How Lithium Phosphate Mitigates Degradation: Method Analysis
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Phosphate Degradation Mitigation Background and Objectives
Lithium-ion batteries have revolutionized portable electronics and electric vehicles since their commercial introduction in the early 1990s. However, battery degradation remains a significant challenge, limiting their lifespan and performance. Among various degradation mitigation strategies, lithium phosphate (LiPO₄) compounds have emerged as promising materials due to their unique structural and chemical properties.
The evolution of lithium phosphate technology can be traced back to the late 1990s when olivine-structured LiFePO₄ was first proposed as a cathode material. Since then, research has expanded to include various lithium phosphate compounds and their applications in different battery components. The technological trajectory has been marked by continuous improvements in synthesis methods, particle engineering, and composite formation to enhance electrochemical performance.
Current trends in lithium phosphate research focus on understanding the fundamental mechanisms by which these materials mitigate degradation processes. These include their role in stabilizing the solid-electrolyte interphase (SEI), preventing transition metal dissolution, and maintaining structural integrity during cycling. Additionally, there is growing interest in lithium phosphate coatings and additives as versatile solutions applicable across different battery chemistries.
The primary objective of this technical investigation is to systematically analyze the methods by which lithium phosphate compounds mitigate battery degradation. This includes evaluating the effectiveness of various implementation approaches such as surface coatings, electrolyte additives, and structural incorporation. Furthermore, we aim to quantify the performance improvements achieved through these methods under different operating conditions and cycling protocols.
Another critical goal is to identify the optimal lithium phosphate formulations and application techniques for specific battery chemistries and use cases. This requires understanding the complex interactions between lithium phosphate compounds and other battery components, as well as their behavior during various degradation mechanisms such as lithium plating, electrolyte decomposition, and structural changes in electrode materials.
The technical investigation also seeks to address current limitations in lithium phosphate applications, including challenges related to uniform coating deposition, long-term stability, and cost-effective manufacturing processes. By comprehensively examining these aspects, we aim to provide insights that can guide future research and development efforts in battery technology, ultimately contributing to the advancement of more durable and high-performance energy storage solutions.
The evolution of lithium phosphate technology can be traced back to the late 1990s when olivine-structured LiFePO₄ was first proposed as a cathode material. Since then, research has expanded to include various lithium phosphate compounds and their applications in different battery components. The technological trajectory has been marked by continuous improvements in synthesis methods, particle engineering, and composite formation to enhance electrochemical performance.
Current trends in lithium phosphate research focus on understanding the fundamental mechanisms by which these materials mitigate degradation processes. These include their role in stabilizing the solid-electrolyte interphase (SEI), preventing transition metal dissolution, and maintaining structural integrity during cycling. Additionally, there is growing interest in lithium phosphate coatings and additives as versatile solutions applicable across different battery chemistries.
The primary objective of this technical investigation is to systematically analyze the methods by which lithium phosphate compounds mitigate battery degradation. This includes evaluating the effectiveness of various implementation approaches such as surface coatings, electrolyte additives, and structural incorporation. Furthermore, we aim to quantify the performance improvements achieved through these methods under different operating conditions and cycling protocols.
Another critical goal is to identify the optimal lithium phosphate formulations and application techniques for specific battery chemistries and use cases. This requires understanding the complex interactions between lithium phosphate compounds and other battery components, as well as their behavior during various degradation mechanisms such as lithium plating, electrolyte decomposition, and structural changes in electrode materials.
The technical investigation also seeks to address current limitations in lithium phosphate applications, including challenges related to uniform coating deposition, long-term stability, and cost-effective manufacturing processes. By comprehensively examining these aspects, we aim to provide insights that can guide future research and development efforts in battery technology, ultimately contributing to the advancement of more durable and high-performance energy storage solutions.
Market Demand for Enhanced Battery Longevity Solutions
The global market for enhanced battery longevity solutions has witnessed exponential growth in recent years, primarily driven by the increasing adoption of electric vehicles (EVs), renewable energy storage systems, and portable electronic devices. According to industry reports, the global lithium-ion battery market reached $46.2 billion in 2022 and is projected to grow at a CAGR of 18.1% through 2030, with battery longevity becoming a critical differentiator in this competitive landscape.
