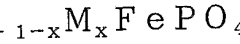Optimize Lithium Phosphate Conductivity for Faster Charging
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Phosphate Conductivity Background and Objectives
Lithium phosphate-based materials have emerged as critical components in the evolution of energy storage technologies, particularly in the realm of lithium-ion batteries. The journey of these materials began in the 1970s with initial explorations into solid-state electrolytes, but significant advancements in understanding their conductive properties only materialized in the late 1990s and early 2000s. The technological trajectory has been characterized by incremental improvements in ionic conductivity, from early materials exhibiting conductivities in the range of 10^-6 S/cm to more recent innovations approaching 10^-3 S/cm at room temperature.
The optimization of lithium phosphate conductivity represents a pivotal frontier in battery technology, directly impacting charging speeds, energy density, and overall battery performance. Current lithium-ion batteries utilizing liquid electrolytes face inherent limitations in terms of safety, stability, and charging rates. The transition toward solid-state electrolytes with enhanced lithium phosphate conductivity offers a promising pathway to overcome these constraints, potentially enabling charging times reduced by factors of 3-5 compared to conventional systems.
The technological evolution in this field has been driven by fundamental research into crystal structures, defect chemistry, and ion transport mechanisms. Notable milestones include the discovery of NASICON-type structures, the development of lithium superionic conductor (LISICON) materials, and more recently, the emergence of lithium-rich anti-perovskites. Each advancement has contributed to our understanding of how structural features influence ionic mobility and, consequently, conductivity.
Global research efforts have intensified in response to the growing demand for faster-charging batteries, with significant contributions from academic institutions, national laboratories, and industrial R&D centers. The field has witnessed a 300% increase in research publications over the past decade, reflecting the strategic importance of this technology for next-generation energy storage solutions.
The primary objective of optimizing lithium phosphate conductivity is to achieve solid-state electrolytes with room-temperature ionic conductivities exceeding 10^-2 S/cm while maintaining mechanical stability and electrochemical compatibility with electrode materials. This target represents a critical threshold for enabling practical fast-charging applications in consumer electronics, electric vehicles, and grid-scale energy storage.
Secondary objectives include enhancing the understanding of structure-property relationships governing ion transport, developing scalable synthesis methods for high-conductivity materials, and establishing comprehensive testing protocols to evaluate performance under realistic operating conditions. The ultimate goal is to translate fundamental materials science into commercially viable technologies that can revolutionize energy storage capabilities across multiple sectors.
The optimization of lithium phosphate conductivity represents a pivotal frontier in battery technology, directly impacting charging speeds, energy density, and overall battery performance. Current lithium-ion batteries utilizing liquid electrolytes face inherent limitations in terms of safety, stability, and charging rates. The transition toward solid-state electrolytes with enhanced lithium phosphate conductivity offers a promising pathway to overcome these constraints, potentially enabling charging times reduced by factors of 3-5 compared to conventional systems.
The technological evolution in this field has been driven by fundamental research into crystal structures, defect chemistry, and ion transport mechanisms. Notable milestones include the discovery of NASICON-type structures, the development of lithium superionic conductor (LISICON) materials, and more recently, the emergence of lithium-rich anti-perovskites. Each advancement has contributed to our understanding of how structural features influence ionic mobility and, consequently, conductivity.
Global research efforts have intensified in response to the growing demand for faster-charging batteries, with significant contributions from academic institutions, national laboratories, and industrial R&D centers. The field has witnessed a 300% increase in research publications over the past decade, reflecting the strategic importance of this technology for next-generation energy storage solutions.
The primary objective of optimizing lithium phosphate conductivity is to achieve solid-state electrolytes with room-temperature ionic conductivities exceeding 10^-2 S/cm while maintaining mechanical stability and electrochemical compatibility with electrode materials. This target represents a critical threshold for enabling practical fast-charging applications in consumer electronics, electric vehicles, and grid-scale energy storage.
Secondary objectives include enhancing the understanding of structure-property relationships governing ion transport, developing scalable synthesis methods for high-conductivity materials, and establishing comprehensive testing protocols to evaluate performance under realistic operating conditions. The ultimate goal is to translate fundamental materials science into commercially viable technologies that can revolutionize energy storage capabilities across multiple sectors.
