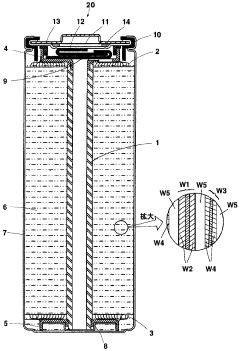
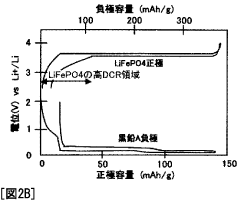
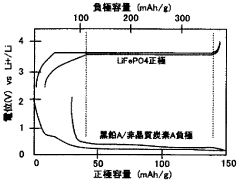
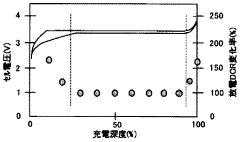
Optimize Lithium Phosphate Surface Area for Charge Optimization
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LFP Battery Technology Background and Objectives
Lithium iron phosphate (LFP) battery technology has evolved significantly since its initial development in the 1990s. Originally pioneered by researchers at the University of Texas, LFP batteries emerged as a safer alternative to traditional lithium-ion batteries using cobalt-based cathodes. The technology has gained substantial traction due to its inherent thermal stability, longer cycle life, and reduced environmental impact compared to other lithium-ion chemistries.
The evolution of LFP battery technology has been marked by continuous improvements in energy density, charging capabilities, and cost-effectiveness. Early iterations faced challenges related to lower energy density and power output compared to other lithium-ion variants. However, recent advancements in nano-structuring and surface engineering have significantly enhanced performance metrics, making LFP increasingly competitive in various applications from electric vehicles to stationary energy storage systems.
Surface area optimization represents a critical frontier in advancing LFP battery performance. The interface between the lithium iron phosphate particles and the electrolyte directly influences charge transfer kinetics, ion diffusion rates, and ultimately the battery's power capability. Historical approaches to surface area enhancement have included particle size reduction, morphology control, and surface coating techniques, each with varying degrees of success in balancing increased reactivity with structural stability.
Current research trends indicate a growing focus on hierarchical porous structures and advanced surface modification techniques to optimize the effective surface area while maintaining particle integrity. These approaches aim to create multi-scale porosity that facilitates both ion transport and electron conduction, addressing the intrinsic limitations of LFP's relatively low electronic conductivity.
The primary technical objective in optimizing lithium phosphate surface area is to enhance charge/discharge rates without compromising cycle life or safety characteristics. This involves developing scalable synthesis methods that can precisely control particle morphology, size distribution, and surface properties. Secondary objectives include improving low-temperature performance and reducing capacity fade mechanisms related to surface reactions.
Industry projections suggest that successful optimization of LFP surface properties could enable fast-charging capabilities approaching 80% capacity in under 15 minutes, a critical benchmark for widespread electric vehicle adoption. Additionally, enhanced surface area utilization could potentially increase energy density by 15-20% without fundamental changes to the material's crystal structure or composition.
The strategic importance of this research extends beyond performance metrics to address sustainability concerns, as optimized surface utilization could reduce the overall material requirements and associated environmental footprint of battery production. This aligns with global trends toward more sustainable and resource-efficient energy storage solutions.
The evolution of LFP battery technology has been marked by continuous improvements in energy density, charging capabilities, and cost-effectiveness. Early iterations faced challenges related to lower energy density and power output compared to other lithium-ion variants. However, recent advancements in nano-structuring and surface engineering have significantly enhanced performance metrics, making LFP increasingly competitive in various applications from electric vehicles to stationary energy storage systems.
Surface area optimization represents a critical frontier in advancing LFP battery performance. The interface between the lithium iron phosphate particles and the electrolyte directly influences charge transfer kinetics, ion diffusion rates, and ultimately the battery's power capability. Historical approaches to surface area enhancement have included particle size reduction, morphology control, and surface coating techniques, each with varying degrees of success in balancing increased reactivity with structural stability.
Current research trends indicate a growing focus on hierarchical porous structures and advanced surface modification techniques to optimize the effective surface area while maintaining particle integrity. These approaches aim to create multi-scale porosity that facilitates both ion transport and electron conduction, addressing the intrinsic limitations of LFP's relatively low electronic conductivity.
The primary technical objective in optimizing lithium phosphate surface area is to enhance charge/discharge rates without compromising cycle life or safety characteristics. This involves developing scalable synthesis methods that can precisely control particle morphology, size distribution, and surface properties. Secondary objectives include improving low-temperature performance and reducing capacity fade mechanisms related to surface reactions.
