How to Assess Lithium Phosphate Electrochemical Performance
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LFP Battery Technology Background and Objectives
Lithium iron phosphate (LFP) battery technology has evolved significantly since its initial development in the 1990s. Originally pioneered by researchers at the University of Texas, LFP cathode materials emerged as a promising alternative to traditional lithium cobalt oxide (LCO) batteries due to their enhanced safety profile, longer cycle life, and reduced environmental impact. The technology has progressed through several developmental phases, from laboratory research to commercial implementation, with continuous improvements in energy density, rate capability, and manufacturing processes.
The global transition toward renewable energy systems and electric mobility has accelerated interest in LFP technology over the past decade. This shift has been particularly pronounced since 2018, when major automotive manufacturers began incorporating LFP batteries into their electric vehicle portfolios. The technology's evolution has been characterized by incremental improvements in electrochemical performance, with recent innovations focusing on nano-structuring, doping strategies, and carbon coating techniques to overcome inherent limitations in electronic conductivity.
Current technological trends indicate a growing emphasis on developing advanced assessment methodologies for LFP electrochemical performance. These assessment frameworks are crucial for optimizing battery design, predicting lifetime performance, and ensuring quality control in manufacturing processes. The industry is moving toward more sophisticated characterization techniques that can provide insights into degradation mechanisms, rate-limiting steps, and performance under various operating conditions.
The primary objectives of LFP battery technology assessment include establishing standardized testing protocols that accurately reflect real-world usage scenarios, developing predictive models for performance and aging, and identifying key performance indicators that correlate with long-term reliability. Additionally, there is a growing need for rapid screening methods that can accelerate materials development and quality control processes in production environments.
From a strategic perspective, comprehensive electrochemical performance assessment serves multiple purposes: it enables manufacturers to optimize cell design and manufacturing processes, helps system integrators select appropriate cells for specific applications, and provides end-users with reliable information about expected performance and lifetime. The development of robust assessment methodologies is therefore essential for the continued advancement and widespread adoption of LFP battery technology.
As the technology continues to mature, the focus is shifting from basic performance metrics to more nuanced evaluations that consider the interplay between electrochemical performance, safety characteristics, and economic factors. This holistic approach to assessment is expected to drive the next wave of innovations in LFP battery technology, potentially enabling its application in increasingly diverse energy storage scenarios.
The global transition toward renewable energy systems and electric mobility has accelerated interest in LFP technology over the past decade. This shift has been particularly pronounced since 2018, when major automotive manufacturers began incorporating LFP batteries into their electric vehicle portfolios. The technology's evolution has been characterized by incremental improvements in electrochemical performance, with recent innovations focusing on nano-structuring, doping strategies, and carbon coating techniques to overcome inherent limitations in electronic conductivity.
Current technological trends indicate a growing emphasis on developing advanced assessment methodologies for LFP electrochemical performance. These assessment frameworks are crucial for optimizing battery design, predicting lifetime performance, and ensuring quality control in manufacturing processes. The industry is moving toward more sophisticated characterization techniques that can provide insights into degradation mechanisms, rate-limiting steps, and performance under various operating conditions.
The primary objectives of LFP battery technology assessment include establishing standardized testing protocols that accurately reflect real-world usage scenarios, developing predictive models for performance and aging, and identifying key performance indicators that correlate with long-term reliability. Additionally, there is a growing need for rapid screening methods that can accelerate materials development and quality control processes in production environments.
From a strategic perspective, comprehensive electrochemical performance assessment serves multiple purposes: it enables manufacturers to optimize cell design and manufacturing processes, helps system integrators select appropriate cells for specific applications, and provides end-users with reliable information about expected performance and lifetime. The development of robust assessment methodologies is therefore essential for the continued advancement and widespread adoption of LFP battery technology.
As the technology continues to mature, the focus is shifting from basic performance metrics to more nuanced evaluations that consider the interplay between electrochemical performance, safety characteristics, and economic factors. This holistic approach to assessment is expected to drive the next wave of innovations in LFP battery technology, potentially enabling its application in increasingly diverse energy storage scenarios.
Market Analysis of LFP Battery Applications
The global LFP (Lithium Iron Phosphate) battery market has experienced remarkable growth in recent years, primarily driven by the expanding electric vehicle (EV) sector. As of 2023, the market size reached approximately $15 billion, with projections indicating a compound annual growth rate of 20-25% through 2030. This growth trajectory is supported by the inherent advantages of LFP batteries, including enhanced safety profiles, longer cycle life, and improved thermal stability compared to alternative lithium-ion chemistries.
