Measure Thermal Stability of Lithium Phosphate in Lab Scenarios
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Phosphate Thermal Stability Background & Objectives
Lithium phosphate compounds have emerged as critical materials in modern energy storage systems, particularly in lithium-ion batteries. The evolution of these materials spans several decades, beginning with fundamental research in the 1970s that explored the intercalation properties of lithium in various host structures. By the 1990s, lithium phosphate-based cathode materials, especially lithium iron phosphate (LiFePO4), gained significant attention due to their promising stability characteristics compared to other lithium-containing compounds.
The thermal stability of lithium phosphate compounds represents a cornerstone property that directly impacts battery safety, longevity, and performance under various operating conditions. Historical incidents involving lithium battery thermal runaway have underscored the critical importance of understanding these stability parameters. The 2013 Boeing 787 Dreamliner battery fires and numerous consumer electronics incidents have accelerated research into more thermally stable lithium compounds.
Recent technological advancements have focused on enhancing the intrinsic thermal stability of lithium phosphate materials through various approaches, including doping with stabilizing elements, surface modifications, and novel synthesis methods. These developments aim to push the thermal stability envelope while maintaining or improving electrochemical performance characteristics.
The scientific literature demonstrates a clear progression in measurement techniques for thermal stability, evolving from basic differential scanning calorimetry (DSC) to more sophisticated approaches combining multiple analytical methods. This evolution reflects the growing recognition of thermal stability as a multifaceted property requiring comprehensive characterization methodologies.
The primary objective of measuring thermal stability in laboratory scenarios is to establish standardized protocols that accurately predict real-world behavior of lithium phosphate materials under thermal stress. This includes quantifying decomposition temperatures, heat generation rates, gas evolution profiles, and structural changes during thermal events.
Secondary objectives include identifying specific failure mechanisms and their triggers, establishing safety thresholds for various applications, and developing accelerated testing methods that correlate with long-term stability performance. These measurements aim to bridge the gap between fundamental material properties and practical application requirements.
The ultimate goal is to develop a comprehensive thermal stability profile for lithium phosphate compounds that enables rational material design, informs safety protocols, and supports regulatory frameworks. This knowledge directly contributes to the advancement of safer, more reliable energy storage technologies across multiple sectors, from consumer electronics to electric vehicles and grid-scale storage systems.
The thermal stability of lithium phosphate compounds represents a cornerstone property that directly impacts battery safety, longevity, and performance under various operating conditions. Historical incidents involving lithium battery thermal runaway have underscored the critical importance of understanding these stability parameters. The 2013 Boeing 787 Dreamliner battery fires and numerous consumer electronics incidents have accelerated research into more thermally stable lithium compounds.
Recent technological advancements have focused on enhancing the intrinsic thermal stability of lithium phosphate materials through various approaches, including doping with stabilizing elements, surface modifications, and novel synthesis methods. These developments aim to push the thermal stability envelope while maintaining or improving electrochemical performance characteristics.
The scientific literature demonstrates a clear progression in measurement techniques for thermal stability, evolving from basic differential scanning calorimetry (DSC) to more sophisticated approaches combining multiple analytical methods. This evolution reflects the growing recognition of thermal stability as a multifaceted property requiring comprehensive characterization methodologies.
The primary objective of measuring thermal stability in laboratory scenarios is to establish standardized protocols that accurately predict real-world behavior of lithium phosphate materials under thermal stress. This includes quantifying decomposition temperatures, heat generation rates, gas evolution profiles, and structural changes during thermal events.
Secondary objectives include identifying specific failure mechanisms and their triggers, establishing safety thresholds for various applications, and developing accelerated testing methods that correlate with long-term stability performance. These measurements aim to bridge the gap between fundamental material properties and practical application requirements.
The ultimate goal is to develop a comprehensive thermal stability profile for lithium phosphate compounds that enables rational material design, informs safety protocols, and supports regulatory frameworks. This knowledge directly contributes to the advancement of safer, more reliable energy storage technologies across multiple sectors, from consumer electronics to electric vehicles and grid-scale storage systems.