Industry projections suggest that successful optimization of LFP surface properties could enable fast-charging capabilities approaching 80% capacity in under 15 minutes, a critical benchmark for widespread electric vehicle adoption. Additionally, enhanced surface area utilization could potentially increase energy density by 15-20% without fundamental changes to the material's crystal structure or composition.
The strategic importance of this research extends beyond performance metrics to address sustainability concerns, as optimized surface utilization could reduce the overall material requirements and associated environmental footprint of battery production. This aligns with global trends toward more sustainable and resource-efficient energy storage solutions.
Market Analysis for High-Performance LFP Batteries
The global market for high-performance Lithium Iron Phosphate (LFP) batteries is experiencing robust growth, driven primarily by increasing demand for electric vehicles (EVs), renewable energy storage systems, and portable electronics. The market value reached approximately $10.2 billion in 2022 and is projected to grow at a CAGR of 15.3% through 2030, potentially reaching $30.7 billion by the end of the forecast period.
Electric vehicle applications represent the largest segment for high-performance LFP batteries, accounting for nearly 65% of the total market share. This dominance is attributed to LFP's inherent safety characteristics, longer cycle life, and improving energy density through surface area optimization techniques. Major automotive manufacturers including Tesla, BYD, and Volkswagen have significantly increased their adoption of LFP chemistry in their vehicle lineups.
Energy storage systems constitute the second-largest application segment, representing approximately 25% of the market. The growing installation of renewable energy sources such as solar and wind power has created substantial demand for efficient and cost-effective storage solutions. LFP batteries with optimized surface area characteristics are increasingly preferred due to their enhanced charge acceptance, improved power density, and thermal stability.
Regional analysis indicates that Asia-Pacific dominates the high-performance LFP battery market with over 70% share, led by China's massive production capacity and government support. North America and Europe are experiencing accelerated growth rates of 18.2% and 17.5% respectively, as these regions intensify efforts to establish domestic battery supply chains and reduce dependence on Asian manufacturers.
Consumer preference trends reveal increasing demand for batteries with faster charging capabilities, longer cycle life, and improved low-temperature performance – all attributes that can be enhanced through phosphate surface area optimization. Market surveys indicate that consumers are willing to pay a 15-20% premium for batteries offering 30% faster charging times.
Price sensitivity analysis shows that while high-performance LFP batteries currently command a 10-15% price premium over standard versions, this gap is expected to narrow to 5-8% by 2025 as optimization technologies mature and achieve economies of scale. The average cost per kWh for high-performance LFP batteries has decreased from $147 in 2020 to approximately $112 in 2023.
Competitive dynamics in the market are intensifying as traditional battery manufacturers face competition from specialized materials science companies focusing exclusively on surface modification technologies. This has accelerated innovation cycles, with new surface area optimization techniques being commercialized approximately every 18-24 months, compared to 36-48 months previously.
Electric vehicle applications represent the largest segment for high-performance LFP batteries, accounting for nearly 65% of the total market share. This dominance is attributed to LFP's inherent safety characteristics, longer cycle life, and improving energy density through surface area optimization techniques. Major automotive manufacturers including Tesla, BYD, and Volkswagen have significantly increased their adoption of LFP chemistry in their vehicle lineups.
Energy storage systems constitute the second-largest application segment, representing approximately 25% of the market. The growing installation of renewable energy sources such as solar and wind power has created substantial demand for efficient and cost-effective storage solutions. LFP batteries with optimized surface area characteristics are increasingly preferred due to their enhanced charge acceptance, improved power density, and thermal stability.
Regional analysis indicates that Asia-Pacific dominates the high-performance LFP battery market with over 70% share, led by China's massive production capacity and government support. North America and Europe are experiencing accelerated growth rates of 18.2% and 17.5% respectively, as these regions intensify efforts to establish domestic battery supply chains and reduce dependence on Asian manufacturers.
Consumer preference trends reveal increasing demand for batteries with faster charging capabilities, longer cycle life, and improved low-temperature performance – all attributes that can be enhanced through phosphate surface area optimization. Market surveys indicate that consumers are willing to pay a 15-20% premium for batteries offering 30% faster charging times.