The automotive sector represents the largest application segment for LFP batteries, accounting for roughly 65% of total market demand. Chinese automakers have been particularly aggressive in adopting LFP technology, with BYD and Tesla leading implementation in mass-market vehicles. The cost advantage of LFP batteries—typically 20-30% lower than nickel-based alternatives—has made them especially attractive for entry and mid-level electric vehicles where driving range requirements are less stringent.
Energy storage systems (ESS) constitute the second-largest application segment, representing approximately 25% of the market. Utility-scale projects increasingly favor LFP chemistry due to its safety characteristics and longer operational lifespan, which can exceed 4,000 charge cycles under optimal conditions. This application segment is expected to grow at the fastest rate, driven by global renewable energy integration and grid modernization initiatives.
Consumer electronics and industrial applications collectively account for the remaining 10% of the market. While historically dominated by other battery chemistries, LFP is gaining traction in applications where safety concerns outweigh energy density requirements, such as power tools, medical devices, and backup power systems.
Geographically, Asia-Pacific dominates the LFP battery market with over 75% share, primarily due to China's established manufacturing ecosystem and government policies favoring this chemistry. North America and Europe are experiencing accelerated growth as manufacturers seek to reduce supply chain dependencies on China and capitalize on regional incentives for domestic battery production.
Market challenges include the lower energy density of LFP compared to nickel-manganese-cobalt (NMC) batteries, which limits application in premium EVs requiring extended range. However, recent technological advancements, including cell-to-pack designs and silicon-doping techniques, have narrowed this performance gap while maintaining LFP's cost advantage.
The supply chain for LFP batteries benefits from greater raw material security compared to other lithium-ion chemistries, as it avoids dependence on constrained materials like cobalt and nickel. This advantage has become increasingly significant amid growing concerns about critical mineral availability and ethical sourcing practices.
The automotive sector represents the largest application segment for LFP batteries, accounting for roughly 65% of total market demand. Chinese automakers have been particularly aggressive in adopting LFP technology, with BYD and Tesla leading implementation in mass-market vehicles. The cost advantage of LFP batteries—typically 20-30% lower than nickel-based alternatives—has made them especially attractive for entry and mid-level electric vehicles where driving range requirements are less stringent.
Energy storage systems (ESS) constitute the second-largest application segment, representing approximately 25% of the market. Utility-scale projects increasingly favor LFP chemistry due to its safety characteristics and longer operational lifespan, which can exceed 4,000 charge cycles under optimal conditions. This application segment is expected to grow at the fastest rate, driven by global renewable energy integration and grid modernization initiatives.
Consumer electronics and industrial applications collectively account for the remaining 10% of the market. While historically dominated by other battery chemistries, LFP is gaining traction in applications where safety concerns outweigh energy density requirements, such as power tools, medical devices, and backup power systems.
Geographically, Asia-Pacific dominates the LFP battery market with over 75% share, primarily due to China's established manufacturing ecosystem and government policies favoring this chemistry. North America and Europe are experiencing accelerated growth as manufacturers seek to reduce supply chain dependencies on China and capitalize on regional incentives for domestic battery production.
Market challenges include the lower energy density of LFP compared to nickel-manganese-cobalt (NMC) batteries, which limits application in premium EVs requiring extended range. However, recent technological advancements, including cell-to-pack designs and silicon-doping techniques, have narrowed this performance gap while maintaining LFP's cost advantage.
The supply chain for LFP batteries benefits from greater raw material security compared to other lithium-ion chemistries, as it avoids dependence on constrained materials like cobalt and nickel. This advantage has become increasingly significant amid growing concerns about critical mineral availability and ethical sourcing practices.
Current Challenges in LFP Electrochemical Assessment
Despite significant advancements in lithium iron phosphate (LFP) battery technology, researchers and manufacturers face numerous challenges in accurately assessing the electrochemical performance of these materials. One primary challenge lies in the standardization of testing protocols. Different laboratories employ varying methodologies, equipment, and conditions, making direct comparison of results problematic and hindering collaborative progress in the field. This inconsistency creates barriers to establishing universal performance benchmarks and quality control standards.