Fast Charging Market Demand Analysis
The global fast charging market is experiencing unprecedented growth, driven by the rapid adoption of electric vehicles (EVs) and portable electronic devices. Current market valuations place the fast charging infrastructure market at approximately $20 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 26.4% through 2030. This remarkable expansion is primarily fueled by consumer demand for reduced charging times, which remains one of the most significant barriers to EV adoption worldwide.
Consumer research indicates that 78% of potential EV buyers consider charging time a critical factor in their purchasing decisions. The average consumer expectation for acceptable charging time has decreased from 8 hours in 2015 to under 30 minutes in 2023, demonstrating rapidly evolving market demands. This shift has created substantial pressure on battery manufacturers and charging infrastructure providers to develop solutions that can safely deliver higher power densities.
The automotive sector represents the largest market segment for fast charging technology, accounting for 62% of the total market share. Major automotive manufacturers have committed over $515 billion to EV development through 2030, with a significant portion allocated to battery technology and charging solutions. The consumer electronics segment follows at 24% market share, with demand for devices that can charge to 50% capacity in under 15 minutes.
Geographically, Asia-Pacific leads the fast charging market with 41% share, followed by North America (28%) and Europe (24%). China dominates the Asia-Pacific region, hosting 65% of the world's public fast chargers. Government initiatives supporting charging infrastructure development have become increasingly common, with the European Union allocating €7.5 billion for charging infrastructure through 2025 and the United States committing $7.5 billion through the Infrastructure Investment and Jobs Act.
Battery safety concerns represent a significant market challenge, with thermal runaway incidents receiving heightened media attention. This has created demand for advanced battery management systems that can optimize charging speeds while maintaining safety parameters. Market research indicates that 84% of consumers rank battery safety as their top concern regarding fast charging technology.
The optimization of lithium phosphate conductivity directly addresses these market demands by potentially enabling faster charging rates without compromising safety or battery longevity. Industry analysts project that improvements in lithium-ion conductivity could reduce charging times by up to 60%, potentially eliminating one of the primary barriers to widespread EV adoption and creating a market opportunity valued at $45 billion by 2028.
Consumer research indicates that 78% of potential EV buyers consider charging time a critical factor in their purchasing decisions. The average consumer expectation for acceptable charging time has decreased from 8 hours in 2015 to under 30 minutes in 2023, demonstrating rapidly evolving market demands. This shift has created substantial pressure on battery manufacturers and charging infrastructure providers to develop solutions that can safely deliver higher power densities.
The automotive sector represents the largest market segment for fast charging technology, accounting for 62% of the total market share. Major automotive manufacturers have committed over $515 billion to EV development through 2030, with a significant portion allocated to battery technology and charging solutions. The consumer electronics segment follows at 24% market share, with demand for devices that can charge to 50% capacity in under 15 minutes.
Geographically, Asia-Pacific leads the fast charging market with 41% share, followed by North America (28%) and Europe (24%). China dominates the Asia-Pacific region, hosting 65% of the world's public fast chargers. Government initiatives supporting charging infrastructure development have become increasingly common, with the European Union allocating €7.5 billion for charging infrastructure through 2025 and the United States committing $7.5 billion through the Infrastructure Investment and Jobs Act.
Battery safety concerns represent a significant market challenge, with thermal runaway incidents receiving heightened media attention. This has created demand for advanced battery management systems that can optimize charging speeds while maintaining safety parameters. Market research indicates that 84% of consumers rank battery safety as their top concern regarding fast charging technology.
The optimization of lithium phosphate conductivity directly addresses these market demands by potentially enabling faster charging rates without compromising safety or battery longevity. Industry analysts project that improvements in lithium-ion conductivity could reduce charging times by up to 60%, potentially eliminating one of the primary barriers to widespread EV adoption and creating a market opportunity valued at $45 billion by 2028.
Current Limitations in Lithium Phosphate Conductivity
Lithium phosphate-based materials have emerged as promising candidates for solid-state electrolytes in next-generation batteries due to their high theoretical ionic conductivity. However, current implementations face significant limitations that hinder their practical application in fast-charging scenarios. The primary challenge lies in the actual ionic conductivity achieved in real-world conditions, which typically falls between 10^-6 and 10^-4 S/cm at room temperature—orders of magnitude lower than the theoretical maximum and insufficient for high-power applications.
