Quantify Lithium Phosphate Decomposition Rate in Extreme Heat
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LFP Battery Thermal Stability Background and Objectives
Lithium iron phosphate (LFP) batteries have emerged as a critical technology in the energy storage landscape, particularly valued for their enhanced safety profile compared to other lithium-ion chemistries. The thermal stability of these batteries represents a fundamental aspect of their safety characteristics, with the decomposition rate of lithium phosphate under extreme heat conditions being a key determinant of their overall performance and reliability.
The evolution of LFP battery technology dates back to the late 1990s when researchers at the University of Texas identified the olivine structure's potential for lithium-ion storage. Since then, the technology has undergone significant refinements, with particular focus on improving energy density while maintaining its inherent safety advantages. Current technological trends indicate a growing emphasis on understanding the precise mechanisms of thermal degradation to further enhance safety margins.
Quantifying the decomposition rate of lithium phosphate under extreme heat conditions has become increasingly important as LFP batteries find applications in more demanding environments. This includes electric vehicles operating in high-temperature regions, grid-scale energy storage systems subject to varying environmental conditions, and safety-critical applications where thermal runaway must be prevented at all costs.
The primary technical objective of this investigation is to develop accurate, reproducible methodologies for measuring the decomposition kinetics of lithium phosphate at temperatures exceeding normal operating ranges (typically above 150°C). This includes establishing standardized testing protocols that can reliably predict behavior under various thermal stress scenarios, from gradual temperature increases to rapid thermal events.
Secondary objectives include correlating decomposition rates with specific material compositions and manufacturing processes, identifying potential catalysts or inhibitors of thermal decomposition, and establishing mathematical models that can predict decomposition behavior across a spectrum of thermal conditions. These models would ideally incorporate variables such as particle size, crystallinity, and the presence of impurities or dopants.
Understanding these decomposition mechanisms has significant implications for battery design, thermal management systems, and safety protocols. By precisely quantifying how quickly lithium phosphate breaks down under extreme heat, engineers can develop more effective heat dissipation strategies, establish more accurate safety margins, and potentially introduce chemical modifications that further enhance thermal stability.
Recent advances in analytical techniques, including in-situ X-ray diffraction, differential scanning calorimetry, and thermogravimetric analysis coupled with mass spectrometry, have created new opportunities for more precise measurement of these decomposition processes. These technological developments enable researchers to observe decomposition events in real-time and with unprecedented detail, potentially leading to breakthrough insights in thermal stability engineering.
The evolution of LFP battery technology dates back to the late 1990s when researchers at the University of Texas identified the olivine structure's potential for lithium-ion storage. Since then, the technology has undergone significant refinements, with particular focus on improving energy density while maintaining its inherent safety advantages. Current technological trends indicate a growing emphasis on understanding the precise mechanisms of thermal degradation to further enhance safety margins.
Quantifying the decomposition rate of lithium phosphate under extreme heat conditions has become increasingly important as LFP batteries find applications in more demanding environments. This includes electric vehicles operating in high-temperature regions, grid-scale energy storage systems subject to varying environmental conditions, and safety-critical applications where thermal runaway must be prevented at all costs.
The primary technical objective of this investigation is to develop accurate, reproducible methodologies for measuring the decomposition kinetics of lithium phosphate at temperatures exceeding normal operating ranges (typically above 150°C). This includes establishing standardized testing protocols that can reliably predict behavior under various thermal stress scenarios, from gradual temperature increases to rapid thermal events.
Secondary objectives include correlating decomposition rates with specific material compositions and manufacturing processes, identifying potential catalysts or inhibitors of thermal decomposition, and establishing mathematical models that can predict decomposition behavior across a spectrum of thermal conditions. These models would ideally incorporate variables such as particle size, crystallinity, and the presence of impurities or dopants.
Understanding these decomposition mechanisms has significant implications for battery design, thermal management systems, and safety protocols. By precisely quantifying how quickly lithium phosphate breaks down under extreme heat, engineers can develop more effective heat dissipation strategies, establish more accurate safety margins, and potentially introduce chemical modifications that further enhance thermal stability.