Market Applications and Demand Analysis
The thermal stability measurement of lithium phosphate compounds has gained significant market traction across multiple industries, driven primarily by the exponential growth in lithium-ion battery applications. The global lithium-ion battery market, valued at over $40 billion in 2020, is projected to reach $116 billion by 2030, with a compound annual growth rate exceeding 12%. This growth directly correlates with increased demand for thermal stability testing of battery components, including lithium phosphate materials.
Energy storage systems represent the largest application segment, with electric vehicles (EVs) and grid storage solutions driving demand for thermally stable lithium phosphate compounds. The automotive sector particularly values thermal stability data as safety concerns remain paramount in consumer adoption of EVs. Major automotive manufacturers have established dedicated thermal testing protocols for battery materials, creating a specialized market for laboratory testing equipment and methodologies specific to lithium phosphate thermal characterization.
Consumer electronics manufacturers constitute another significant market segment requiring thermal stability measurements. With incidents of battery thermal runaway in portable devices causing product recalls and safety concerns, manufacturers are implementing more rigorous testing protocols during product development phases. This has created a growing market for specialized laboratory testing services and equipment focused on lithium phosphate thermal behavior analysis.
The renewable energy sector presents an emerging market opportunity as grid-scale storage solutions increasingly utilize lithium phosphate-based technologies. Utility companies and energy infrastructure developers require comprehensive thermal stability data to ensure long-term reliability and safety of these installations, particularly in variable climate conditions.
Research institutions and academic laboratories form a stable market segment with consistent demand for thermal stability measurement capabilities. Government funding for energy storage research has increased substantially, with programs specifically targeting safety improvements in lithium-based energy storage systems. This has created a specialized market for advanced thermal analysis equipment capable of precise measurements under controlled laboratory conditions.
Regulatory compliance represents a significant market driver as safety standards for lithium-based products continue to evolve globally. Testing laboratories that can provide certified thermal stability measurements for lithium phosphate materials are experiencing increased demand as manufacturers seek to meet these regulatory requirements across international markets.
The market landscape shows regional variations, with Asia-Pacific dominating manufacturing-related testing demand while North American and European markets focus more on research applications and safety certification services. This geographical distribution creates diverse market opportunities for thermal stability measurement technologies tailored to specific regional requirements and applications.
Energy storage systems represent the largest application segment, with electric vehicles (EVs) and grid storage solutions driving demand for thermally stable lithium phosphate compounds. The automotive sector particularly values thermal stability data as safety concerns remain paramount in consumer adoption of EVs. Major automotive manufacturers have established dedicated thermal testing protocols for battery materials, creating a specialized market for laboratory testing equipment and methodologies specific to lithium phosphate thermal characterization.
Consumer electronics manufacturers constitute another significant market segment requiring thermal stability measurements. With incidents of battery thermal runaway in portable devices causing product recalls and safety concerns, manufacturers are implementing more rigorous testing protocols during product development phases. This has created a growing market for specialized laboratory testing services and equipment focused on lithium phosphate thermal behavior analysis.
The renewable energy sector presents an emerging market opportunity as grid-scale storage solutions increasingly utilize lithium phosphate-based technologies. Utility companies and energy infrastructure developers require comprehensive thermal stability data to ensure long-term reliability and safety of these installations, particularly in variable climate conditions.
Research institutions and academic laboratories form a stable market segment with consistent demand for thermal stability measurement capabilities. Government funding for energy storage research has increased substantially, with programs specifically targeting safety improvements in lithium-based energy storage systems. This has created a specialized market for advanced thermal analysis equipment capable of precise measurements under controlled laboratory conditions.
Regulatory compliance represents a significant market driver as safety standards for lithium-based products continue to evolve globally. Testing laboratories that can provide certified thermal stability measurements for lithium phosphate materials are experiencing increased demand as manufacturers seek to meet these regulatory requirements across international markets.
The market landscape shows regional variations, with Asia-Pacific dominating manufacturing-related testing demand while North American and European markets focus more on research applications and safety certification services. This geographical distribution creates diverse market opportunities for thermal stability measurement technologies tailored to specific regional requirements and applications.
Current Thermal Stability Testing Challenges
The thermal stability testing of lithium phosphate compounds presents several significant challenges in laboratory environments. Traditional calorimetric methods, while established, often struggle with the unique properties of lithium-based materials. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA), commonly employed for thermal stability assessment, frequently yield inconsistent results when applied to lithium phosphate due to its complex phase transition behavior and sensitivity to environmental conditions.