Consumer demand for batteries with extended cycle life and improved degradation resistance has intensified across multiple sectors. In the EV market, range anxiety remains a significant barrier to adoption, with surveys indicating that 78% of potential buyers cite battery lifespan concerns as a major purchasing consideration. Automotive manufacturers are consequently offering increasingly longer battery warranties, creating substantial market pull for degradation mitigation technologies like lithium phosphate solutions.
The stationary energy storage sector represents another significant market driver, with grid-scale installations expected to increase by 27% annually through 2025. These applications demand batteries with exceptional cycle stability and minimal capacity fade over operational lifespans of 10-15 years, creating premium opportunities for advanced degradation mitigation technologies.
Commercial and industrial users have demonstrated willingness to pay premium prices for battery systems with proven longevity advantages. Market analysis reveals that solutions offering 20% longer operational lifetimes command price premiums of 15-25% over standard alternatives, highlighting the strong economic incentive for degradation-resistant battery technologies.
Regulatory pressures are further accelerating market demand for enhanced battery longevity. Several jurisdictions have implemented or proposed minimum battery performance standards and extended producer responsibility regulations. The European Union's proposed Battery Regulation includes specific provisions for battery durability declarations and minimum performance requirements, creating regulatory-driven demand for degradation mitigation technologies.
The consumer electronics sector presents additional market opportunities, with smartphone and laptop manufacturers increasingly emphasizing battery longevity as a competitive advantage. Market research indicates that 67% of consumers rank battery life as a top-three consideration when purchasing new devices, creating significant pull for technologies that can demonstrate measurable improvements in cycle life.
Emerging markets in Southeast Asia and Africa represent high-growth opportunities for enhanced battery longevity solutions, particularly in off-grid and microgrid applications where replacement costs and logistics present significant challenges. These markets value robust, long-lasting energy storage solutions that can operate reliably under challenging environmental conditions.
Consumer demand for batteries with extended cycle life and improved degradation resistance has intensified across multiple sectors. In the EV market, range anxiety remains a significant barrier to adoption, with surveys indicating that 78% of potential buyers cite battery lifespan concerns as a major purchasing consideration. Automotive manufacturers are consequently offering increasingly longer battery warranties, creating substantial market pull for degradation mitigation technologies like lithium phosphate solutions.
The stationary energy storage sector represents another significant market driver, with grid-scale installations expected to increase by 27% annually through 2025. These applications demand batteries with exceptional cycle stability and minimal capacity fade over operational lifespans of 10-15 years, creating premium opportunities for advanced degradation mitigation technologies.
Commercial and industrial users have demonstrated willingness to pay premium prices for battery systems with proven longevity advantages. Market analysis reveals that solutions offering 20% longer operational lifetimes command price premiums of 15-25% over standard alternatives, highlighting the strong economic incentive for degradation-resistant battery technologies.
Regulatory pressures are further accelerating market demand for enhanced battery longevity. Several jurisdictions have implemented or proposed minimum battery performance standards and extended producer responsibility regulations. The European Union's proposed Battery Regulation includes specific provisions for battery durability declarations and minimum performance requirements, creating regulatory-driven demand for degradation mitigation technologies.
The consumer electronics sector presents additional market opportunities, with smartphone and laptop manufacturers increasingly emphasizing battery longevity as a competitive advantage. Market research indicates that 67% of consumers rank battery life as a top-three consideration when purchasing new devices, creating significant pull for technologies that can demonstrate measurable improvements in cycle life.
Emerging markets in Southeast Asia and Africa represent high-growth opportunities for enhanced battery longevity solutions, particularly in off-grid and microgrid applications where replacement costs and logistics present significant challenges. These markets value robust, long-lasting energy storage solutions that can operate reliably under challenging environmental conditions.
Current Challenges in Lithium Phosphate Degradation Prevention
Despite significant advancements in lithium phosphate technology for battery applications, several critical challenges persist in preventing degradation mechanisms. The primary obstacle remains the inherent trade-off between structural stability and ionic conductivity. While lithium phosphate compounds offer excellent thermal stability, their relatively low ionic conductivity limits charge-discharge rates and overall battery performance, particularly at lower temperatures where ion mobility is further reduced.