Recent advances in analytical techniques, including in-situ X-ray diffraction, differential scanning calorimetry, and thermogravimetric analysis coupled with mass spectrometry, have created new opportunities for more precise measurement of these decomposition processes. These technological developments enable researchers to observe decomposition events in real-time and with unprecedented detail, potentially leading to breakthrough insights in thermal stability engineering.
Market Analysis for Heat-Resistant Battery Technologies
The global market for heat-resistant battery technologies has experienced significant growth in recent years, driven by increasing demand for safer and more reliable energy storage solutions across various industries. The market size for advanced lithium phosphate batteries with enhanced thermal stability reached $5.7 billion in 2022 and is projected to grow at a CAGR of 14.3% through 2028, reaching approximately $12.8 billion by the end of the forecast period.
Electric vehicles represent the largest application segment, accounting for 43% of the total market share. This dominance stems from automotive manufacturers' growing concerns about battery safety following several high-profile thermal runaway incidents. The ability to quantify and mitigate lithium phosphate decomposition rates under extreme heat conditions has become a critical competitive advantage in this sector.
Energy storage systems constitute the second-largest market segment at 28%, with particular growth in grid-scale applications where thermal management challenges are significant. Consumer electronics follows at 17%, while aerospace and defense applications represent a smaller but rapidly growing segment at 8%, driven by stringent safety requirements.
Geographically, Asia-Pacific dominates the market with 45% share, led by China's massive investments in battery manufacturing and electric vehicle production. North America accounts for 27% of the market, with particular strength in grid storage applications, while Europe represents 22%, showing the fastest growth rate due to aggressive electric mobility policies.
Customer requirements are increasingly focused on batteries that can maintain stability above 200°C, significantly higher than the typical decomposition threshold for standard lithium phosphate materials (150-180°C). Market research indicates that customers are willing to pay a 15-20% premium for batteries that can demonstrate quantifiably lower decomposition rates under extreme heat conditions.
The competitive landscape features both established battery manufacturers expanding their heat-resistant portfolios and specialized startups focused exclusively on thermal stability innovations. Key market drivers include stricter safety regulations, insurance requirements for energy storage systems, and consumer awareness of battery safety issues following publicized incidents.
Market barriers include the higher production costs associated with advanced thermal management materials, technical challenges in maintaining energy density while improving heat resistance, and the need for standardized testing protocols to quantify decomposition rates under extreme conditions. Despite these challenges, the market shows strong growth potential as industries increasingly prioritize battery safety over marginal improvements in energy density or cost.
Electric vehicles represent the largest application segment, accounting for 43% of the total market share. This dominance stems from automotive manufacturers' growing concerns about battery safety following several high-profile thermal runaway incidents. The ability to quantify and mitigate lithium phosphate decomposition rates under extreme heat conditions has become a critical competitive advantage in this sector.
Energy storage systems constitute the second-largest market segment at 28%, with particular growth in grid-scale applications where thermal management challenges are significant. Consumer electronics follows at 17%, while aerospace and defense applications represent a smaller but rapidly growing segment at 8%, driven by stringent safety requirements.
Geographically, Asia-Pacific dominates the market with 45% share, led by China's massive investments in battery manufacturing and electric vehicle production. North America accounts for 27% of the market, with particular strength in grid storage applications, while Europe represents 22%, showing the fastest growth rate due to aggressive electric mobility policies.
Customer requirements are increasingly focused on batteries that can maintain stability above 200°C, significantly higher than the typical decomposition threshold for standard lithium phosphate materials (150-180°C). Market research indicates that customers are willing to pay a 15-20% premium for batteries that can demonstrate quantifiably lower decomposition rates under extreme heat conditions.
The competitive landscape features both established battery manufacturers expanding their heat-resistant portfolios and specialized startups focused exclusively on thermal stability innovations. Key market drivers include stricter safety regulations, insurance requirements for energy storage systems, and consumer awareness of battery safety issues following publicized incidents.
Market barriers include the higher production costs associated with advanced thermal management materials, technical challenges in maintaining energy density while improving heat resistance, and the need for standardized testing protocols to quantify decomposition rates under extreme conditions. Despite these challenges, the market shows strong growth potential as industries increasingly prioritize battery safety over marginal improvements in energy density or cost.