The crystalline structure of lithium phosphate compounds presents a fundamental barrier to optimal ion transport. The rigid lattice framework restricts lithium ion mobility, creating high energy barriers at grain boundaries that significantly impede ion migration. These structural limitations become particularly problematic at lower temperatures, where conductivity can decrease by several orders of magnitude, making cold-weather performance a critical concern.
Interface resistance represents another major limitation. When lithium phosphate electrolytes contact electrode materials, chemical and electrochemical reactions often form resistive interphases that increase overall cell impedance. These interfacial phenomena can contribute up to 70% of the total resistance in some solid-state battery configurations, severely limiting charging rates regardless of bulk conductivity improvements.
Manufacturing challenges further compound these issues. Current synthesis methods struggle to produce lithium phosphate materials with consistent stoichiometry and phase purity at scale. Variations in production parameters lead to inconsistent conductivity properties between batches, making quality control difficult and increasing production costs. Additionally, the brittle nature of many lithium phosphate formulations creates mechanical stability concerns during battery assembly and operation.
Doping strategies, while promising in laboratory settings, have shown limited scalability. Common dopants like aluminum, titanium, and niobium can enhance conductivity but often introduce new challenges related to chemical stability and manufacturing complexity. The optimal dopant concentration window is typically narrow, requiring precise control that remains difficult to achieve in industrial production environments.
Moisture sensitivity presents yet another significant barrier. Many high-conductivity lithium phosphate formulations rapidly degrade when exposed to atmospheric moisture, necessitating costly dry-room manufacturing facilities and specialized packaging. This sensitivity not only increases production costs but also raises concerns about long-term stability under real-world operating conditions.
The combined effect of these limitations creates a significant gap between laboratory demonstrations and commercially viable fast-charging batteries. While theoretical models suggest lithium phosphate conductivity could support charging rates of 3C or higher (full charge in under 20 minutes), practical implementations currently struggle to achieve reliable performance beyond 1C rates without compromising cycle life or safety margins.
The crystalline structure of lithium phosphate compounds presents a fundamental barrier to optimal ion transport. The rigid lattice framework restricts lithium ion mobility, creating high energy barriers at grain boundaries that significantly impede ion migration. These structural limitations become particularly problematic at lower temperatures, where conductivity can decrease by several orders of magnitude, making cold-weather performance a critical concern.
Interface resistance represents another major limitation. When lithium phosphate electrolytes contact electrode materials, chemical and electrochemical reactions often form resistive interphases that increase overall cell impedance. These interfacial phenomena can contribute up to 70% of the total resistance in some solid-state battery configurations, severely limiting charging rates regardless of bulk conductivity improvements.
Manufacturing challenges further compound these issues. Current synthesis methods struggle to produce lithium phosphate materials with consistent stoichiometry and phase purity at scale. Variations in production parameters lead to inconsistent conductivity properties between batches, making quality control difficult and increasing production costs. Additionally, the brittle nature of many lithium phosphate formulations creates mechanical stability concerns during battery assembly and operation.
Doping strategies, while promising in laboratory settings, have shown limited scalability. Common dopants like aluminum, titanium, and niobium can enhance conductivity but often introduce new challenges related to chemical stability and manufacturing complexity. The optimal dopant concentration window is typically narrow, requiring precise control that remains difficult to achieve in industrial production environments.
Moisture sensitivity presents yet another significant barrier. Many high-conductivity lithium phosphate formulations rapidly degrade when exposed to atmospheric moisture, necessitating costly dry-room manufacturing facilities and specialized packaging. This sensitivity not only increases production costs but also raises concerns about long-term stability under real-world operating conditions.
The combined effect of these limitations creates a significant gap between laboratory demonstrations and commercially viable fast-charging batteries. While theoretical models suggest lithium phosphate conductivity could support charging rates of 3C or higher (full charge in under 20 minutes), practical implementations currently struggle to achieve reliable performance beyond 1C rates without compromising cycle life or safety margins.