A primary challenge lies in maintaining controlled atmospheric conditions during testing. Lithium phosphate's reactivity with moisture and oxygen necessitates specialized equipment capable of creating and maintaining inert environments. Many laboratories lack these advanced setups, resulting in data contamination and reduced reliability. Even minor atmospheric variations can significantly alter thermal stability profiles, making standardization across different testing facilities exceptionally difficult.
Sample preparation introduces another layer of complexity. The particle size distribution, crystallinity, and moisture content of lithium phosphate samples dramatically influence thermal behavior measurements. Current protocols lack standardized preparation methods, leading to substantial variations in test results between laboratories and even between different batches within the same facility. This inconsistency hampers meaningful comparison of research findings across the scientific community.
Temperature ramp rates present a particular challenge in thermal stability assessment. Too rapid heating can mask subtle phase transitions or decomposition events, while excessively slow rates extend testing times impractically. The optimal ramp rate varies depending on the specific lithium phosphate composition being tested, yet standardized guidelines remain inadequate across the industry.
Data interpretation poses significant difficulties due to the complex thermal profiles exhibited by lithium phosphate compounds. Multiple overlapping thermal events often occur simultaneously, making it challenging to isolate and quantify individual reactions. Current analytical software lacks specialized algorithms for lithium phosphate thermal profile deconvolution, forcing researchers to rely on subjective interpretations that reduce reproducibility.
Scale-up considerations represent another major challenge. Laboratory-scale thermal stability results frequently fail to predict behavior in larger industrial applications accurately. The heat transfer dynamics change substantially with increased sample size, yet methodologies for extrapolating small-scale test results to industrial applications remain underdeveloped.
Finally, safety concerns complicate testing procedures. Some lithium phosphate formulations can release toxic gases or undergo energetic decomposition when heated, requiring specialized containment and monitoring equipment that many laboratories cannot afford or lack expertise to operate properly. This limitation restricts comprehensive thermal stability assessment to a small number of specialized facilities.
A primary challenge lies in maintaining controlled atmospheric conditions during testing. Lithium phosphate's reactivity with moisture and oxygen necessitates specialized equipment capable of creating and maintaining inert environments. Many laboratories lack these advanced setups, resulting in data contamination and reduced reliability. Even minor atmospheric variations can significantly alter thermal stability profiles, making standardization across different testing facilities exceptionally difficult.
Sample preparation introduces another layer of complexity. The particle size distribution, crystallinity, and moisture content of lithium phosphate samples dramatically influence thermal behavior measurements. Current protocols lack standardized preparation methods, leading to substantial variations in test results between laboratories and even between different batches within the same facility. This inconsistency hampers meaningful comparison of research findings across the scientific community.
Temperature ramp rates present a particular challenge in thermal stability assessment. Too rapid heating can mask subtle phase transitions or decomposition events, while excessively slow rates extend testing times impractically. The optimal ramp rate varies depending on the specific lithium phosphate composition being tested, yet standardized guidelines remain inadequate across the industry.
Data interpretation poses significant difficulties due to the complex thermal profiles exhibited by lithium phosphate compounds. Multiple overlapping thermal events often occur simultaneously, making it challenging to isolate and quantify individual reactions. Current analytical software lacks specialized algorithms for lithium phosphate thermal profile deconvolution, forcing researchers to rely on subjective interpretations that reduce reproducibility.
Scale-up considerations represent another major challenge. Laboratory-scale thermal stability results frequently fail to predict behavior in larger industrial applications accurately. The heat transfer dynamics change substantially with increased sample size, yet methodologies for extrapolating small-scale test results to industrial applications remain underdeveloped.
Finally, safety concerns complicate testing procedures. Some lithium phosphate formulations can release toxic gases or undergo energetic decomposition when heated, requiring specialized containment and monitoring equipment that many laboratories cannot afford or lack expertise to operate properly. This limitation restricts comprehensive thermal stability assessment to a small number of specialized facilities.