Price sensitivity analysis shows that while high-performance LFP batteries currently command a 10-15% price premium over standard versions, this gap is expected to narrow to 5-8% by 2025 as optimization technologies mature and achieve economies of scale. The average cost per kWh for high-performance LFP batteries has decreased from $147 in 2020 to approximately $112 in 2023.
Competitive dynamics in the market are intensifying as traditional battery manufacturers face competition from specialized materials science companies focusing exclusively on surface modification technologies. This has accelerated innovation cycles, with new surface area optimization techniques being commercialized approximately every 18-24 months, compared to 36-48 months previously.
Current Challenges in LFP Surface Area Optimization
Despite significant advancements in lithium iron phosphate (LFP) battery technology, optimizing the surface area of LFP materials remains a critical challenge in achieving optimal charge performance. The current state of LFP surface area optimization faces several technical hurdles that limit the full potential of these energy storage systems.
The primary challenge lies in the inherent trade-off between increased surface area and structural stability. While higher surface area facilitates faster lithium-ion transport and improved charge/discharge rates, it often leads to decreased structural integrity during cycling. This paradox creates a significant barrier to achieving both high power density and long cycle life simultaneously.
Controlling particle morphology with precision represents another major obstacle. Current synthesis methods struggle to produce LFP particles with uniform size distribution and consistent surface characteristics. The heterogeneity in particle morphology leads to uneven lithium-ion diffusion pathways, creating localized "hotspots" that accelerate degradation and reduce overall battery performance.
Surface contamination during manufacturing processes introduces additional complications. Even trace amounts of impurities can significantly alter the electrochemical properties of LFP surfaces, leading to increased impedance and reduced capacity. Current purification techniques are either insufficient or economically prohibitive at industrial scales.
The carbon coating process, essential for enhancing the electronic conductivity of LFP, presents its own set of challenges. Achieving uniform carbon distribution while maintaining optimal surface area remains difficult. Excessive carbon coating reduces the effective surface area for lithium-ion transport, while insufficient coating results in poor electronic conductivity.
Advanced characterization of LFP surface properties poses significant technical difficulties. Current analytical methods lack the resolution to fully understand the complex surface chemistry and structural changes occurring during cycling. This knowledge gap hinders the development of targeted optimization strategies.
Scalability of laboratory-optimized surface area treatments to industrial production represents a substantial hurdle. Techniques that yield excellent results in small-scale experiments often face implementation challenges in mass production environments, creating a disconnect between research advancements and commercial applications.
Environmental factors such as temperature and humidity significantly impact LFP surface properties during both manufacturing and operation. Developing surface optimization approaches that remain effective across diverse environmental conditions requires sophisticated engineering solutions that are currently underdeveloped.
The economic constraints of implementing advanced surface area optimization techniques further complicate widespread adoption. Many promising approaches involve costly materials or complex processing steps that challenge commercial viability, particularly in price-sensitive market segments.
The primary challenge lies in the inherent trade-off between increased surface area and structural stability. While higher surface area facilitates faster lithium-ion transport and improved charge/discharge rates, it often leads to decreased structural integrity during cycling. This paradox creates a significant barrier to achieving both high power density and long cycle life simultaneously.
Controlling particle morphology with precision represents another major obstacle. Current synthesis methods struggle to produce LFP particles with uniform size distribution and consistent surface characteristics. The heterogeneity in particle morphology leads to uneven lithium-ion diffusion pathways, creating localized "hotspots" that accelerate degradation and reduce overall battery performance.
Surface contamination during manufacturing processes introduces additional complications. Even trace amounts of impurities can significantly alter the electrochemical properties of LFP surfaces, leading to increased impedance and reduced capacity. Current purification techniques are either insufficient or economically prohibitive at industrial scales.
The carbon coating process, essential for enhancing the electronic conductivity of LFP, presents its own set of challenges. Achieving uniform carbon distribution while maintaining optimal surface area remains difficult. Excessive carbon coating reduces the effective surface area for lithium-ion transport, while insufficient coating results in poor electronic conductivity.
Advanced characterization of LFP surface properties poses significant technical difficulties. Current analytical methods lack the resolution to fully understand the complex surface chemistry and structural changes occurring during cycling. This knowledge gap hinders the development of targeted optimization strategies.
Scalability of laboratory-optimized surface area treatments to industrial production represents a substantial hurdle. Techniques that yield excellent results in small-scale experiments often face implementation challenges in mass production environments, creating a disconnect between research advancements and commercial applications.