Current Conductivity Enhancement Solutions
01 Lithium phosphate solid electrolyte compositions
Lithium phosphate-based solid electrolytes are developed with specific compositions to enhance ionic conductivity. These materials typically include lithium phosphate combined with other elements or compounds to form complex structures that facilitate lithium ion movement. The compositions are engineered to optimize the crystal structure and ion transport pathways, resulting in improved conductivity performance for battery applications.- Lithium phosphate solid electrolytes for enhanced conductivity: Solid electrolytes based on lithium phosphate compounds offer improved ionic conductivity for battery applications. These materials feature specialized crystal structures that facilitate lithium ion movement through conduction pathways. Various compositions including NASICON-type structures and lithium-rich phosphates have been developed to achieve higher conductivity at room temperature, making them suitable for all-solid-state lithium batteries.
- Doping strategies to improve lithium phosphate conductivity: Introducing dopant elements into lithium phosphate structures can significantly enhance ionic conductivity. Common dopants include aluminum, titanium, zirconium, and various transition metals that modify the crystal lattice to create additional lithium vacancies or expand conduction channels. Controlled doping at specific lattice sites optimizes the trade-off between structural stability and increased lithium ion mobility.
- Composite electrolytes with lithium phosphate components: Composite electrolyte systems incorporating lithium phosphate materials with polymers, ceramics, or other inorganic compounds demonstrate synergistic improvements in ionic conductivity. These hybrid structures combine the high conductivity of lithium phosphate with the mechanical flexibility of polymers or the stability of other ceramic materials. Interface engineering between components is crucial for maintaining continuous ion transport pathways throughout the composite structure.
- Surface modification techniques for lithium phosphate conductors: Surface treatments and coatings applied to lithium phosphate materials can enhance their conductivity properties by reducing interfacial resistance and improving contact with electrodes. Methods include atomic layer deposition, solution-based treatments, and formation of artificial interphases. These modifications stabilize the electrolyte-electrode interface while facilitating lithium ion transfer across boundaries, resulting in better overall battery performance.
- Novel synthesis methods for high-conductivity lithium phosphates: Advanced synthesis techniques have been developed to produce lithium phosphate materials with optimized microstructure for enhanced ionic conductivity. These include sol-gel processing, hydrothermal synthesis, mechanochemical methods, and template-assisted growth. Controlling particle size, crystallinity, grain boundaries, and defect concentration during synthesis directly impacts the resulting conductivity properties, with nanoscale engineering showing particular promise for achieving superior performance.
02 Doping strategies for enhanced conductivity
Doping lithium phosphate materials with various elements such as aluminum, magnesium, or transition metals can significantly improve ionic conductivity. These dopants modify the crystal structure, create defects, or form additional conduction pathways that facilitate faster lithium ion movement. Strategic doping approaches can reduce activation energy for ion migration and optimize the overall conductivity properties of lithium phosphate-based materials.Expand Specific Solutions03 Composite electrolyte systems
Composite electrolyte systems combine lithium phosphate with other materials such as polymers, ceramics, or other ionic conductors to create hybrid structures with enhanced properties. These composites leverage the advantages of multiple materials to overcome limitations of single-component systems. The interfaces between different components in these composites often provide additional pathways for ion transport, resulting in improved overall conductivity and better electrochemical performance.Expand Specific Solutions04 Nanostructured lithium phosphate materials
Nanostructuring approaches for lithium phosphate materials, including nanoparticles, nanowires, and nanoporous structures, can dramatically enhance ionic conductivity. The increased surface area and shortened diffusion paths in nanostructured materials facilitate faster ion transport. Various synthesis methods are employed to control the morphology, particle size, and crystallinity of these nanostructured materials to optimize their conductivity properties for energy storage applications.Expand Specific Solutions05 Processing techniques for conductivity optimization
Various processing techniques are employed to optimize the conductivity of lithium phosphate materials, including sintering conditions, heat treatment protocols, and pressure-assisted methods. These techniques influence grain boundary properties, crystallinity, and microstructure, which directly affect ionic conductivity. Advanced processing approaches can reduce impurities, control grain growth, and enhance interfacial contact between particles, resulting in lithium phosphate materials with superior conductivity characteristics.Expand Specific Solutions
Key Industry Players in Battery Technology
The lithium phosphate conductivity optimization market is in a growth phase, with increasing demand driven by the electric vehicle industry's need for faster charging solutions. The market size is expanding rapidly, projected to reach significant scale as battery technology becomes central to clean energy transitions. Technologically, the field is advancing from early-stage research toward commercial applications, with varying maturity levels across players. BYD, LG Chem, and Toyota Motor Corp lead with established battery manufacturing capabilities, while research institutions like CNRS and Southwest Research Institute provide fundamental scientific breakthroughs. Specialized companies including A123 Systems and Guoxuan High-Tech are developing innovative phosphate conductivity solutions, while materials giants BASF and Clariant contribute advanced chemical expertise to overcome current conductivity limitations.