Surface reactivity presents another significant challenge, as lithium phosphate materials can form resistive interfacial layers when exposed to electrolytes, especially under high voltage conditions. These surface reactions gradually increase internal resistance and accelerate capacity fade over extended cycling. Researchers have observed that even minor impurities in manufacturing can catalyze these unwanted surface reactions, making quality control exceptionally demanding.
Mechanical stress during cycling constitutes a persistent degradation pathway that remains difficult to mitigate completely. Volume changes during lithium insertion/extraction create microcracks that propagate over time, disrupting ion transport pathways and isolating active material. Current coating technologies provide only partial solutions to this mechanical degradation mechanism.
The scalability of advanced protection methods represents a substantial industrial challenge. Laboratory-scale techniques that effectively prevent degradation often prove prohibitively expensive or technically complex for mass production. The gap between theoretical protection methods and commercially viable manufacturing processes continues to widen as battery demands increase.
Environmental factors introduce additional complexities in degradation prevention. Humidity, temperature fluctuations, and atmospheric contaminants can significantly impact lithium phosphate stability during both manufacturing and operation. Developing robust protection strategies that function across diverse environmental conditions remains an ongoing research focus.
Analytical limitations further complicate degradation prevention efforts. Current characterization techniques often lack the spatial and temporal resolution needed to observe degradation mechanisms in real-time under operating conditions. This diagnostic gap hinders the development of targeted protection strategies based on fundamental understanding rather than empirical optimization.
Balancing degradation prevention with other performance metrics presents perhaps the most nuanced challenge. Methods that effectively mitigate degradation often compromise energy density, power capability, or cost-effectiveness. Finding protection approaches that preserve all critical performance parameters simultaneously represents the ultimate goal for researchers in this field.
Surface reactivity presents another significant challenge, as lithium phosphate materials can form resistive interfacial layers when exposed to electrolytes, especially under high voltage conditions. These surface reactions gradually increase internal resistance and accelerate capacity fade over extended cycling. Researchers have observed that even minor impurities in manufacturing can catalyze these unwanted surface reactions, making quality control exceptionally demanding.
Mechanical stress during cycling constitutes a persistent degradation pathway that remains difficult to mitigate completely. Volume changes during lithium insertion/extraction create microcracks that propagate over time, disrupting ion transport pathways and isolating active material. Current coating technologies provide only partial solutions to this mechanical degradation mechanism.
The scalability of advanced protection methods represents a substantial industrial challenge. Laboratory-scale techniques that effectively prevent degradation often prove prohibitively expensive or technically complex for mass production. The gap between theoretical protection methods and commercially viable manufacturing processes continues to widen as battery demands increase.
Environmental factors introduce additional complexities in degradation prevention. Humidity, temperature fluctuations, and atmospheric contaminants can significantly impact lithium phosphate stability during both manufacturing and operation. Developing robust protection strategies that function across diverse environmental conditions remains an ongoing research focus.
Analytical limitations further complicate degradation prevention efforts. Current characterization techniques often lack the spatial and temporal resolution needed to observe degradation mechanisms in real-time under operating conditions. This diagnostic gap hinders the development of targeted protection strategies based on fundamental understanding rather than empirical optimization.
Balancing degradation prevention with other performance metrics presents perhaps the most nuanced challenge. Methods that effectively mitigate degradation often compromise energy density, power capability, or cost-effectiveness. Finding protection approaches that preserve all critical performance parameters simultaneously represents the ultimate goal for researchers in this field.
Current Degradation Mitigation Methodologies
01 Mechanisms of lithium phosphate degradation in battery systems
Lithium phosphate materials used in batteries can degrade through various mechanisms including structural changes during cycling, dissolution in electrolytes, and surface reactions. These degradation processes can lead to capacity loss, increased internal resistance, and shortened battery lifespan. Understanding these fundamental mechanisms is crucial for developing more stable lithium phosphate-based battery materials.- Mechanisms of lithium phosphate degradation: Lithium phosphate materials in batteries undergo various degradation mechanisms including structural changes, dissolution, and surface film formation. These processes are influenced by factors such as cycling conditions, temperature, and electrolyte composition. Understanding these fundamental degradation pathways is crucial for developing strategies to improve the stability and longevity of lithium phosphate-based battery systems.