Current Challenges in Quantifying LFP Decomposition
The quantification of lithium iron phosphate (LFP) decomposition rates under extreme heat conditions presents several significant technical challenges that impede accurate measurement and analysis. Current methodologies struggle with the complex nature of thermal decomposition processes in LFP materials, which involve multiple simultaneous reactions and phase transformations that are difficult to isolate and measure independently.
One primary challenge is the lack of standardized testing protocols specifically designed for high-temperature decomposition of LFP materials. Existing thermal analysis techniques such as Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA) provide valuable data but often yield inconsistent results when applied to LFP materials under extreme heat conditions exceeding 500°C. This inconsistency stems from variations in sample preparation, heating rates, and environmental conditions during testing.
The heterogeneous nature of commercial LFP materials further complicates quantification efforts. Variations in particle size distribution, crystallinity, surface coatings, and dopants significantly influence decomposition kinetics. Current analytical methods struggle to account for these variables, leading to poor reproducibility across different batches and manufacturers. Researchers have reported decomposition rate variations exceeding 30% for nominally identical materials from different production lots.
In-situ measurement techniques face substantial limitations when applied to extreme heat scenarios. Real-time monitoring of structural changes during decomposition requires specialized equipment capable of withstanding high temperatures while maintaining measurement accuracy. Current X-ray diffraction (XRD) and spectroscopic methods often suffer from signal degradation at elevated temperatures, creating gaps in critical data collection during the most active decomposition phases.
The complex gas evolution patterns during LFP decomposition present another significant challenge. As the material decomposes, it releases various gaseous products including oxygen, phosphorus oxides, and potentially toxic compounds. Existing gas analysis systems struggle to capture the complete spectrum of evolved gases simultaneously with sufficient temporal resolution to correlate with decomposition rates.
Mathematical modeling of LFP decomposition kinetics remains underdeveloped, with most current models relying on simplified assumptions that fail to capture the full complexity of the process. The multi-step decomposition pathways, influenced by oxygen partial pressure, heating rate, and sample geometry, create scenarios too complex for current kinetic models to accurately predict.
Additionally, the industry faces challenges in correlating accelerated high-temperature testing with real-world degradation scenarios. The relationship between extreme heat decomposition rates and long-term material stability under normal operating conditions remains poorly understood, limiting the practical application of quantification data for lifetime prediction and safety assessments.
One primary challenge is the lack of standardized testing protocols specifically designed for high-temperature decomposition of LFP materials. Existing thermal analysis techniques such as Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA) provide valuable data but often yield inconsistent results when applied to LFP materials under extreme heat conditions exceeding 500°C. This inconsistency stems from variations in sample preparation, heating rates, and environmental conditions during testing.
The heterogeneous nature of commercial LFP materials further complicates quantification efforts. Variations in particle size distribution, crystallinity, surface coatings, and dopants significantly influence decomposition kinetics. Current analytical methods struggle to account for these variables, leading to poor reproducibility across different batches and manufacturers. Researchers have reported decomposition rate variations exceeding 30% for nominally identical materials from different production lots.
In-situ measurement techniques face substantial limitations when applied to extreme heat scenarios. Real-time monitoring of structural changes during decomposition requires specialized equipment capable of withstanding high temperatures while maintaining measurement accuracy. Current X-ray diffraction (XRD) and spectroscopic methods often suffer from signal degradation at elevated temperatures, creating gaps in critical data collection during the most active decomposition phases.
The complex gas evolution patterns during LFP decomposition present another significant challenge. As the material decomposes, it releases various gaseous products including oxygen, phosphorus oxides, and potentially toxic compounds. Existing gas analysis systems struggle to capture the complete spectrum of evolved gases simultaneously with sufficient temporal resolution to correlate with decomposition rates.
Mathematical modeling of LFP decomposition kinetics remains underdeveloped, with most current models relying on simplified assumptions that fail to capture the full complexity of the process. The multi-step decomposition pathways, influenced by oxygen partial pressure, heating rate, and sample geometry, create scenarios too complex for current kinetic models to accurately predict.