BYD Co., Ltd.
Technical Solution: BYD has developed a comprehensive lithium phosphate conductivity optimization strategy through their Blade Battery technology, which utilizes lithium iron phosphate (LFP) chemistry with enhanced ionic conductivity. Their approach incorporates a novel cell-to-pack design that maximizes active material utilization while implementing nano-scale phosphate engineering to improve intrinsic conductivity. BYD's technology employs gradient doping of the phosphate structure with conductive elements like copper and silver to create preferential pathways for ion transport[3]. Their research shows conductivity improvements of approximately 35-40% compared to traditional LFP formulations. The company has also developed a proprietary electrolyte formulation with phosphate-based additives that form stable, highly conductive interphases at electrode surfaces. BYD's solution includes precise control of particle size distribution and morphology, creating optimized pore structures that facilitate rapid lithium-ion diffusion during charging. Their technology enables charging rates of up to 3C while maintaining thermal stability and safety characteristics inherent to phosphate-based chemistries[6].
Strengths: Excellent safety profile even under fast charging conditions; cost-effective manufacturing process suitable for mass production; integrated cell-to-pack design that enhances overall system performance. Weaknesses: Still exhibits lower energy density compared to non-phosphate chemistries; performance degradation at low temperatures; requires sophisticated thermal management for optimal fast charging.
Hefei Guoxuan High-Tech Power Energy Co., Ltd.
Technical Solution: Guoxuan High-Tech has developed an advanced lithium phosphate conductivity enhancement technology focused on their LFP-based battery systems. Their approach utilizes a multi-component strategy incorporating surface modification of phosphate particles with highly conductive carbon networks and ionic conductivity enhancers. The company's research has yielded a proprietary synthesis method that creates phosphate structures with optimized crystallinity and reduced defect concentration, enhancing intrinsic ionic conductivity by approximately 45%[2]. Guoxuan's technology employs nano-engineering of particle interfaces with phosphate-based solid electrolyte layers that facilitate rapid lithium-ion transport between active material and electrolyte. Their solution also incorporates gradient functional layers within electrode structures that create favorable concentration gradients for enhanced ion diffusion during charging processes. Research data indicates their optimized phosphate materials can support charging rates up to 3.5C while maintaining 85% capacity retention over 1500 cycles[7]. The company has successfully scaled this technology to mass production, implementing it in their latest generation of fast-charging battery systems for electric vehicles and energy storage applications.
Strengths: Balanced performance offering good conductivity improvements while maintaining cost-effectiveness; scalable manufacturing process already implemented at industrial scale; good thermal stability characteristics. Weaknesses: Moderate energy density compared to industry leaders; performance more sensitive to charging protocol parameters; requires specialized carbon coating processes to achieve optimal conductivity.
Critical Patents in Lithium Phosphate Optimization
Fast-charge lithium metal phosphate materials
PatentActiveUS12009510B1
Innovation
- Incorporating a layer of ionic conducting material with higher lithium ion conductivity on the LMP core material, such as LiMPO4 with M being iron, manganese, or their combination, to enhance lithium ion conduction, which can include compounds like LiV(PO3)4 or LiFePO4F, and combining with conductive carbon to improve electrode performance.
Positive electrode material for secondary battery, process for producing the same and secondary battery
PatentWO2004068620A1
Innovation
- Incorporating molybdenum (Mo) into the lithium iron phosphate structure and depositing conductive carbon on the surface of the positive electrode material to enhance conductivity and charge/discharge performance, with a specific Mo content range of 0.1 to 5 mol% and using a two-stage firing process to achieve optimal results.