The complex interplay between particle morphology, size distribution, and carbon coating quality significantly impacts LFP performance, yet current assessment techniques struggle to isolate and quantify these individual contributions. Conventional electrochemical testing often provides aggregate performance data without revealing the underlying mechanisms or rate-limiting factors, creating a "black box" effect that impedes targeted material optimization.
Temperature sensitivity presents another substantial challenge. LFP materials exhibit markedly different performance characteristics across temperature ranges, particularly at low temperatures where performance degradation becomes pronounced. Current assessment methodologies frequently fail to adequately characterize this temperature-dependent behavior, leading to potential overestimation of real-world performance in variable climate conditions.
Long-term cycling stability evaluation remains problematic due to the time-intensive nature of such testing. Accelerated aging protocols, while time-efficient, often fail to accurately predict real-world degradation mechanisms and lifespans. This disconnect between laboratory assessment and practical application creates uncertainty in lifetime performance projections and warranty determinations for commercial products.
The industry also faces challenges in correlating laboratory-scale assessments with full-cell and pack-level performance. Scaling effects, including thermal management considerations and electrical interconnection resistances, can significantly alter performance metrics when transitioning from coin cells to commercial formats. Current assessment methodologies struggle to account for these scaling factors.
Advanced characterization techniques such as operando X-ray diffraction and neutron imaging offer valuable insights into LFP behavior during operation but remain largely confined to specialized research facilities due to their complexity and cost. This limited accessibility creates a divide between fundamental research capabilities and industrial application requirements.
Finally, the emergence of doped and modified LFP materials introduces additional assessment complexities. These novel compositions often exhibit unique electrochemical signatures that conventional testing protocols may not adequately capture, necessitating the development of tailored assessment methodologies to properly evaluate their performance advantages and potential limitations.
The complex interplay between particle morphology, size distribution, and carbon coating quality significantly impacts LFP performance, yet current assessment techniques struggle to isolate and quantify these individual contributions. Conventional electrochemical testing often provides aggregate performance data without revealing the underlying mechanisms or rate-limiting factors, creating a "black box" effect that impedes targeted material optimization.
Temperature sensitivity presents another substantial challenge. LFP materials exhibit markedly different performance characteristics across temperature ranges, particularly at low temperatures where performance degradation becomes pronounced. Current assessment methodologies frequently fail to adequately characterize this temperature-dependent behavior, leading to potential overestimation of real-world performance in variable climate conditions.
Long-term cycling stability evaluation remains problematic due to the time-intensive nature of such testing. Accelerated aging protocols, while time-efficient, often fail to accurately predict real-world degradation mechanisms and lifespans. This disconnect between laboratory assessment and practical application creates uncertainty in lifetime performance projections and warranty determinations for commercial products.
The industry also faces challenges in correlating laboratory-scale assessments with full-cell and pack-level performance. Scaling effects, including thermal management considerations and electrical interconnection resistances, can significantly alter performance metrics when transitioning from coin cells to commercial formats. Current assessment methodologies struggle to account for these scaling factors.
Advanced characterization techniques such as operando X-ray diffraction and neutron imaging offer valuable insights into LFP behavior during operation but remain largely confined to specialized research facilities due to their complexity and cost. This limited accessibility creates a divide between fundamental research capabilities and industrial application requirements.
Finally, the emergence of doped and modified LFP materials introduces additional assessment complexities. These novel compositions often exhibit unique electrochemical signatures that conventional testing protocols may not adequately capture, necessitating the development of tailored assessment methodologies to properly evaluate their performance advantages and potential limitations.
Standard Testing Protocols for LFP Batteries
01 Doping and surface modification of lithium phosphate materials
Doping lithium phosphate materials with various elements such as metal ions (e.g., Mg, Al, Ni, Co) can significantly enhance electrochemical performance by improving ionic conductivity and structural stability. Surface modification techniques, including coating with carbon materials or metal oxides, can reduce interfacial resistance and protect the active material from electrolyte degradation, resulting in improved cycling stability and rate capability of lithium phosphate-based electrodes.- Doping and modification of lithium phosphate materials: Various doping and modification strategies can enhance the electrochemical performance of lithium phosphate materials. Introducing metal ions or other elements into the crystal structure can improve conductivity and stability. Surface modifications with conductive coatings or functional groups can enhance electron transfer and ion diffusion. These approaches effectively address the inherent limitations of lithium phosphate materials, such as low electronic conductivity, resulting in improved capacity, rate capability, and cycling stability.