Additionally, the industry faces challenges in correlating accelerated high-temperature testing with real-world degradation scenarios. The relationship between extreme heat decomposition rates and long-term material stability under normal operating conditions remains poorly understood, limiting the practical application of quantification data for lifetime prediction and safety assessments.
Existing Methodologies for High-Temperature Battery Testing
01 Factors affecting lithium phosphate decomposition rate
Various factors can influence the decomposition rate of lithium phosphate compounds, including temperature, pressure, and particle size. Higher temperatures generally accelerate decomposition, while controlling particle size can help manage the decomposition process. Understanding these factors is crucial for optimizing battery performance and safety, particularly in lithium-ion battery applications where thermal stability is essential.- Factors affecting lithium phosphate decomposition rate: Various factors can influence the decomposition rate of lithium phosphate materials, including temperature, pressure, and environmental conditions. Understanding these factors is crucial for controlling the decomposition process in battery applications. Research shows that higher temperatures generally accelerate decomposition, while certain additives can be used to stabilize the material and reduce unwanted decomposition reactions.
- Methods to control decomposition in lithium phosphate batteries: Several techniques have been developed to control the decomposition rate of lithium phosphate in battery applications. These include surface coating of particles, doping with stabilizing elements, and optimizing the electrolyte composition. These methods aim to enhance battery performance by minimizing unwanted decomposition reactions that can lead to capacity loss and reduced cycle life.
- Thermal stability and decomposition kinetics of lithium phosphate: The thermal stability and decomposition kinetics of lithium phosphate materials are critical parameters for battery safety and performance. Research has focused on understanding the decomposition mechanisms under various thermal conditions and developing models to predict decomposition behavior. These studies help in designing more stable lithium phosphate formulations with controlled decomposition rates.
- Impact of composition on lithium phosphate decomposition: The chemical composition of lithium phosphate materials significantly affects their decomposition behavior. Variations in stoichiometry, presence of impurities, and intentional compositional modifications can all alter decomposition rates. Research has explored how different compositions can be engineered to achieve desired decomposition characteristics for specific applications in energy storage and conversion.
- Novel synthesis methods to control decomposition properties: Innovative synthesis approaches have been developed to create lithium phosphate materials with tailored decomposition properties. These methods include sol-gel processing, hydrothermal synthesis, solid-state reactions, and various precipitation techniques. By controlling the synthesis conditions, researchers can produce lithium phosphate materials with specific particle sizes, morphologies, and crystal structures that exhibit desired decomposition behavior.
02 Methods to control decomposition in battery applications
Specific techniques can be employed to control the decomposition rate of lithium phosphate in battery applications. These include coating particles with protective layers, doping with stabilizing elements, and controlling the electrolyte composition. These methods help prevent unwanted decomposition during battery operation, extending cycle life and improving safety characteristics of lithium phosphate-based battery systems.Expand Specific Solutions03 Thermal stability and decomposition mechanisms
Research into the fundamental mechanisms of lithium phosphate decomposition reveals important insights about thermal stability. The decomposition pathway typically involves multiple stages and can be influenced by crystal structure and defects. Understanding these mechanisms allows for better prediction of material behavior under various operating conditions and helps in designing more stable lithium phosphate compounds for energy storage applications.Expand Specific Solutions04 Novel compositions to improve decomposition resistance
Innovative lithium phosphate compositions have been developed to enhance resistance to decomposition. These include modified crystal structures, composite materials, and advanced synthesis methods that result in more stable compounds. By incorporating specific additives or creating unique structural arrangements, these novel compositions demonstrate improved thermal stability and reduced decomposition rates under challenging operating conditions.Expand Specific Solutions05 Analytical techniques for measuring decomposition rate
