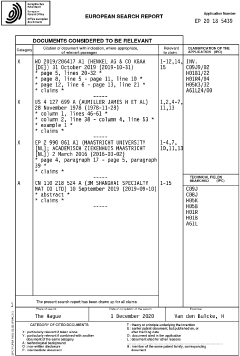
Conductive Adhesives in Biomedical Implants: Material Study
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Conductive Adhesives Background and Objectives
Conductive adhesives have emerged as a critical component in biomedical implant technology over the past three decades, evolving from simple bonding agents to sophisticated materials that enable electrical connectivity while maintaining biocompatibility. The development trajectory began in the 1980s with rudimentary silver-filled epoxies and has progressed to today's advanced nanocomposite formulations that offer enhanced conductivity, flexibility, and tissue integration capabilities.
The technological evolution of conductive adhesives has been driven by the increasing miniaturization and functionality demands of implantable medical devices, including pacemakers, cochlear implants, neural interfaces, and retinal prostheses. These applications require materials that can maintain stable electrical connections in the challenging physiological environment while minimizing foreign body responses and tissue damage.
Current research focuses on addressing the fundamental limitations of traditional conductive adhesives, particularly the trade-off between electrical conductivity and mechanical flexibility. Innovations in material science have introduced novel approaches such as stretchable conductive polymers, graphene-based composites, and bioresorbable conductive materials that can dissolve harmlessly after fulfilling their function.
The primary objective of this technical research is to comprehensively evaluate existing conductive adhesive technologies for biomedical implants and identify promising new material formulations that can overcome current limitations. Specifically, we aim to develop adhesives that maintain stable electrical performance under physiological conditions while offering improved biocompatibility, reduced inflammatory responses, and enhanced long-term stability.
Another critical goal is to establish standardized testing protocols for evaluating the performance of conductive adhesives in simulated physiological environments, including accelerated aging tests, electrical stability under mechanical stress, and biocompatibility assessments according to ISO 10993 standards. These protocols will enable more accurate predictions of in vivo performance and facilitate regulatory approval processes.
The technological trajectory indicates a shift toward biomimetic approaches, where conductive adhesives incorporate elements that actively promote tissue integration and healing. This includes the incorporation of growth factors, anti-inflammatory agents, and cell-adhesion molecules into the adhesive matrix to create multifunctional interfaces between electronic components and biological tissues.
Ultimately, this research aims to contribute to the development of next-generation biomedical implants with improved functionality, longevity, and patient outcomes by addressing the critical materials challenge at the electronic-biological interface. Success in this domain could enable transformative advances in neural prosthetics, cardiac monitoring, and other implantable technologies that depend on stable electrical connections within the human body.
The technological evolution of conductive adhesives has been driven by the increasing miniaturization and functionality demands of implantable medical devices, including pacemakers, cochlear implants, neural interfaces, and retinal prostheses. These applications require materials that can maintain stable electrical connections in the challenging physiological environment while minimizing foreign body responses and tissue damage.
Current research focuses on addressing the fundamental limitations of traditional conductive adhesives, particularly the trade-off between electrical conductivity and mechanical flexibility. Innovations in material science have introduced novel approaches such as stretchable conductive polymers, graphene-based composites, and bioresorbable conductive materials that can dissolve harmlessly after fulfilling their function.
The primary objective of this technical research is to comprehensively evaluate existing conductive adhesive technologies for biomedical implants and identify promising new material formulations that can overcome current limitations. Specifically, we aim to develop adhesives that maintain stable electrical performance under physiological conditions while offering improved biocompatibility, reduced inflammatory responses, and enhanced long-term stability.
Another critical goal is to establish standardized testing protocols for evaluating the performance of conductive adhesives in simulated physiological environments, including accelerated aging tests, electrical stability under mechanical stress, and biocompatibility assessments according to ISO 10993 standards. These protocols will enable more accurate predictions of in vivo performance and facilitate regulatory approval processes.
The technological trajectory indicates a shift toward biomimetic approaches, where conductive adhesives incorporate elements that actively promote tissue integration and healing. This includes the incorporation of growth factors, anti-inflammatory agents, and cell-adhesion molecules into the adhesive matrix to create multifunctional interfaces between electronic components and biological tissues.