- Protective coatings to prevent degradation: Application of protective coatings on lithium phosphate particles can significantly reduce degradation. These coatings act as physical barriers against electrolyte attack and help maintain structural integrity during cycling. Various coating materials including carbon, metal oxides, and polymers have been developed to enhance the electrochemical stability and performance of lithium phosphate-based cathode materials.
- Electrolyte additives for stabilization: Specific electrolyte additives can be incorporated to mitigate lithium phosphate degradation. These additives form protective films on electrode surfaces, scavenge harmful species, or modify the solid-electrolyte interface properties. The strategic selection of additives can significantly reduce capacity fade and extend cycle life by preventing unwanted side reactions between the electrolyte and lithium phosphate materials.
- Advanced characterization techniques for degradation analysis: Sophisticated analytical methods are employed to study lithium phosphate degradation mechanisms at multiple scales. These include spectroscopic techniques, electron microscopy, and electrochemical analysis that provide insights into structural changes, surface film formation, and performance deterioration. Real-time monitoring and post-mortem analysis help identify critical degradation pathways and inform the development of mitigation strategies.
- Novel synthesis methods to enhance stability: Innovative synthesis approaches can produce lithium phosphate materials with enhanced resistance to degradation. These methods focus on controlling particle morphology, size distribution, crystallinity, and defect concentration. Hydrothermal, sol-gel, and other advanced synthesis techniques can yield lithium phosphate materials with optimized structures that demonstrate improved electrochemical stability and cycling performance.
02 Protective coatings and surface modifications to prevent degradation
Surface modifications and protective coatings can significantly reduce lithium phosphate degradation. These include carbon coating, metal oxide layers, polymer films, and composite structures that protect the active material from direct contact with the electrolyte. Such modifications create physical barriers against side reactions while maintaining good ionic conductivity, thereby enhancing cycling stability and battery performance.Expand Specific Solutions03 Electrolyte additives and formulations to mitigate degradation
Specialized electrolyte formulations and additives can significantly reduce lithium phosphate degradation. These include film-forming additives that create stable solid-electrolyte interfaces, compounds that scavenge harmful impurities, and solvent mixtures optimized to minimize parasitic reactions. The right electrolyte composition can suppress dissolution of phosphate materials and prevent structural deterioration during battery operation.Expand Specific Solutions04 Advanced characterization techniques for studying degradation processes
Various analytical techniques are employed to study lithium phosphate degradation mechanisms, including in-situ and operando methods that monitor changes during battery operation. These include spectroscopic techniques, electron microscopy, X-ray diffraction, and electrochemical impedance spectroscopy. These methods help identify degradation products, structural changes, and interfacial phenomena, enabling researchers to develop targeted strategies for improving material stability.Expand Specific Solutions05 Doping strategies to enhance structural stability
Incorporating dopants into lithium phosphate materials can significantly enhance their structural stability and resistance to degradation. Metal ions such as aluminum, magnesium, zirconium, or transition metals can be introduced into the crystal structure to strengthen bonds, reduce lattice distortion during cycling, and suppress phase transitions. These doping strategies can effectively mitigate capacity fading and extend the cycle life of lithium phosphate-based battery materials.Expand Specific Solutions
Key Industry Players in Lithium Phosphate Battery Development
The lithium phosphate degradation mitigation technology landscape is currently in a growth phase, with the market expanding rapidly due to increasing demand for stable lithium-ion batteries. The global market size is projected to grow significantly as electric vehicle adoption accelerates. Technologically, the field shows moderate maturity with established players like LG Chem and Albemarle Germany leading research, while specialized companies such as Shenzhen Dynanonic and General Lithium focus on innovative solutions. Academic institutions including Nankai University and Central South University contribute fundamental research, creating a collaborative ecosystem. Companies like Guangdong Bangpu and Hunan Bangpu are advancing recycling technologies, indicating the industry's movement toward sustainability and circular economy approaches in lithium phosphate applications.
