery systems during calcination stages can further improve cost structures by 7-10% while reducing carbon footprint.
For optimized high-energy-density LFP formulations, specialized coating and doping processes add 15-25% to production costs but can yield energy density improvements of 10-20%, presenting favorable cost-performance trade-offs for premium applications where volumetric efficiency is prioritized over absolute cost minimization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!