State-of-the-Art Thermal Analysis Methods
01 Thermal stability enhancement through doping and coating
Lithium phosphate materials can be stabilized thermally through various doping and coating strategies. Introducing dopants such as metal ions (e.g., aluminum, magnesium, or zinc) into the crystal structure can improve the thermal stability by strengthening chemical bonds and reducing phase transitions at elevated temperatures. Additionally, applying protective coatings like carbon, metal oxides, or polymers on lithium phosphate particles creates a barrier against thermal degradation and prevents unwanted reactions during high-temperature exposure.- Thermal stability enhancement through doping and coating: Lithium phosphate materials can be stabilized thermally through doping with various elements such as metal ions or coating with protective layers. These modifications help to prevent structural degradation at elevated temperatures, reduce thermal runaway risks, and maintain electrochemical performance under thermal stress. The coating layers act as protective barriers against thermal decomposition while dopants can strengthen the crystal structure and improve thermal conductivity.
- Synthesis methods affecting thermal stability: Different synthesis methods significantly impact the thermal stability of lithium phosphate materials. Hydrothermal, solid-state, sol-gel, and other preparation techniques can be optimized to produce lithium phosphate with enhanced thermal resistance. Parameters such as reaction temperature, time, precursor selection, and post-synthesis treatment all contribute to the final product's ability to withstand thermal stress without decomposition or phase transformation.
- Composite structures for improved thermal performance: Creating composite structures by combining lithium phosphate with other materials can significantly enhance thermal stability. These composites often incorporate carbon-based materials, metal oxides, or other thermally stable compounds that synergistically improve heat dissipation and structural integrity under thermal stress. The composite approach allows for customized thermal management solutions while maintaining or enhancing the electrochemical properties of lithium phosphate.
- Thermal stability testing and characterization methods: Various analytical techniques are employed to evaluate the thermal stability of lithium phosphate materials, including differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), temperature-controlled X-ray diffraction, and accelerated aging tests. These methods help quantify thermal decomposition temperatures, phase transition points, and structural changes under thermal stress. Understanding these properties is crucial for developing lithium phosphate materials with enhanced thermal stability for applications in batteries and other high-temperature environments.
- Application-specific thermal stability requirements: Different applications of lithium phosphate materials require specific thermal stability profiles. For lithium-ion batteries, thermal stability is critical for safety and longevity, particularly in high-power applications. In catalysis, thermal stability at reaction temperatures is essential. For solid-state electrolytes, maintaining structural integrity across wide temperature ranges is required. These application-specific requirements drive the development of tailored lithium phosphate formulations with optimized thermal stability characteristics for each use case.
02 Synthesis methods affecting thermal stability
The thermal stability of lithium phosphate materials is significantly influenced by the synthesis method employed. Hydrothermal synthesis, solid-state reactions, sol-gel processes, and precipitation methods each produce materials with different particle sizes, crystallinity, and defect concentrations, all of which affect thermal behavior. Controlled cooling rates, precise temperature management during synthesis, and post-synthesis heat treatments can optimize the crystal structure and reduce internal stresses, resulting in lithium phosphate materials with enhanced thermal stability properties.Expand Specific Solutions03 Composite materials for improved thermal performance
Creating composite materials by combining lithium phosphate with other thermally stable compounds can significantly enhance overall thermal stability. These composites often incorporate materials like silicon dioxide, aluminum oxide, or specialized polymers that act as thermal buffers. The composite structure helps distribute thermal stress, prevent crack propagation, and maintain structural integrity at elevated temperatures. This approach is particularly valuable in battery applications where thermal management is critical for safety and performance.Expand Specific Solutions04 Surface modification techniques
Surface modification of lithium phosphate particles can dramatically improve their thermal stability. Techniques include surface functionalization with organic molecules, phosphate groups, or silanes that create protective barriers against thermal degradation. Other approaches involve creating core-shell structures or gradient compositions that shield the core material from thermal stress. These modifications can prevent unwanted surface reactions, reduce gas evolution at high temperatures, and maintain structural integrity under thermal cycling conditions.Expand Specific Solutions05 Thermal stability testing and characterization methods