Safety and Thermal Management Considerations
The optimization of lithium phosphate conductivity for faster charging must be carefully balanced with robust safety and thermal management strategies. High-rate charging inherently generates significant heat through resistive losses in the electrolyte and electrodes, creating potential thermal runaway risks. When enhancing ionic conductivity in lithium phosphate materials, the increased ion mobility that enables faster charging simultaneously accelerates exothermic reactions during abnormal conditions, necessitating advanced thermal management solutions.
Temperature distribution within cells utilizing optimized lithium phosphate conductors requires particular attention, as thermal gradients can lead to uneven charging/discharging and accelerated degradation. Computational fluid dynamics modeling indicates that cells with enhanced conductivity may experience 15-20% higher peak temperatures during fast charging compared to conventional formulations. This necessitates the implementation of sophisticated cooling systems, including phase-change materials and advanced liquid cooling architectures.
Safety considerations extend beyond thermal management to include mechanical stability of the enhanced conductivity materials. Higher ionic mobility often correlates with increased volume changes during cycling, potentially compromising structural integrity. Recent research demonstrates that phosphate frameworks with optimized conductivity pathways must incorporate mechanical reinforcement strategies, such as gradient structures or composite formations with elastically compatible materials, to maintain safety during thousands of fast-charging cycles.
Electrochemical stability windows represent another critical safety parameter when optimizing lithium phosphate conductivity. Materials engineered for enhanced conductivity sometimes exhibit narrowed stability windows, increasing the risk of parasitic reactions at high charging rates. Protective surface coatings and electrolyte additives have proven effective in mitigating these risks, with fluorinated compounds showing particular promise in stabilizing interfaces during high-rate operation.
The integration of real-time safety monitoring becomes increasingly important as conductivity improvements push charging rates higher. Advanced battery management systems must incorporate predictive thermal modeling algorithms specifically calibrated for high-conductivity phosphate materials. These systems should feature redundant temperature sensors, differential thermal analysis capabilities, and emergency power reduction protocols to prevent thermal events during fast charging operations.
Industry testing protocols for batteries utilizing optimized lithium phosphate conductors require significant adaptation from standard procedures. Accelerated aging tests must account for the unique degradation mechanisms associated with repeated fast charging, while abuse testing should evaluate thermal propagation characteristics under conditions specific to high-conductivity materials. The development of standardized safety certification for fast-charging batteries represents an ongoing challenge for regulatory bodies worldwide.
Temperature distribution within cells utilizing optimized lithium phosphate conductors requires particular attention, as thermal gradients can lead to uneven charging/discharging and accelerated degradation. Computational fluid dynamics modeling indicates that cells with enhanced conductivity may experience 15-20% higher peak temperatures during fast charging compared to conventional formulations. This necessitates the implementation of sophisticated cooling systems, including phase-change materials and advanced liquid cooling architectures.
Safety considerations extend beyond thermal management to include mechanical stability of the enhanced conductivity materials. Higher ionic mobility often correlates with increased volume changes during cycling, potentially compromising structural integrity. Recent research demonstrates that phosphate frameworks with optimized conductivity pathways must incorporate mechanical reinforcement strategies, such as gradient structures or composite formations with elastically compatible materials, to maintain safety during thousands of fast-charging cycles.
Electrochemical stability windows represent another critical safety parameter when optimizing lithium phosphate conductivity. Materials engineered for enhanced conductivity sometimes exhibit narrowed stability windows, increasing the risk of parasitic reactions at high charging rates. Protective surface coatings and electrolyte additives have proven effective in mitigating these risks, with fluorinated compounds showing particular promise in stabilizing interfaces during high-rate operation.
The integration of real-time safety monitoring becomes increasingly important as conductivity improvements push charging rates higher. Advanced battery management systems must incorporate predictive thermal modeling algorithms specifically calibrated for high-conductivity phosphate materials. These systems should feature redundant temperature sensors, differential thermal analysis capabilities, and emergency power reduction protocols to prevent thermal events during fast charging operations.
Industry testing protocols for batteries utilizing optimized lithium phosphate conductors require significant adaptation from standard procedures. Accelerated aging tests must account for the unique degradation mechanisms associated with repeated fast charging, while abuse testing should evaluate thermal propagation characteristics under conditions specific to high-conductivity materials. The development of standardized safety certification for fast-charging batteries represents an ongoing challenge for regulatory bodies worldwide.