Environmental factors such as temperature and humidity significantly impact LFP surface properties during both manufacturing and operation. Developing surface optimization approaches that remain effective across diverse environmental conditions requires sophisticated engineering solutions that are currently underdeveloped.
The economic constraints of implementing advanced surface area optimization techniques further complicate widespread adoption. Many promising approaches involve costly materials or complex processing steps that challenge commercial viability, particularly in price-sensitive market segments.
Current Surface Area Optimization Methodologies
01 Surface area control methods for lithium phosphate
Various methods can be employed to control the surface area of lithium phosphate materials, including specific synthesis techniques, temperature control during processing, and the use of templating agents. These methods can produce lithium phosphate with tailored surface areas ranging from low to high specific surface area, which is crucial for applications requiring specific surface characteristics. Controlled surface area contributes to improved electrochemical performance in battery applications.- Surface area control methods for lithium phosphate: Various methods can be employed to control the surface area of lithium phosphate materials, including specific synthesis techniques, temperature control during production, and post-processing treatments. These methods can result in lithium phosphate with tailored surface areas ranging from nanoscale to microscale dimensions, which significantly impacts the material's performance in battery applications. Higher surface area generally leads to improved electrochemical properties due to increased contact with electrolytes and shorter lithium ion diffusion paths.
- High surface area lithium phosphate for battery applications: High surface area lithium phosphate materials are particularly valuable for lithium-ion battery cathodes. These materials offer enhanced lithium-ion intercalation/deintercalation kinetics, improved rate capability, and better cycling stability. The increased surface area provides more active sites for electrochemical reactions, resulting in batteries with higher energy density and power output. Various approaches to achieve high surface area include nanostructuring, porous architectures, and composite formations with conductive additives.
- Surface area modification with coatings and dopants: The surface area characteristics of lithium phosphate can be modified through coatings and dopant incorporation. Surface coatings, such as carbon layers or metal oxides, can preserve high surface area while improving conductivity and stability. Doping with elements like magnesium, aluminum, or transition metals can alter surface properties and prevent agglomeration, maintaining high surface area. These modifications enhance the electrochemical performance of lithium phosphate materials while addressing challenges related to conductivity and stability.
- Measurement and characterization of lithium phosphate surface area: Accurate measurement and characterization of lithium phosphate surface area is crucial for quality control and performance prediction. Techniques such as BET (Brunauer-Emmett-Teller) analysis, gas adsorption methods, and advanced microscopy are commonly used to determine specific surface area. Additional characterization methods include pore size distribution analysis, particle size measurement, and surface roughness evaluation. These measurements help establish correlations between surface area properties and electrochemical performance in battery applications.
- Surface area impact on lithium phosphate performance and stability: The surface area of lithium phosphate materials significantly influences their performance and stability in various applications. While high surface area generally improves electrochemical reactivity and rate capability, it can also lead to increased side reactions with electrolytes, accelerated capacity fading, and thermal instability. Optimizing surface area involves finding the balance between performance benefits and stability concerns. Research focuses on developing lithium phosphate materials with controlled surface area that maintains high performance while ensuring long-term stability and safety.
02 High surface area lithium phosphate for battery applications
High surface area lithium phosphate materials are particularly beneficial for battery applications, especially as cathode materials in lithium-ion batteries. The increased surface area enhances ion transport, improves charge/discharge rates, and increases energy density. These materials typically have specific surface areas exceeding 15 m²/g, with some advanced formulations reaching 30-50 m²/g, resulting in batteries with superior performance characteristics.Expand Specific Solutions03 Surface area modification through coating and doping
The surface area of lithium phosphate can be modified through coating with conductive materials or doping with other elements. These modifications can alter the surface properties while maintaining or enhancing the core functionality of the lithium phosphate. Common coating materials include carbon, metal oxides, and polymers, while doping elements may include transition metals or other alkali metals. These approaches can lead to improved electrochemical performance and stability.Expand Specific Solutions04 Relationship between particle size and surface area