- Carbon coating and composite formation: Carbon coating is a widely used technique to enhance the electrochemical performance of lithium phosphate materials. By forming a conductive carbon layer on the surface of lithium phosphate particles, electron transport is significantly improved. Additionally, creating composites with carbon materials such as graphene, carbon nanotubes, or conductive polymers can further enhance conductivity and structural stability. These carbon-based strategies effectively address the poor electronic conductivity of lithium phosphate while maintaining its inherent advantages.
- Nanostructuring and morphology control: Controlling the size, shape, and morphology of lithium phosphate materials at the nanoscale can significantly improve their electrochemical performance. Nanostructured materials offer shorter lithium-ion diffusion paths, larger electrode-electrolyte contact areas, and better accommodation of volume changes during cycling. Various synthesis methods, including hydrothermal, sol-gel, and template-assisted approaches, can be employed to create nanoparticles, nanowires, nanoplates, or hierarchical structures with optimized performance characteristics.
- Electrolyte optimization and interface engineering: The electrolyte composition and electrode-electrolyte interface play crucial roles in the electrochemical performance of lithium phosphate batteries. Optimizing electrolyte formulations with appropriate solvents, lithium salts, and additives can enhance ionic conductivity and form stable solid electrolyte interphase (SEI) layers. Interface engineering approaches, such as surface coatings or functional electrolyte additives, can reduce unwanted side reactions, improve lithium-ion transport, and enhance the overall cycling stability and rate capability of lithium phosphate-based battery systems.
- Advanced synthesis methods and processing techniques: Novel synthesis methods and processing techniques can significantly improve the electrochemical performance of lithium phosphate materials. These include solution-based methods, solid-state reactions, microwave-assisted synthesis, and mechanochemical approaches. Post-synthesis treatments such as annealing under controlled atmospheres, high-pressure processing, or activation procedures can further optimize crystal structure, particle size distribution, and surface properties. These advanced methods enable precise control over material characteristics, resulting in enhanced capacity, rate capability, and cycling stability.
02 Nanostructured lithium phosphate composites
Developing nanostructured lithium phosphate materials, such as nanoparticles, nanowires, and nanoplates, can significantly enhance electrochemical performance by shortening lithium ion diffusion paths and increasing the electrode/electrolyte contact area. Composite structures combining lithium phosphate with conductive materials like graphene, carbon nanotubes, or conductive polymers can overcome the inherent low electronic conductivity of lithium phosphate, resulting in improved rate capability and capacity retention.Expand Specific Solutions03 Advanced synthesis methods for high-performance lithium phosphate
Novel synthesis methods including hydrothermal/solvothermal processes, sol-gel techniques, and microwave-assisted synthesis can produce lithium phosphate materials with controlled morphology, particle size, and crystallinity. These advanced preparation techniques can optimize the microstructure and physicochemical properties of lithium phosphate materials, leading to enhanced electrochemical performance including higher capacity, better rate capability, and improved cycling stability.Expand Specific Solutions04 Electrolyte optimization for lithium phosphate batteries
Tailoring electrolyte compositions specifically for lithium phosphate-based electrodes can significantly improve electrochemical performance. This includes developing novel electrolyte additives that form stable solid electrolyte interphase (SEI) layers, using ionic liquid-based electrolytes with wider electrochemical windows, and formulating high-concentration electrolytes that suppress unwanted side reactions. These electrolyte innovations can enhance the cycling stability, rate capability, and temperature performance of lithium phosphate battery systems.Expand Specific Solutions05 Multi-component lithium phosphate systems
Developing multi-component lithium phosphate systems, such as mixed-metal phosphates or phosphate-based composite cathodes, can provide synergistic effects that enhance electrochemical performance. These systems often combine the advantages of different materials, such as the high capacity of one component with the structural stability of another. Strategic combinations of lithium phosphate with other active materials can result in electrodes with improved energy density, power capability, and cycling life compared to single-component systems.Expand Specific Solutions
Leading Companies in LFP Battery Technology
The lithium phosphate electrochemical performance assessment landscape is currently in a growth phase, with the market expanding rapidly due to increasing demand for sustainable energy storage solutions. Major players include established automotive manufacturers (Tesla, Renault, Nissan, BMW) investing heavily in battery technology, specialized battery producers (LG Energy Solution, LG Chem, Saft Groupe), and emerging Chinese companies (Guoxuan High-Tech, Jiangsu Haiji). The technology shows varying maturity levels, with companies like Tesla and LG Energy Solution leading commercial applications while academic institutions (Tsinghua University, Nanyang Technological University) focus on fundamental research. Recycling specialists like Guangdong Bangpu are developing complementary technologies to address full lifecycle performance, indicating the industry's progression toward more sustainable and efficient energy storage solutions.