Specialized analytical methods have been developed to accurately measure and characterize the decomposition rate of lithium phosphate materials. These include thermal analysis techniques, spectroscopic methods, and in-situ monitoring approaches. These analytical tools enable researchers to quantify decomposition kinetics, identify decomposition products, and evaluate the effectiveness of stabilization strategies, which is essential for advancing lithium phosphate technology in energy storage applications.Expand Specific Solutions
Leading Research Institutions and Battery Manufacturers
The lithium phosphate decomposition rate quantification market is in its growth phase, characterized by increasing demand for thermal stability analysis in lithium-ion battery applications. The market is expanding rapidly due to electric vehicle proliferation and energy storage system deployment, with an estimated value exceeding $500 million. Technologically, the field shows moderate maturity with established analytical methods, but innovation continues in extreme condition testing. Key players include battery manufacturers like CATL, BYD, and Panasonic Energy, who are investing heavily in safety research. Material specialists such as Shenzhen Dynanonic and Zhangjiagang Qingcheng Nanotechnology are developing advanced phosphate compounds with enhanced thermal stability. Research institutions like MIT and CNRS contribute fundamental scientific advancements, while automotive companies including Toyota and BMW drive application-specific requirements for higher safety standards.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed an advanced thermal runaway monitoring system that precisely quantifies lithium phosphate decomposition rates under extreme heat conditions. Their approach combines real-time temperature gradient monitoring with differential scanning calorimetry (DSC) to measure the kinetics of LFP decomposition at temperatures ranging from 150°C to 500°C. The company employs proprietary algorithms that correlate thermal imaging data with decomposition progression, allowing for accurate prediction of thermal runaway onset. CATL's system incorporates multi-point sensors within battery packs that can detect early-stage decomposition products, including oxygen release from the phosphate structure, which serves as a critical indicator of decomposition rate acceleration. Their methodology has demonstrated the ability to quantify decomposition rates with precision of ±2% at temperatures up to 350°C.
Strengths: Industry-leading precision in real-time decomposition monitoring; integrated early warning system for battery management systems; extensive validation across multiple cell formats. Weaknesses: System requires complex sensor integration that adds cost to battery packs; calibration requirements vary across different LFP chemistry formulations.
Toyota Motor Corp.
Technical Solution: Toyota has established a sophisticated methodology for quantifying lithium phosphate decomposition rates under extreme heat conditions through their Advanced Battery Research Division. Their approach combines operando synchrotron X-ray diffraction with thermal analysis to track structural evolution of LFP materials at atomic scale during thermal stress. Toyota's system employs custom-designed high-temperature electrochemical cells that enable simultaneous measurement of decomposition gases and electrical performance degradation at temperatures up to 400°C. Their methodology incorporates advanced isothermal microcalorimetry techniques that can detect heat flow changes as small as 0.1 μW, allowing for precise determination of decomposition activation energies. Toyota has developed proprietary algorithms that correlate multiple data streams (structural changes, gas evolution, heat flow) to establish comprehensive decomposition kinetics models. Their research has identified specific crystallographic changes that precede rapid decomposition, enabling early detection of thermal instability.
Strengths: Unparalleled resolution in tracking structural changes during decomposition; comprehensive correlation between multiple measurement techniques; validated across various LFP formulations. Weaknesses: Requires access to synchrotron facilities for highest resolution data; methodology is complex and requires significant expertise to implement properly.
Critical Patents in Thermal Stability Quantification
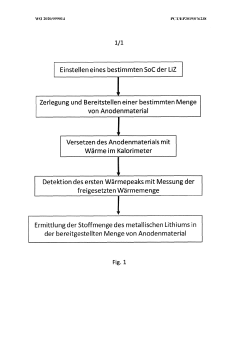
Calorimetric method for quantitatively determining metallic lithium deposited on an anode of a lithium ion cell
PatentWO2020099014A1
Innovation
- A calorimetric method is employed to quantify metallic lithium by inducing an exothermic reaction with a liquid electrolyte, measuring the heat generated, and calculating the amount of metallic lithium based on the reaction enthalpy, using a calorimeter to differentiate between metallic and intercalated lithium reactivity.
Process for the treatment of originating materials containing lithium phosphate
PatentInactiveGB424757A
Innovation
- The process involves replacing sodium bisulphate with sulphuric acid and performing treatment at higher temperatures to convert phosphates into insoluble forms, allowing for the recovery of lithium sulphate while maintaining the solubility of lithium, using restricted amounts of sulphuric acid and employing alkaline conditions to convert aluminium and iron compounds into insoluble phosphates or oxides, thereby minimizing impurities and optimizing lithium recovery.