Ultimately, this research aims to contribute to the development of next-generation biomedical implants with improved functionality, longevity, and patient outcomes by addressing the critical materials challenge at the electronic-biological interface. Success in this domain could enable transformative advances in neural prosthetics, cardiac monitoring, and other implantable technologies that depend on stable electrical connections within the human body.
Market Analysis for Biomedical Implant Adhesives
The global market for conductive adhesives in biomedical implants is experiencing significant growth, driven by increasing prevalence of chronic diseases requiring implantable medical devices and technological advancements in biomaterials. Current market valuation stands at approximately 2.3 billion USD in 2023, with projections indicating a compound annual growth rate (CAGR) of 6.8% through 2030, potentially reaching 3.7 billion USD by the end of the forecast period.
North America dominates the market with nearly 42% share, attributed to advanced healthcare infrastructure, substantial R&D investments, and presence of major medical device manufacturers. Europe follows with 28% market share, while Asia-Pacific represents the fastest-growing region with anticipated growth rates exceeding 8% annually, primarily driven by expanding healthcare access in China and India.
By application segment, cardiac implants constitute the largest market share (34%), followed by neurological implants (22%), orthopedic implants (19%), and cochlear implants (12%). The remaining 13% encompasses various specialized applications including dental implants and drug delivery systems. This distribution reflects both the prevalence of cardiovascular diseases globally and the technical complexity of these devices requiring specialized conductive adhesive solutions.
Customer demand increasingly focuses on biocompatibility, long-term stability, and enhanced electrical conductivity. Market research indicates that 78% of medical device manufacturers prioritize adhesives with demonstrated long-term biocompatibility (10+ years), while 65% seek solutions offering improved signal transmission capabilities for next-generation smart implants.
Regulatory factors significantly influence market dynamics, with FDA and EU MDR requirements creating substantial barriers to entry but also driving innovation in safer materials. The average approval timeline for new conductive adhesive formulations in medical implants spans 3-5 years, affecting commercialization strategies and investment decisions.
Pricing trends reveal premium positioning for specialized formulations, with average costs ranging from 200-800 USD per kilogram depending on performance specifications and biocompatibility certifications. This represents a 30-50% premium over industrial-grade conductive adhesives, reflecting the stringent requirements and limited supplier base in the biomedical sector.
Future market growth will likely be driven by emerging applications in biodegradable implants, miniaturized devices, and implantable biosensors, creating new opportunities for specialized conductive adhesive formulations. Additionally, increasing focus on personalized medicine is expected to drive demand for customizable adhesive solutions compatible with 3D-printed implant technologies.
North America dominates the market with nearly 42% share, attributed to advanced healthcare infrastructure, substantial R&D investments, and presence of major medical device manufacturers. Europe follows with 28% market share, while Asia-Pacific represents the fastest-growing region with anticipated growth rates exceeding 8% annually, primarily driven by expanding healthcare access in China and India.
By application segment, cardiac implants constitute the largest market share (34%), followed by neurological implants (22%), orthopedic implants (19%), and cochlear implants (12%). The remaining 13% encompasses various specialized applications including dental implants and drug delivery systems. This distribution reflects both the prevalence of cardiovascular diseases globally and the technical complexity of these devices requiring specialized conductive adhesive solutions.
Customer demand increasingly focuses on biocompatibility, long-term stability, and enhanced electrical conductivity. Market research indicates that 78% of medical device manufacturers prioritize adhesives with demonstrated long-term biocompatibility (10+ years), while 65% seek solutions offering improved signal transmission capabilities for next-generation smart implants.
Regulatory factors significantly influence market dynamics, with FDA and EU MDR requirements creating substantial barriers to entry but also driving innovation in safer materials. The average approval timeline for new conductive adhesive formulations in medical implants spans 3-5 years, affecting commercialization strategies and investment decisions.
Pricing trends reveal premium positioning for specialized formulations, with average costs ranging from 200-800 USD per kilogram depending on performance specifications and biocompatibility certifications. This represents a 30-50% premium over industrial-grade conductive adhesives, reflecting the stringent requirements and limited supplier base in the biomedical sector.