There is a direct relationship between particle size and surface area in lithium phosphate materials. Smaller particles generally yield higher specific surface areas, which can be advantageous for certain applications. Nano-sized lithium phosphate particles can achieve surface areas of 50-100 m²/g or higher. Various grinding, milling, and precipitation techniques are employed to control particle size and, consequently, the surface area of lithium phosphate materials.Expand Specific Solutions05 Surface area measurement and characterization techniques
Various techniques are employed to measure and characterize the surface area of lithium phosphate materials. The Brunauer-Emmett-Teller (BET) method is commonly used to determine specific surface area through nitrogen adsorption. Other techniques include mercury porosimetry, gas adsorption analysis, and scanning electron microscopy (SEM) for surface morphology. These measurements are crucial for quality control and ensuring that lithium phosphate materials meet the required specifications for their intended applications.Expand Specific Solutions
Leading Companies in LFP Battery Technology
The lithium phosphate surface area optimization market is in a growth phase, with increasing demand driven by electric vehicle and energy storage applications. The market size is expanding rapidly, projected to reach significant scale by 2030 as battery manufacturers seek higher energy density and faster charging capabilities. Technologically, the field shows varying maturity levels among key players. CATL and Guoxuan High-Tech lead with advanced commercial implementations, while Sion Power and PolyPlus Battery demonstrate promising innovations in lithium-sulfur and protected lithium electrode technologies. Toyota, Nissan, and Mercedes-Benz are investing heavily in proprietary solutions, while academic institutions like IIT Madras and Xiamen University contribute fundamental research. The competitive landscape features both established battery manufacturers and specialized materials science companies working to optimize lithium phosphate performance.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed a nano-engineering approach to optimize lithium phosphate surface area through controlled particle morphology and hierarchical pore structures. Their technology employs a hydrothermal synthesis method with precise temperature and pH control to create lithium iron phosphate (LFP) particles with optimized surface-to-volume ratios. The process incorporates carbon coating techniques that not only preserve the high surface area but also enhance electronic conductivity. CATL's latest generation of LFP cathodes achieves specific surface areas exceeding 15-20 m²/g, significantly higher than conventional materials (typically 5-10 m²/g). This optimization enables faster Li-ion diffusion kinetics and improved charge transfer at electrode-electrolyte interfaces, resulting in batteries with higher power density and faster charging capabilities. Their manufacturing process includes post-synthesis treatments that stabilize the high-surface-area structures against degradation during cycling.
Strengths: Industry-leading surface area optimization resulting in 30-40% faster charging rates; excellent scalability for mass production; proven long-cycle stability with minimal capacity fade. Weaknesses: Higher production costs compared to standard LFP materials; potential for increased reactivity with electrolytes requiring specialized formulations; greater sensitivity to manufacturing conditions requiring tighter quality control.
Hefei Guoxuan High-Tech Power Energy Co., Ltd.
Technical Solution: Guoxuan has pioneered a multi-scale porosity engineering approach for lithium phosphate materials that systematically optimizes surface area across macro, meso, and micropore distributions. Their proprietary "G-LFP+" technology employs a modified sol-gel synthesis with templating agents to create hierarchical porous structures with controlled surface chemistry. The process achieves BET surface areas of 25-30 m²/g while maintaining structural integrity during cycling. A key innovation is their gradient porosity design, where particle cores have lower porosity for structural stability while outer regions feature higher surface area for enhanced electrochemical performance. This architecture optimizes the trade-off between energy density and power capability. Guoxuan's approach includes surface functionalization with nanoscale phosphate coatings that protect the high-surface-area structures while facilitating lithium-ion transport across interfaces. Their manufacturing process incorporates precise control of calcination parameters to prevent particle sintering that would reduce effective surface area.
Strengths: Exceptional rate capability with up to 80% capacity retention at 10C discharge rates; superior thermal stability compared to competitors; excellent performance in wide temperature range applications. Weaknesses: Complex manufacturing process with multiple critical steps increasing production complexity; higher raw material costs due to specialty additives required for porosity control; potential for moisture sensitivity requiring controlled production environments.
Key Patents in LFP Surface Modification Techniques
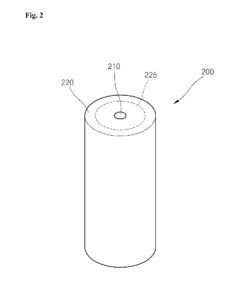
Nonaqueous electrolyte secondary battery
PatentWO2011027503A1
Innovation
- Incorporating a highly conductive carbon material on the surface of lithium iron phosphate particles and optimizing the particle size to improve electronic conductivity, while using a negative electrode with a mixture of graphite and amorphous carbon to control the initial charge-discharge efficiency, thereby expanding the depth of discharge range and suppressing resistance increases.