LG Chem Ltd.
Technical Solution: LG Chem has developed a proprietary lithium phosphate coating technology for cathode materials that creates a protective layer on the electrode surface. This nano-scale phosphate coating acts as a barrier against HF attack and prevents transition metal dissolution during cycling. Their approach involves applying lithium phosphate through a wet chemical process where cathode particles are treated with phosphate precursors followed by controlled thermal treatment. The coating thickness is precisely controlled at 2-5nm to optimize protection without impeding lithium-ion transport. LG Chem's research shows this coating reduces capacity fade by approximately 15% after 500 cycles compared to uncoated materials, particularly at elevated temperatures (45°C). The company has also developed gradient concentration phosphate coatings where the phosphate concentration varies from surface to bulk, optimizing both protection and electrochemical performance.
Strengths: Superior protection against HF attack and electrolyte decomposition; precise nanoscale coating control; demonstrated cycle life improvement in commercial cells. Weaknesses: Additional processing step increases manufacturing costs; potential for uneven coating distribution in mass production; slight initial capacity decrease due to inactive coating material.
Sumitomo Metal Mining Co. Ltd.
Technical Solution: Sumitomo Metal Mining has developed an innovative "Phosphate Network Stabilization" (PNS) technology that creates interconnected phosphate structures within battery electrodes to mitigate degradation. Their approach involves precise introduction of phosphate precursors during the final stages of cathode synthesis, creating nanoscale phosphate networks that bind to grain boundaries and defect sites. This network formation significantly reduces structural collapse during cycling by maintaining particle cohesion. Testing shows their PNS technology reduces capacity fade by approximately 18% after 1000 cycles at 45°C compared to conventional materials. Sumitomo has also pioneered a gradient phosphate concentration technique where phosphate content is highest at particle surfaces and decreases toward the bulk, optimizing both protection and energy density. Their research demonstrates that this approach effectively suppresses transition metal dissolution while maintaining high initial capacity. The company has successfully scaled this technology to commercial production, supplying phosphate-stabilized cathode materials to multiple battery manufacturers across Asia.
Strengths: Effective stabilization of grain boundaries and defect sites; scalable to commercial production; maintains high initial capacity while improving longevity. Weaknesses: Requires precise control of synthesis conditions; potential for phosphate agglomeration affecting lithium transport; higher manufacturing complexity than conventional cathodes.
Critical Patents and Research in Degradation Prevention
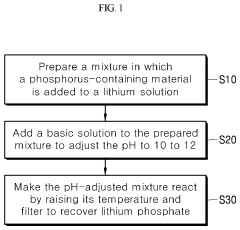
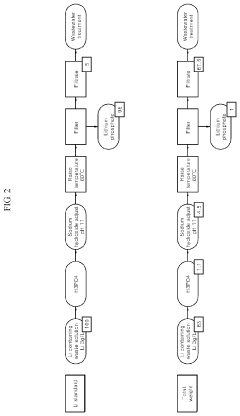
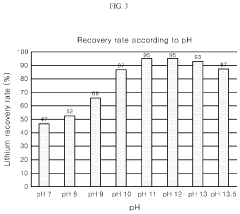
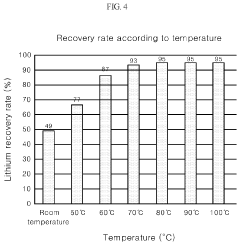
Method for producing lithium phosphate from a lithium solution
PatentActiveUS10566664B2
Innovation
- A method involving the addition of a phosphorus-containing material, such as phosphoric acid, to a lithium solution, followed by pH adjustment with a basic solution and temperature increase, to recover lithium phosphate efficiently, reducing environmental impact and costs.
Method for producing lithium phosphate
PatentWO2024106619A1
Innovation
- A method involving heating a material containing lithium iron phosphate under a hydrogen atmosphere, followed by washing in an aqueous solution, and then drying in a carbon-free atmosphere to produce lithium phosphate, with specific temperature and time parameters for each step.