Tsinghua University
Technical Solution: Tsinghua University has pioneered advanced methodologies for assessing lithium phosphate electrochemical performance through a combination of experimental techniques and theoretical modeling. Their approach employs synchrotron-based X-ray absorption spectroscopy (XAS) and in-situ X-ray diffraction (XRD) to monitor structural changes in LFP cathodes during cycling at the atomic level. Tsinghua researchers have developed a multi-scale characterization framework that links nano-scale phenomena to macroscopic performance metrics[1]. Their assessment protocol includes electrochemical quartz crystal microbalance (EQCM) measurements to quantify mass changes during lithiation/delithiation processes with nanogram sensitivity. For practical performance evaluation, they utilize galvanostatic intermittent titration technique (GITT) and potentiostatic intermittent titration technique (PITT) to determine lithium diffusion coefficients within LFP particles[3]. Tsinghua's approach also incorporates density functional theory (DFT) calculations to predict and interpret experimental results, creating a feedback loop between theoretical predictions and experimental validation[5]. Their facilities include custom-built testing equipment capable of operando neutron diffraction for non-destructive analysis of lithium transport mechanisms.
Strengths: Tsinghua's methodology provides exceptional fundamental insights into LFP performance mechanisms, with particular strength in correlating atomic/molecular level phenomena with cell-level performance. Their integration of computational modeling with experimental techniques accelerates materials optimization. Weaknesses: Their highly academic approach sometimes emphasizes fundamental understanding over practical application metrics, potentially limiting direct industrial relevance without translation work.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has established a multi-tiered assessment framework for lithium phosphate electrochemical performance evaluation. Their approach begins with material-level characterization using X-ray diffraction (XRD) and scanning electron microscopy (SEM) to analyze crystal structure, particle morphology, and surface properties of LFP cathodes. For electrochemical assessment, they employ galvanostatic charge-discharge testing at various C-rates to determine rate capability, capacity retention, and voltage profiles. LG's proprietary three-electrode cell configuration allows isolation of cathode performance from full cell behavior[2]. Their assessment protocol includes accelerated aging tests under elevated temperatures (45-60°C) and high current densities to predict long-term performance. LG has developed specialized electrolyte formulations to enhance LFP performance at low temperatures, with assessment protocols specifically designed to evaluate cold-weather operation down to -30°C[4]. Their facilities include high-precision calorimetry to measure heat generation during cycling, critical for thermal management system design.
Strengths: LG's assessment methodology excels in translating laboratory results to mass production, with robust quality control processes that ensure consistency across large-scale manufacturing. Their three-electrode testing provides detailed insights into cathode-specific performance limitations. Weaknesses: Their assessment approach tends to be more conservative in pushing performance boundaries compared to some competitors, potentially missing opportunities for breakthrough innovations in LFP technology.
Key Research Advances in LFP Characterization
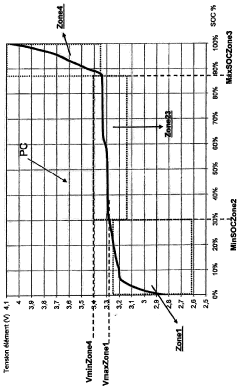
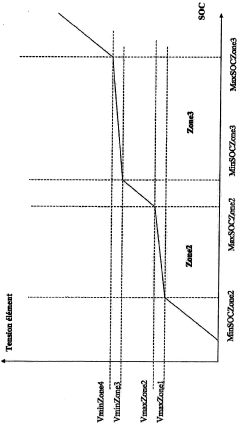
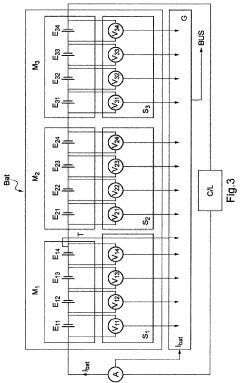
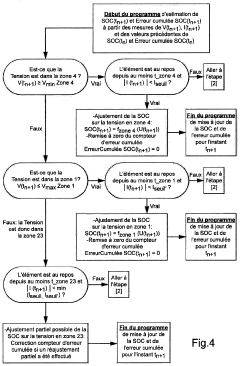
Method and system for estimating the charge level of a lithium electrochemical element including a lithium phosphate positive electrode
PatentActiveFR2987703A1
Innovation
- A method for estimating SOC using a charge profile optimization based on voltage and charge limits, combined with coulometry and current measurement, to adjust and correct for errors in current sensor measurements, ensuring precise SOC determination across various charge zones.