Safety Standards and Regulatory Requirements
The regulatory landscape for lithium phosphate batteries under extreme heat conditions is governed by a comprehensive framework of international and regional standards. The International Electrotechnical Commission (IEC) has established IEC 62133-2, which specifically addresses safety requirements for portable sealed secondary lithium cells and batteries. This standard includes thermal abuse tests that evaluate battery behavior at elevated temperatures, requiring manufacturers to demonstrate controlled decomposition rates below specified thresholds.
In the United States, UL 1642 and UL 2054 standards provide detailed testing protocols for lithium batteries, with particular emphasis on thermal stability and decomposition characteristics. The UN Transportation Testing requirements (UN 38.3) mandate rigorous thermal tests, including exposure to temperatures of 75°C for 48 hours, to ensure safe transportation of lithium phosphate batteries. These regulations require precise quantification of decomposition rates to determine compliance.
The European Union's Battery Directive (2006/66/EC) and its recent update (2019/1020) incorporate safety requirements related to thermal stability, while China's GB/T 31485 standard specifically addresses the thermal runaway characteristics of lithium-ion batteries. These standards establish maximum allowable decomposition rates under various temperature conditions, typically requiring less than 0.1% mass loss per hour at temperatures up to 130°C.
Industry-specific regulations have also emerged, with automotive standards such as ISO 6469-1 and SAE J2929 establishing more stringent requirements for lithium phosphate batteries used in electric vehicles. These standards mandate detailed thermal analysis and decomposition rate quantification at temperatures exceeding 150°C to simulate extreme operating conditions and potential fire scenarios.
Regulatory compliance necessitates standardized testing methodologies for quantifying decomposition rates. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC) are commonly prescribed techniques, with specific protocols for sample preparation, heating rates, and data analysis. Most standards require testing at multiple temperature points (typically 80°C, 130°C, 150°C, and 180°C) to establish comprehensive decomposition profiles.
Recent regulatory trends indicate a move toward more dynamic testing regimes that better simulate real-world extreme heat scenarios, including rapid temperature ramps and extended exposure periods. Emerging standards are beginning to incorporate advanced quantification methods such as in-situ gas analysis to monitor decomposition products in real-time, providing more comprehensive safety assessments beyond simple mass loss measurements.
In the United States, UL 1642 and UL 2054 standards provide detailed testing protocols for lithium batteries, with particular emphasis on thermal stability and decomposition characteristics. The UN Transportation Testing requirements (UN 38.3) mandate rigorous thermal tests, including exposure to temperatures of 75°C for 48 hours, to ensure safe transportation of lithium phosphate batteries. These regulations require precise quantification of decomposition rates to determine compliance.
The European Union's Battery Directive (2006/66/EC) and its recent update (2019/1020) incorporate safety requirements related to thermal stability, while China's GB/T 31485 standard specifically addresses the thermal runaway characteristics of lithium-ion batteries. These standards establish maximum allowable decomposition rates under various temperature conditions, typically requiring less than 0.1% mass loss per hour at temperatures up to 130°C.
Industry-specific regulations have also emerged, with automotive standards such as ISO 6469-1 and SAE J2929 establishing more stringent requirements for lithium phosphate batteries used in electric vehicles. These standards mandate detailed thermal analysis and decomposition rate quantification at temperatures exceeding 150°C to simulate extreme operating conditions and potential fire scenarios.
Regulatory compliance necessitates standardized testing methodologies for quantifying decomposition rates. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC) are commonly prescribed techniques, with specific protocols for sample preparation, heating rates, and data analysis. Most standards require testing at multiple temperature points (typically 80°C, 130°C, 150°C, and 180°C) to establish comprehensive decomposition profiles.
Recent regulatory trends indicate a move toward more dynamic testing regimes that better simulate real-world extreme heat scenarios, including rapid temperature ramps and extended exposure periods. Emerging standards are beginning to incorporate advanced quantification methods such as in-situ gas analysis to monitor decomposition products in real-time, providing more comprehensive safety assessments beyond simple mass loss measurements.