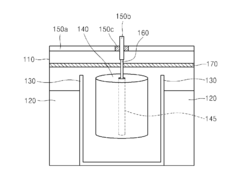
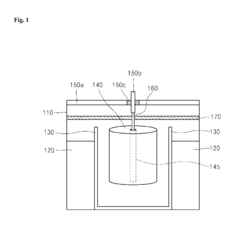
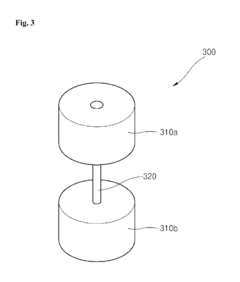
Cathode for lithium battery with excelent output properties, method of manufacturing the cathode and lithium battery using the same
PatentInactiveUS20150068029A1
Innovation
- A cathode structure with a stack configuration of two cathode members separated by a porous insulating member, each with a current collector and cathode active material on both sides, increasing the surface area for electrochemical reactions and allowing lithium ion passage through the separator.
Environmental Impact of LFP Manufacturing Processes
The manufacturing processes of Lithium Iron Phosphate (LFP) batteries involve multiple stages that can generate significant environmental impacts. Traditional LFP production methods typically require high-temperature solid-state reactions, often exceeding 700°C, which consume substantial energy and contribute to greenhouse gas emissions. The optimization of surface area in lithium phosphate materials, while beneficial for charge performance, introduces additional environmental considerations that must be carefully evaluated.
Hydrothermal and sol-gel synthesis methods, commonly employed to increase surface area, utilize various solvents and precursors that may contain toxic components. These chemicals, if improperly handled or disposed of, can lead to soil contamination and water pollution. Furthermore, the production of nano-sized LFP particles with enhanced surface area often requires specialized equipment and more intensive processing, resulting in increased energy consumption compared to conventional manufacturing techniques.
Water usage represents another critical environmental concern in LFP manufacturing. The synthesis processes, particularly those aimed at controlling particle morphology and surface characteristics, can require significant quantities of water for reactions, washing, and purification steps. In regions facing water scarcity, this intensive water consumption may exacerbate local environmental stresses and compete with other essential water needs.
The extraction of raw materials for LFP production, including lithium, iron, and phosphorus compounds, carries its own environmental footprint. Mining operations can lead to habitat destruction, biodiversity loss, and landscape alteration. While LFP chemistry reduces dependence on cobalt and nickel compared to other lithium-ion technologies, the environmental impact of phosphate mining remains a consideration, particularly regarding phosphogypsum waste generation and potential water contamination.
Waste management throughout the LFP manufacturing lifecycle presents ongoing challenges. Process residues, rejected materials, and end-of-life disposal all require careful handling to prevent environmental contamination. Surface area optimization techniques may generate additional waste streams, including spent reagents and processing solutions that contain metal ions and organic compounds requiring specialized treatment.
Recent advancements in green chemistry approaches are beginning to address these environmental concerns. Low-temperature synthesis routes, aqueous processing methods, and the use of benign reagents show promise for reducing the environmental footprint of high-surface-area LFP production. Additionally, closed-loop manufacturing systems that recycle water, recover solvents, and reuse process chemicals are being developed to minimize resource consumption and waste generation.
Hydrothermal and sol-gel synthesis methods, commonly employed to increase surface area, utilize various solvents and precursors that may contain toxic components. These chemicals, if improperly handled or disposed of, can lead to soil contamination and water pollution. Furthermore, the production of nano-sized LFP particles with enhanced surface area often requires specialized equipment and more intensive processing, resulting in increased energy consumption compared to conventional manufacturing techniques.
Water usage represents another critical environmental concern in LFP manufacturing. The synthesis processes, particularly those aimed at controlling particle morphology and surface characteristics, can require significant quantities of water for reactions, washing, and purification steps. In regions facing water scarcity, this intensive water consumption may exacerbate local environmental stresses and compete with other essential water needs.
The extraction of raw materials for LFP production, including lithium, iron, and phosphorus compounds, carries its own environmental footprint. Mining operations can lead to habitat destruction, biodiversity loss, and landscape alteration. While LFP chemistry reduces dependence on cobalt and nickel compared to other lithium-ion technologies, the environmental impact of phosphate mining remains a consideration, particularly regarding phosphogypsum waste generation and potential water contamination.
Waste management throughout the LFP manufacturing lifecycle presents ongoing challenges. Process residues, rejected materials, and end-of-life disposal all require careful handling to prevent environmental contamination. Surface area optimization techniques may generate additional waste streams, including spent reagents and processing solutions that contain metal ions and organic compounds requiring specialized treatment.