Advanced characterization techniques are essential for evaluating the thermal stability of lithium phosphate materials. Differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and temperature-controlled X-ray diffraction (XRD) provide critical data on phase transitions, decomposition temperatures, and structural changes under thermal stress. Accelerated aging tests and thermal cycling protocols help predict long-term stability. These characterization methods enable researchers to quantify thermal stability improvements and optimize formulations for specific application requirements.Expand Specific Solutions
Leading Research Institutions and Industry Players
The thermal stability measurement of lithium phosphate in laboratory settings is currently in a growth phase, with increasing market demand driven by the expanding lithium battery industry. The global market is experiencing significant expansion as electric vehicle adoption accelerates, creating a projected $5-7 billion market for related testing technologies by 2028. Leading research institutions like Centre National de la Recherche Scientifique and California Institute of Technology are advancing fundamental understanding, while commercial players such as Hefei Guoxuan High-Tech, Svolt Energy, and Toyota Motor Corp are developing proprietary testing methodologies. Chinese companies including BST Power and Zhejiang Geely are particularly active in scaling up technologies, while established players like Honeywell and Mitsubishi Electric focus on integrating thermal stability testing into comprehensive battery management systems, indicating the technology is approaching commercial maturity but still requires standardization.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed advanced differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) protocols specifically optimized for lithium phosphate compounds. Their approach combines in-situ X-ray diffraction during thermal ramping to monitor crystallographic changes while simultaneously measuring heat flow. This allows precise determination of phase transition temperatures, decomposition pathways, and activation energies for lithium phosphate materials. Their methodology incorporates controlled atmosphere testing (inert, oxygen-rich, and humidity-controlled environments) to simulate various degradation scenarios relevant to battery applications. CNRS researchers have established correlations between synthesis parameters and resulting thermal stability profiles, creating a comprehensive database of structure-property relationships for various lithium phosphate compositions[1][3].
Strengths: Exceptional precision in thermal analysis with custom-built equipment allowing for simultaneous multi-parameter measurements. Their extensive database enables predictive modeling of thermal behavior. Weaknesses: Their highly specialized equipment requirements limit widespread adoption of their protocols, and their methods typically require longer testing periods compared to industry standard approaches.
Hefei Guoxuan High-Tech Power Energy Co., Ltd.
Technical Solution: Hefei Guoxuan has pioneered an accelerated thermal stability assessment platform specifically for lithium phosphate materials used in their LFP battery production. Their approach combines high-throughput differential scanning calorimetry with automated sample handling systems capable of processing up to 100 samples daily. The company employs proprietary algorithms to analyze thermal runaway onset temperatures and heat generation rates, correlating these with battery safety performance. Their methodology includes cyclic heating protocols that simulate repeated thermal stress conditions experienced during battery operation. Guoxuan's system incorporates real-time gas analysis during thermal decomposition, providing insights into potential toxic byproduct formation. This comprehensive approach enables rapid screening of various lithium phosphate compositions and dopants to optimize thermal stability while maintaining electrochemical performance[2][5].
Strengths: High-throughput capabilities allow rapid screening of multiple material variations, significantly accelerating R&D cycles. Their integrated approach connects material-level thermal properties directly to cell-level safety metrics. Weaknesses: Their proprietary algorithms and correlation models are highly calibrated to their specific manufacturing processes, potentially limiting applicability to other production environments or novel lithium phosphate formulations.
Critical Research Findings on Lithium Phosphate Stability
Electrical storage system comprising a sheet-type discrete element, discrete sheet-type element, method for the production thereof, and use thereof
PatentInactiveUS20170263900A1
Innovation
- A sheet-type discrete element with a composition of SiO2, B2O3, Al2O3, and controlled Li2O content, designed for high thermal stability and resistance to alkali metal attack, featuring a barrier layer for moisture and UV radiation, and optimized for uniform thickness and adhesion to prevent layer detachment and cracking.
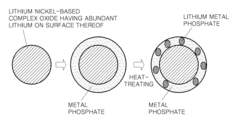
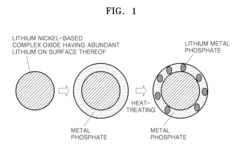
Positive active material including lithium nickel composite oxide core and coating layer containing lithium metal phosphate and metal phosphate, manufacturing method thereof, and electrode and lithium battery containing the same
PatentActiveUS9343739B2
Innovation
- A positive active material is developed comprising a lithium nickel-based composite oxide with a composite coating layer of lithium metal phosphate and metal phosphate, formed through a method involving mixing, stirring, and heat treatment between 300° C to 800° C, enhancing thermal stability and capacity retention.