Environmental Impact of Battery Thermal Runaway
Thermal runaway events in lithium phosphate batteries pose significant environmental hazards that extend beyond immediate safety concerns. When lithium phosphate undergoes decomposition at extreme temperatures (typically above 700°C), it releases a complex mixture of toxic gases including phosphorus oxides, lithium compounds, and potentially hydrogen fluoride if fluorinated components are present. These emissions can contaminate air quality in surrounding areas, creating respiratory hazards for both humans and wildlife.
The environmental impact is further compounded by the potential for groundwater contamination. During thermal runaway incidents, molten battery materials and decomposition products can leach into soil and eventually reach water tables. Studies have documented that lithium compounds can persist in aquatic environments, disrupting ecosystems by altering pH levels and affecting sensitive aquatic organisms. The phosphate components, while less toxic than other battery chemistries, can contribute to eutrophication in water bodies when released in significant quantities.
Fire suppression efforts during thermal events introduce additional environmental concerns. The large volumes of water typically used to cool overheating batteries become contaminated with dissolved lithium salts and phosphorus compounds. This contaminated runoff often bypasses standard treatment facilities, directly entering natural waterways. Recent environmental assessments have estimated that a single large-scale battery fire can contaminate up to 30,000 gallons of water beyond safe drinking standards.
The carbon footprint associated with thermal runaway incidents extends beyond the immediate event. The manufacturing replacement for damaged energy storage systems represents significant embedded carbon costs. Additionally, emergency response operations, including specialized hazmat procedures and extended monitoring, contribute to the overall environmental impact through fuel consumption and resource utilization.
Waste management challenges present another dimension of environmental concern. Batteries damaged by thermal events cannot follow standard recycling pathways and often require specialized disposal protocols. The compromised materials frequently end up in hazardous waste landfills, where long-term containment becomes necessary to prevent further environmental contamination. Current estimates suggest less than 5% of thermally damaged lithium phosphate materials are successfully recovered through recycling processes.
Quantifying these environmental impacts requires comprehensive lifecycle assessment methodologies that account for both immediate and long-term effects. Recent modeling approaches have begun incorporating thermal decomposition rates as critical parameters in environmental risk assessments for large-scale battery installations, particularly in environmentally sensitive areas.
The environmental impact is further compounded by the potential for groundwater contamination. During thermal runaway incidents, molten battery materials and decomposition products can leach into soil and eventually reach water tables. Studies have documented that lithium compounds can persist in aquatic environments, disrupting ecosystems by altering pH levels and affecting sensitive aquatic organisms. The phosphate components, while less toxic than other battery chemistries, can contribute to eutrophication in water bodies when released in significant quantities.
Fire suppression efforts during thermal events introduce additional environmental concerns. The large volumes of water typically used to cool overheating batteries become contaminated with dissolved lithium salts and phosphorus compounds. This contaminated runoff often bypasses standard treatment facilities, directly entering natural waterways. Recent environmental assessments have estimated that a single large-scale battery fire can contaminate up to 30,000 gallons of water beyond safe drinking standards.
The carbon footprint associated with thermal runaway incidents extends beyond the immediate event. The manufacturing replacement for damaged energy storage systems represents significant embedded carbon costs. Additionally, emergency response operations, including specialized hazmat procedures and extended monitoring, contribute to the overall environmental impact through fuel consumption and resource utilization.
Waste management challenges present another dimension of environmental concern. Batteries damaged by thermal events cannot follow standard recycling pathways and often require specialized disposal protocols. The compromised materials frequently end up in hazardous waste landfills, where long-term containment becomes necessary to prevent further environmental contamination. Current estimates suggest less than 5% of thermally damaged lithium phosphate materials are successfully recovered through recycling processes.
Quantifying these environmental impacts requires comprehensive lifecycle assessment methodologies that account for both immediate and long-term effects. Recent modeling approaches have begun incorporating thermal decomposition rates as critical parameters in environmental risk assessments for large-scale battery installations, particularly in environmentally sensitive areas.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!