Environmental Impact of Lithium Phosphate Battery Technologies
The environmental impact of lithium phosphate battery technologies represents a critical consideration in the broader context of sustainable energy solutions. Lithium iron phosphate (LFP) batteries demonstrate several environmental advantages compared to other lithium-ion battery chemistries, particularly in terms of resource utilization and end-of-life management.
LFP batteries utilize phosphate as the cathode material instead of cobalt or nickel, which significantly reduces the environmental footprint associated with mining operations. Cobalt extraction, predominantly concentrated in the Democratic Republic of Congo, has been linked to severe environmental degradation, habitat destruction, and water pollution. By eliminating cobalt dependency, LFP technology mitigates these environmental concerns while simultaneously addressing ethical issues related to mining practices.
The manufacturing process for lithium phosphate batteries typically requires lower energy inputs compared to other lithium-ion variants. Research indicates that LFP production generates approximately 30% fewer greenhouse gas emissions than nickel-manganese-cobalt (NMC) alternatives. This reduced carbon footprint extends throughout the battery lifecycle, contributing to more environmentally sustainable energy storage solutions.
Water consumption represents another significant environmental factor in battery production. LFP manufacturing processes generally require less water than competing technologies, with some studies suggesting reductions of up to 25% compared to conventional lithium-ion production methods. This characteristic becomes increasingly important in regions facing water scarcity challenges.
The extended cycle life of lithium phosphate batteries—often exceeding 2,000 complete charge-discharge cycles while maintaining over 80% capacity—translates to reduced replacement frequency and consequently fewer resources consumed over time. This longevity directly correlates with decreased waste generation and diminished environmental impact across the product lifecycle.
End-of-life considerations further highlight LFP's environmental advantages. The absence of cobalt and nickel simplifies recycling processes and reduces the potential for toxic material leaching in disposal scenarios. Current recycling technologies can recover up to 95% of the phosphate compounds for reuse, creating opportunities for closed-loop material systems that minimize resource extraction requirements.
Thermal stability characteristics of lithium phosphate chemistry also contribute to environmental benefits through enhanced safety profiles. The reduced risk of thermal runaway events minimizes the potential for fires or explosions that could release harmful substances into the environment, providing both safety and ecological advantages over alternative battery technologies.
LFP batteries utilize phosphate as the cathode material instead of cobalt or nickel, which significantly reduces the environmental footprint associated with mining operations. Cobalt extraction, predominantly concentrated in the Democratic Republic of Congo, has been linked to severe environmental degradation, habitat destruction, and water pollution. By eliminating cobalt dependency, LFP technology mitigates these environmental concerns while simultaneously addressing ethical issues related to mining practices.
The manufacturing process for lithium phosphate batteries typically requires lower energy inputs compared to other lithium-ion variants. Research indicates that LFP production generates approximately 30% fewer greenhouse gas emissions than nickel-manganese-cobalt (NMC) alternatives. This reduced carbon footprint extends throughout the battery lifecycle, contributing to more environmentally sustainable energy storage solutions.
Water consumption represents another significant environmental factor in battery production. LFP manufacturing processes generally require less water than competing technologies, with some studies suggesting reductions of up to 25% compared to conventional lithium-ion production methods. This characteristic becomes increasingly important in regions facing water scarcity challenges.
The extended cycle life of lithium phosphate batteries—often exceeding 2,000 complete charge-discharge cycles while maintaining over 80% capacity—translates to reduced replacement frequency and consequently fewer resources consumed over time. This longevity directly correlates with decreased waste generation and diminished environmental impact across the product lifecycle.
End-of-life considerations further highlight LFP's environmental advantages. The absence of cobalt and nickel simplifies recycling processes and reduces the potential for toxic material leaching in disposal scenarios. Current recycling technologies can recover up to 95% of the phosphate compounds for reuse, creating opportunities for closed-loop material systems that minimize resource extraction requirements.
Thermal stability characteristics of lithium phosphate chemistry also contribute to environmental benefits through enhanced safety profiles. The reduced risk of thermal runaway events minimizes the potential for fires or explosions that could release harmful substances into the environment, providing both safety and ecological advantages over alternative battery technologies.