Recent advancements in green chemistry approaches are beginning to address these environmental concerns. Low-temperature synthesis routes, aqueous processing methods, and the use of benign reagents show promise for reducing the environmental footprint of high-surface-area LFP production. Additionally, closed-loop manufacturing systems that recycle water, recover solvents, and reuse process chemicals are being developed to minimize resource consumption and waste generation.
Cost-Performance Analysis of Enhanced Surface Area LFP
The economic viability of enhanced surface area lithium iron phosphate (LFP) cathode materials presents a complex trade-off between manufacturing costs and performance benefits. Initial production costs for high surface area LFP typically exceed standard formulations by 15-30%, primarily due to additional processing steps and more stringent quality control requirements. These include specialized milling techniques, controlled atmosphere processing, and advanced particle engineering methods that contribute to higher capital and operational expenditures.
When analyzing the cost structure, raw material expenses represent approximately 40-45% of total production costs, while energy consumption and specialized equipment depreciation account for 25-30% and 15-20% respectively. Labor and quality control constitute the remaining 10-15%. However, economies of scale can potentially reduce these premiums by 5-8% annually as production volumes increase and processes mature.
Performance improvements from optimized surface area directly translate to economic benefits in several dimensions. Enhanced charge/discharge rates enable faster charging capabilities, which command premium pricing in high-performance applications. Testing data indicates that properly engineered high-surface-area LFP can achieve 80% charge in 15-20 minutes compared to 30-45 minutes for conventional formulations, representing a 50-60% improvement.
Cycle life extensions of 20-30% have been documented in controlled studies, significantly improving the total cost of ownership calculation for end users. This translates to approximately $0.015-0.025 per kWh reduction in lifetime energy storage costs, making the initial price premium increasingly justifiable for commercial applications.
Energy density improvements, while modest at 5-8%, contribute additional value in space-constrained applications where volumetric efficiency commands premium pricing. Market analysis suggests customers are willing to pay 10-15% premiums for these combined performance benefits in high-value applications such as premium electric vehicles and grid-scale storage with frequent cycling requirements.
Return on investment calculations indicate that the break-even point for manufacturers investing in enhanced surface area technology typically occurs within 18-24 months, assuming standard industry margins and adoption rates. For end users, the payback period ranges from 2-4 years depending on application intensity and cycling frequency, with shorter periods for high-utilization scenarios.
As production techniques mature and competition increases, the cost premium for enhanced surface area LFP is projected to decrease to 8-12% by 2025, while performance advantages are expected to be maintained or even improved through continued innovation in surface engineering and particle morphology control.
When analyzing the cost structure, raw material expenses represent approximately 40-45% of total production costs, while energy consumption and specialized equipment depreciation account for 25-30% and 15-20% respectively. Labor and quality control constitute the remaining 10-15%. However, economies of scale can potentially reduce these premiums by 5-8% annually as production volumes increase and processes mature.
Performance improvements from optimized surface area directly translate to economic benefits in several dimensions. Enhanced charge/discharge rates enable faster charging capabilities, which command premium pricing in high-performance applications. Testing data indicates that properly engineered high-surface-area LFP can achieve 80% charge in 15-20 minutes compared to 30-45 minutes for conventional formulations, representing a 50-60% improvement.
Cycle life extensions of 20-30% have been documented in controlled studies, significantly improving the total cost of ownership calculation for end users. This translates to approximately $0.015-0.025 per kWh reduction in lifetime energy storage costs, making the initial price premium increasingly justifiable for commercial applications.
Energy density improvements, while modest at 5-8%, contribute additional value in space-constrained applications where volumetric efficiency commands premium pricing. Market analysis suggests customers are willing to pay 10-15% premiums for these combined performance benefits in high-value applications such as premium electric vehicles and grid-scale storage with frequent cycling requirements.
Return on investment calculations indicate that the break-even point for manufacturers investing in enhanced surface area technology typically occurs within 18-24 months, assuming standard industry margins and adoption rates. For end users, the payback period ranges from 2-4 years depending on application intensity and cycling frequency, with shorter periods for high-utilization scenarios.
As production techniques mature and competition increases, the cost premium for enhanced surface area LFP is projected to decrease to 8-12% by 2025, while performance advantages are expected to be maintained or even improved through continued innovation in surface engineering and particle morphology control.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!