Safety Protocols for Laboratory Testing
Laboratory testing of lithium phosphate thermal stability requires comprehensive safety protocols to protect personnel, equipment, and the environment. These protocols must address the specific hazards associated with lithium compounds, which can be reactive when exposed to heat, moisture, or incompatible materials. All laboratory personnel must undergo specialized training on lithium phosphate handling procedures before participating in thermal stability experiments, with refresher courses scheduled bi-annually to maintain safety awareness.
Personal protective equipment (PPE) requirements for these tests include flame-resistant lab coats, chemical-resistant gloves, safety goggles, and face shields when handling larger quantities. Respiratory protection may be necessary depending on the scale of testing and potential for dust generation. All PPE must be inspected before each use and replaced immediately if damaged.
Laboratory facilities conducting thermal stability measurements must be equipped with appropriate engineering controls, including properly functioning fume hoods, adequate ventilation systems, and thermal monitoring equipment. Emergency equipment such as Class D fire extinguishers (specifically designed for metal fires), emergency showers, and eyewash stations must be readily accessible and regularly maintained.
Material segregation practices are critical when working with lithium phosphate. Incompatible materials, particularly water, acids, and oxidizing agents, must be stored separately. Dedicated storage cabinets for lithium compounds should be clearly labeled and kept dry. Sample preparation areas must be isolated from testing areas to minimize cross-contamination risks.
Emergency response procedures must be clearly documented and prominently displayed in the laboratory. These should include specific instructions for lithium-related incidents such as fires, spills, or personal exposure. Regular emergency drills should be conducted to ensure all personnel can execute these procedures effectively under pressure.
Waste management protocols require special attention, as lithium phosphate waste may present environmental hazards. All waste materials must be collected in designated containers, properly labeled, and disposed of through approved channels. Neutralization procedures should be documented for addressing small spills, while larger incidents may require specialized hazardous materials response teams.
Documentation and reporting systems must be established to record all safety incidents, near-misses, and protocol violations. These records should be regularly reviewed to identify potential improvements to safety procedures. A designated safety officer should oversee compliance with these protocols and coordinate with institutional safety committees to ensure alignment with broader laboratory safety standards.
Personal protective equipment (PPE) requirements for these tests include flame-resistant lab coats, chemical-resistant gloves, safety goggles, and face shields when handling larger quantities. Respiratory protection may be necessary depending on the scale of testing and potential for dust generation. All PPE must be inspected before each use and replaced immediately if damaged.
Laboratory facilities conducting thermal stability measurements must be equipped with appropriate engineering controls, including properly functioning fume hoods, adequate ventilation systems, and thermal monitoring equipment. Emergency equipment such as Class D fire extinguishers (specifically designed for metal fires), emergency showers, and eyewash stations must be readily accessible and regularly maintained.
Material segregation practices are critical when working with lithium phosphate. Incompatible materials, particularly water, acids, and oxidizing agents, must be stored separately. Dedicated storage cabinets for lithium compounds should be clearly labeled and kept dry. Sample preparation areas must be isolated from testing areas to minimize cross-contamination risks.
Emergency response procedures must be clearly documented and prominently displayed in the laboratory. These should include specific instructions for lithium-related incidents such as fires, spills, or personal exposure. Regular emergency drills should be conducted to ensure all personnel can execute these procedures effectively under pressure.
Waste management protocols require special attention, as lithium phosphate waste may present environmental hazards. All waste materials must be collected in designated containers, properly labeled, and disposed of through approved channels. Neutralization procedures should be documented for addressing small spills, while larger incidents may require specialized hazardous materials response teams.
Documentation and reporting systems must be established to record all safety incidents, near-misses, and protocol violations. These records should be regularly reviewed to identify potential improvements to safety procedures. A designated safety officer should oversee compliance with these protocols and coordinate with institutional safety committees to ensure alignment with broader laboratory safety standards.
Environmental Impact Considerations
The environmental implications of lithium phosphate thermal stability testing extend beyond laboratory boundaries, necessitating comprehensive consideration throughout the research process. Laboratory testing of lithium phosphate compounds generates various waste streams including chemical residues, contaminated solvents, and potentially hazardous byproducts from thermal decomposition. These materials require proper handling, treatment, and disposal protocols to prevent environmental contamination and comply with regulatory standards.