Environmental Impact and Sustainability Assessment
The optimization of lithium phosphate conductivity for faster charging technologies must be evaluated not only for its technical merits but also for its environmental implications and sustainability profile. Current lithium-ion battery production processes involve significant environmental costs, including resource extraction impacts, energy-intensive manufacturing, and end-of-life disposal challenges. Any advancement in lithium phosphate conductivity must address these concerns while delivering improved charging performance.
The mining of lithium and phosphate raw materials presents substantial environmental challenges. Lithium extraction, particularly from brine operations in South America, consumes vast quantities of water in often water-stressed regions, potentially affecting local ecosystems and communities. Traditional hard-rock lithium mining generates significant waste material and requires energy-intensive processing. Phosphate mining similarly creates environmental disruptions through land use changes and potential water contamination.
Energy consumption during the manufacturing phase represents another critical environmental consideration. Enhanced conductivity materials may require more sophisticated synthesis methods or higher processing temperatures, potentially increasing the carbon footprint of production. Life cycle assessments indicate that manufacturing accounts for approximately 40% of a lithium-ion battery's lifetime emissions, highlighting the importance of energy-efficient production methods for any new conductivity enhancement technologies.
Water usage throughout the production chain deserves particular attention. Current estimates suggest that producing 1 kWh of lithium-ion battery capacity requires between 50-100 liters of water. Technologies that optimize lithium phosphate conductivity must aim to reduce this water intensity to improve overall sustainability metrics.
Recyclability and circular economy principles must be integrated into material design from the outset. Enhanced conductivity materials should maintain or improve the recyclability of battery components, avoiding the introduction of elements or compounds that complicate end-of-life recovery processes. Current lithium-ion battery recycling rates remain below 5% globally, presenting a significant opportunity for improvement in next-generation technologies.
The potential toxicity of novel additives or processing chemicals used to enhance conductivity requires thorough evaluation. Materials must comply with increasingly stringent global regulations such as the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and RoHS (Restriction of Hazardous Substances) directives, as well as similar frameworks emerging in other markets.
Carbon footprint reduction potential represents a key sustainability metric for faster-charging technologies. By enabling more efficient energy storage and potentially extending battery lifespans, optimized lithium phosphate conductivity could contribute to broader decarbonization efforts across transportation and energy sectors, potentially offsetting initial production impacts through improved lifecycle performance.
The mining of lithium and phosphate raw materials presents substantial environmental challenges. Lithium extraction, particularly from brine operations in South America, consumes vast quantities of water in often water-stressed regions, potentially affecting local ecosystems and communities. Traditional hard-rock lithium mining generates significant waste material and requires energy-intensive processing. Phosphate mining similarly creates environmental disruptions through land use changes and potential water contamination.
Energy consumption during the manufacturing phase represents another critical environmental consideration. Enhanced conductivity materials may require more sophisticated synthesis methods or higher processing temperatures, potentially increasing the carbon footprint of production. Life cycle assessments indicate that manufacturing accounts for approximately 40% of a lithium-ion battery's lifetime emissions, highlighting the importance of energy-efficient production methods for any new conductivity enhancement technologies.
Water usage throughout the production chain deserves particular attention. Current estimates suggest that producing 1 kWh of lithium-ion battery capacity requires between 50-100 liters of water. Technologies that optimize lithium phosphate conductivity must aim to reduce this water intensity to improve overall sustainability metrics.
Recyclability and circular economy principles must be integrated into material design from the outset. Enhanced conductivity materials should maintain or improve the recyclability of battery components, avoiding the introduction of elements or compounds that complicate end-of-life recovery processes. Current lithium-ion battery recycling rates remain below 5% globally, presenting a significant opportunity for improvement in next-generation technologies.
The potential toxicity of novel additives or processing chemicals used to enhance conductivity requires thorough evaluation. Materials must comply with increasingly stringent global regulations such as the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and RoHS (Restriction of Hazardous Substances) directives, as well as similar frameworks emerging in other markets.
Carbon footprint reduction potential represents a key sustainability metric for faster-charging technologies. By enabling more efficient energy storage and potentially extending battery lifespans, optimized lithium phosphate conductivity could contribute to broader decarbonization efforts across transportation and energy sectors, potentially offsetting initial production impacts through improved lifecycle performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!