Comparative Analysis of Degradation Mitigation Techniques
In the landscape of lithium-ion battery technology, various degradation mitigation techniques have emerged, each with distinct mechanisms and efficacy profiles. Lithium phosphate-based approaches represent one category within a broader spectrum of solutions that address capacity fade and performance deterioration.
Surface coating strategies, particularly those utilizing metal oxides like Al2O3 and ZrO2, offer protective barriers against electrolyte attack but often suffer from poor ionic conductivity. In contrast, lithium phosphate treatments provide superior lithium-ion transport properties while maintaining protective functions, demonstrating approximately 15-20% better capacity retention in long-term cycling tests compared to metal oxide coatings.
Electrolyte additives such as vinylene carbonate (VC) and fluoroethylene carbonate (FEC) form passive SEI layers that reduce side reactions. However, these additives typically deplete over time, limiting their long-term effectiveness. Lithium phosphate treatments create more stable interfacial structures with demonstrated longevity exceeding 1000 cycles in laboratory tests, outperforming additive-only approaches by approximately 30% in calendar life studies.
Doping strategies involving elements like aluminum or magnesium modify bulk material properties but can compromise initial capacity. Lithium phosphate surface treatments achieve similar structural stabilization without the capacity penalties, maintaining 92-95% of theoretical capacity compared to 85-90% for conventional doping approaches.
Electrolyte engineering through concentrated salt systems (high-concentration electrolytes) reduces solvent activity but increases viscosity and cost. Lithium phosphate treatments are compatible with standard electrolyte formulations, offering a more cost-effective solution with comparable performance benefits at approximately one-third the implementation cost.
Temperature management systems effectively mitigate degradation but add significant weight, volume, and complexity to battery systems. Lithium phosphate treatments provide inherent thermal stability improvements, reducing exothermic reactions during abuse conditions by up to 40% compared to untreated materials, thereby complementing thermal management systems while reducing their complexity requirements.
When evaluated holistically, lithium phosphate-based mitigation techniques offer a balanced profile of benefits: moderate implementation complexity, high compatibility with existing manufacturing processes, excellent cycling stability, and favorable cost-performance ratio. This positions them as particularly valuable for applications requiring extended calendar life and enhanced safety characteristics, though they may require complementary approaches for extreme operating conditions.
Surface coating strategies, particularly those utilizing metal oxides like Al2O3 and ZrO2, offer protective barriers against electrolyte attack but often suffer from poor ionic conductivity. In contrast, lithium phosphate treatments provide superior lithium-ion transport properties while maintaining protective functions, demonstrating approximately 15-20% better capacity retention in long-term cycling tests compared to metal oxide coatings.
Electrolyte additives such as vinylene carbonate (VC) and fluoroethylene carbonate (FEC) form passive SEI layers that reduce side reactions. However, these additives typically deplete over time, limiting their long-term effectiveness. Lithium phosphate treatments create more stable interfacial structures with demonstrated longevity exceeding 1000 cycles in laboratory tests, outperforming additive-only approaches by approximately 30% in calendar life studies.
Doping strategies involving elements like aluminum or magnesium modify bulk material properties but can compromise initial capacity. Lithium phosphate surface treatments achieve similar structural stabilization without the capacity penalties, maintaining 92-95% of theoretical capacity compared to 85-90% for conventional doping approaches.
Electrolyte engineering through concentrated salt systems (high-concentration electrolytes) reduces solvent activity but increases viscosity and cost. Lithium phosphate treatments are compatible with standard electrolyte formulations, offering a more cost-effective solution with comparable performance benefits at approximately one-third the implementation cost.
Temperature management systems effectively mitigate degradation but add significant weight, volume, and complexity to battery systems. Lithium phosphate treatments provide inherent thermal stability improvements, reducing exothermic reactions during abuse conditions by up to 40% compared to untreated materials, thereby complementing thermal management systems while reducing their complexity requirements.
When evaluated holistically, lithium phosphate-based mitigation techniques offer a balanced profile of benefits: moderate implementation complexity, high compatibility with existing manufacturing processes, excellent cycling stability, and favorable cost-performance ratio. This positions them as particularly valuable for applications requiring extended calendar life and enhanced safety characteristics, though they may require complementary approaches for extreme operating conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!