Sustainability Impact of LFP Battery Technology
The environmental and sustainability implications of Lithium Iron Phosphate (LFP) battery technology represent a critical dimension in evaluating its overall value proposition. LFP batteries demonstrate significant sustainability advantages compared to other lithium-ion chemistries, particularly in terms of resource utilization. The phosphate-based cathode eliminates the need for cobalt and nickel, materials associated with ethical mining concerns and supply chain vulnerabilities in conventional lithium-ion batteries.
From a life-cycle assessment perspective, LFP batteries exhibit a lower carbon footprint during manufacturing compared to nickel-manganese-cobalt (NMC) or lithium-cobalt oxide (LCO) alternatives. This reduced environmental impact stems from both the materials used and the less energy-intensive production processes. Studies indicate that LFP battery production generates approximately 30% fewer greenhouse gas emissions compared to equivalent NMC batteries, representing a substantial improvement in environmental performance.
The extended cycle life of LFP batteries further enhances their sustainability profile. With the capability to withstand 2,000-4,000 charge-discharge cycles without significant degradation, LFP technology effectively reduces the frequency of battery replacement and associated waste generation. This longevity translates to reduced material consumption and waste management challenges over the battery's operational lifetime.
End-of-life considerations also favor LFP chemistry. The absence of cobalt and nickel simplifies recycling processes and reduces the hazardous waste potential. Current recycling technologies can recover up to 95% of the phosphate compounds from spent LFP batteries, enabling more efficient material recovery and circular economy implementation. Additionally, LFP batteries pose reduced fire and thermal runaway risks, minimizing potential environmental contamination incidents during use or disposal.
Water usage represents another important sustainability metric where LFP technology demonstrates advantages. Manufacturing processes for LFP cathode materials typically require 25-40% less water compared to cobalt-based alternatives, contributing to reduced pressure on water resources in production regions. This aspect becomes increasingly significant as battery production scales to meet growing global demand.
The sustainability benefits of LFP technology extend to grid-scale energy storage applications, where their safety characteristics and long cycle life enable more efficient integration of renewable energy sources. By facilitating greater renewable energy penetration, LFP batteries indirectly contribute to broader decarbonization efforts beyond their direct manufacturing impacts.
From a life-cycle assessment perspective, LFP batteries exhibit a lower carbon footprint during manufacturing compared to nickel-manganese-cobalt (NMC) or lithium-cobalt oxide (LCO) alternatives. This reduced environmental impact stems from both the materials used and the less energy-intensive production processes. Studies indicate that LFP battery production generates approximately 30% fewer greenhouse gas emissions compared to equivalent NMC batteries, representing a substantial improvement in environmental performance.
The extended cycle life of LFP batteries further enhances their sustainability profile. With the capability to withstand 2,000-4,000 charge-discharge cycles without significant degradation, LFP technology effectively reduces the frequency of battery replacement and associated waste generation. This longevity translates to reduced material consumption and waste management challenges over the battery's operational lifetime.
End-of-life considerations also favor LFP chemistry. The absence of cobalt and nickel simplifies recycling processes and reduces the hazardous waste potential. Current recycling technologies can recover up to 95% of the phosphate compounds from spent LFP batteries, enabling more efficient material recovery and circular economy implementation. Additionally, LFP batteries pose reduced fire and thermal runaway risks, minimizing potential environmental contamination incidents during use or disposal.
Water usage represents another important sustainability metric where LFP technology demonstrates advantages. Manufacturing processes for LFP cathode materials typically require 25-40% less water compared to cobalt-based alternatives, contributing to reduced pressure on water resources in production regions. This aspect becomes increasingly significant as battery production scales to meet growing global demand.
The sustainability benefits of LFP technology extend to grid-scale energy storage applications, where their safety characteristics and long cycle life enable more efficient integration of renewable energy sources. By facilitating greater renewable energy penetration, LFP batteries indirectly contribute to broader decarbonization efforts beyond their direct manufacturing impacts.