Thermal stability experiments often involve heating lithium phosphate to extreme temperatures, which can release phosphorus oxides, lithium compounds, and other potentially harmful substances into laboratory exhaust systems. Implementation of efficient fume hood systems with appropriate filtration technologies is essential to minimize atmospheric emissions. Additionally, energy consumption during prolonged high-temperature testing contributes to the carbon footprint of research activities, highlighting the importance of energy-efficient experimental designs and equipment selection.
Water usage represents another significant environmental concern, as lithium compounds can contaminate water systems and disrupt aquatic ecosystems. Closed-loop cooling systems and water recycling protocols can substantially reduce consumption and prevent contamination. Furthermore, the sourcing of lithium phosphate materials for testing raises sustainability questions regarding mining practices and supply chain ethics, particularly as lithium demand continues to increase for battery applications worldwide.
Risk assessment frameworks specifically tailored to lithium phosphate thermal testing should incorporate environmental impact evaluations alongside safety considerations. This includes quantifying potential release scenarios, identifying vulnerable environmental receptors, and establishing mitigation measures. Adopting green chemistry principles can guide researchers toward more environmentally responsible experimental designs, including reduced reagent quantities, safer solvent alternatives, and minimized waste generation.
Long-term environmental monitoring programs may be necessary for facilities conducting extensive lithium phosphate thermal stability research, particularly when scaling up from laboratory to pilot plant operations. Such programs should track potential accumulation of lithium compounds in surrounding soil and water systems. Additionally, life cycle assessment methodologies can provide valuable insights into the cumulative environmental impacts of thermal stability research programs, identifying opportunities for sustainability improvements throughout the research workflow.
Regulatory compliance represents a critical dimension of environmental considerations, with requirements varying significantly across jurisdictions. Research facilities must navigate complex regulatory landscapes governing chemical handling, waste management, emissions control, and reporting obligations. Proactive engagement with environmental protection agencies and adoption of best practices beyond minimum compliance standards demonstrates institutional commitment to environmental stewardship while potentially avoiding costly remediation requirements.
Thermal stability experiments often involve heating lithium phosphate to extreme temperatures, which can release phosphorus oxides, lithium compounds, and other potentially harmful substances into laboratory exhaust systems. Implementation of efficient fume hood systems with appropriate filtration technologies is essential to minimize atmospheric emissions. Additionally, energy consumption during prolonged high-temperature testing contributes to the carbon footprint of research activities, highlighting the importance of energy-efficient experimental designs and equipment selection.
Water usage represents another significant environmental concern, as lithium compounds can contaminate water systems and disrupt aquatic ecosystems. Closed-loop cooling systems and water recycling protocols can substantially reduce consumption and prevent contamination. Furthermore, the sourcing of lithium phosphate materials for testing raises sustainability questions regarding mining practices and supply chain ethics, particularly as lithium demand continues to increase for battery applications worldwide.
Risk assessment frameworks specifically tailored to lithium phosphate thermal testing should incorporate environmental impact evaluations alongside safety considerations. This includes quantifying potential release scenarios, identifying vulnerable environmental receptors, and establishing mitigation measures. Adopting green chemistry principles can guide researchers toward more environmentally responsible experimental designs, including reduced reagent quantities, safer solvent alternatives, and minimized waste generation.
Long-term environmental monitoring programs may be necessary for facilities conducting extensive lithium phosphate thermal stability research, particularly when scaling up from laboratory to pilot plant operations. Such programs should track potential accumulation of lithium compounds in surrounding soil and water systems. Additionally, life cycle assessment methodologies can provide valuable insights into the cumulative environmental impacts of thermal stability research programs, identifying opportunities for sustainability improvements throughout the research workflow.
Regulatory compliance represents a critical dimension of environmental considerations, with requirements varying significantly across jurisdictions. Research facilities must navigate complex regulatory landscapes governing chemical handling, waste management, emissions control, and reporting obligations. Proactive engagement with environmental protection agencies and adoption of best practices beyond minimum compliance standards demonstrates institutional commitment to environmental stewardship while potentially avoiding costly remediation requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!