Safety Standards and Compliance Requirements
The assessment of lithium phosphate electrochemical performance must adhere to stringent safety standards and compliance requirements established by international and regional regulatory bodies. These standards are critical for ensuring the safety, reliability, and market acceptance of lithium phosphate batteries across various applications.
IEC 62660 series serves as the cornerstone for performance and safety testing of lithium-ion cells for electric vehicle applications, providing standardized methods for evaluating electrochemical performance characteristics. Complementing this, UL 1642 outlines safety requirements for lithium batteries in consumer electronics, while UN 38.3 establishes mandatory transport testing protocols that lithium phosphate batteries must pass before shipping.
For stationary energy storage applications, IEC 62619 provides essential safety requirements, with specific provisions for lithium phosphate chemistry. The European Union's Battery Directive 2006/66/EC and its amendments impose additional requirements regarding battery labeling, collection, and recycling, directly impacting how lithium phosphate batteries are designed and managed throughout their lifecycle.
In the United States, NFPA 855 addresses the installation of stationary energy storage systems, with specific provisions for lithium-based technologies including phosphate chemistries. This standard is particularly relevant for grid-scale applications where thermal runaway concerns must be addressed. Similarly, IEEE 1625 and 1725 provide frameworks for rechargeable batteries in portable computing and cellular telephone applications respectively.
Laboratory assessment protocols must incorporate safety measures outlined in OSHA standards for handling potentially hazardous materials. These include proper ventilation requirements, personal protective equipment specifications, and emergency response procedures specific to lithium phosphate chemistry. Compliance with these standards is mandatory for research facilities conducting electrochemical performance assessments.
Environmental compliance requirements are increasingly stringent, with RoHS and REACH regulations limiting the use of hazardous substances in battery manufacturing. These regulations impact the assessment methodology by necessitating analysis of trace contaminants and leachable substances that might be present in lithium phosphate electrodes.
Emerging standards from organizations like ASTM International and SAE are developing more specific testing protocols for next-generation lithium phosphate formulations, focusing on cycle life assessment under various temperature conditions and fast-charging capabilities. These evolving standards will shape future assessment methodologies and acceptance criteria for advanced lithium phosphate technologies.
Compliance certification processes typically require third-party validation from accredited laboratories, adding an additional layer of quality assurance to electrochemical performance assessments. This independent verification is essential for market acceptance and regulatory approval across global markets.
IEC 62660 series serves as the cornerstone for performance and safety testing of lithium-ion cells for electric vehicle applications, providing standardized methods for evaluating electrochemical performance characteristics. Complementing this, UL 1642 outlines safety requirements for lithium batteries in consumer electronics, while UN 38.3 establishes mandatory transport testing protocols that lithium phosphate batteries must pass before shipping.
For stationary energy storage applications, IEC 62619 provides essential safety requirements, with specific provisions for lithium phosphate chemistry. The European Union's Battery Directive 2006/66/EC and its amendments impose additional requirements regarding battery labeling, collection, and recycling, directly impacting how lithium phosphate batteries are designed and managed throughout their lifecycle.
In the United States, NFPA 855 addresses the installation of stationary energy storage systems, with specific provisions for lithium-based technologies including phosphate chemistries. This standard is particularly relevant for grid-scale applications where thermal runaway concerns must be addressed. Similarly, IEEE 1625 and 1725 provide frameworks for rechargeable batteries in portable computing and cellular telephone applications respectively.
Laboratory assessment protocols must incorporate safety measures outlined in OSHA standards for handling potentially hazardous materials. These include proper ventilation requirements, personal protective equipment specifications, and emergency response procedures specific to lithium phosphate chemistry. Compliance with these standards is mandatory for research facilities conducting electrochemical performance assessments.
Environmental compliance requirements are increasingly stringent, with RoHS and REACH regulations limiting the use of hazardous substances in battery manufacturing. These regulations impact the assessment methodology by necessitating analysis of trace contaminants and leachable substances that might be present in lithium phosphate electrodes.
Emerging standards from organizations like ASTM International and SAE are developing more specific testing protocols for next-generation lithium phosphate formulations, focusing on cycle life assessment under various temperature conditions and fast-charging capabilities. These evolving standards will shape future assessment methodologies and acceptance criteria for advanced lithium phosphate technologies.
Compliance certification processes typically require third-party validation from accredited laboratories, adding an additional layer of quality assurance to electrochemical performance assessments. This independent verification is essential for market acceptance and regulatory approval across global markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!