Future market growth will likely be driven by emerging applications in biodegradable implants, miniaturized devices, and implantable biosensors, creating new opportunities for specialized conductive adhesive formulations. Additionally, increasing focus on personalized medicine is expected to drive demand for customizable adhesive solutions compatible with 3D-printed implant technologies.
Current Challenges in Conductive Adhesive Technology
Despite significant advancements in conductive adhesive technology for biomedical implants, several critical challenges persist that limit their widespread adoption and long-term efficacy. The primary concern remains the biocompatibility-conductivity trade-off, where materials exhibiting excellent electrical properties often demonstrate poor biocompatibility, and vice versa. This fundamental contradiction has proven difficult to resolve, particularly when considering the diverse tissue environments encountered in implantable applications.
Mechanical stability presents another significant hurdle, as conductive adhesives must maintain consistent electrical performance while withstanding the dynamic mechanical stresses of the human body. Current formulations often experience conductivity degradation over time due to micro-fractures and delamination at the tissue-device interface, compromising long-term device functionality and patient safety.
The biostability of conductive adhesives in the aggressive physiological environment poses substantial challenges. Exposure to body fluids, enzymatic activity, and varying pH levels can accelerate material degradation, leading to potential release of toxic components and premature device failure. Research indicates that even state-of-the-art conductive adhesives show significant performance deterioration after 6-12 months of implantation, falling short of the multi-year lifespans required for many implantable devices.
Manufacturing scalability and reproducibility represent additional technical barriers. Current production methods for high-performance conductive adhesives often involve complex processes that are difficult to standardize and scale. This results in batch-to-batch variations that compromise quality control and regulatory compliance, particularly problematic for medical-grade materials subject to stringent approval processes.
The integration of conductive adhesives with existing implant manufacturing workflows presents compatibility issues. Many advanced formulations require specialized curing conditions or handling protocols that conflict with established production methods for biomedical implants, creating significant implementation barriers for device manufacturers.
Regulatory hurdles further complicate advancement in this field. Novel conductive adhesive formulations face extensive testing requirements and lengthy approval processes, with regulatory frameworks struggling to keep pace with technological innovations. This regulatory landscape often discourages investment in breakthrough technologies that might otherwise address current limitations.
Cost considerations also remain prohibitive for many promising materials. High-performance conductive adhesives frequently incorporate expensive components such as noble metals or specialized polymers, driving up production costs and limiting accessibility for broader medical applications. The economic viability of these materials represents a significant constraint on their development and adoption.
Mechanical stability presents another significant hurdle, as conductive adhesives must maintain consistent electrical performance while withstanding the dynamic mechanical stresses of the human body. Current formulations often experience conductivity degradation over time due to micro-fractures and delamination at the tissue-device interface, compromising long-term device functionality and patient safety.
The biostability of conductive adhesives in the aggressive physiological environment poses substantial challenges. Exposure to body fluids, enzymatic activity, and varying pH levels can accelerate material degradation, leading to potential release of toxic components and premature device failure. Research indicates that even state-of-the-art conductive adhesives show significant performance deterioration after 6-12 months of implantation, falling short of the multi-year lifespans required for many implantable devices.
Manufacturing scalability and reproducibility represent additional technical barriers. Current production methods for high-performance conductive adhesives often involve complex processes that are difficult to standardize and scale. This results in batch-to-batch variations that compromise quality control and regulatory compliance, particularly problematic for medical-grade materials subject to stringent approval processes.
The integration of conductive adhesives with existing implant manufacturing workflows presents compatibility issues. Many advanced formulations require specialized curing conditions or handling protocols that conflict with established production methods for biomedical implants, creating significant implementation barriers for device manufacturers.
Regulatory hurdles further complicate advancement in this field. Novel conductive adhesive formulations face extensive testing requirements and lengthy approval processes, with regulatory frameworks struggling to keep pace with technological innovations. This regulatory landscape often discourages investment in breakthrough technologies that might otherwise address current limitations.
Cost considerations also remain prohibitive for many promising materials. High-performance conductive adhesives frequently incorporate expensive components such as noble metals or specialized polymers, driving up production costs and limiting accessibility for broader medical applications. The economic viability of these materials represents a significant constraint on their development and adoption.
Existing Conductive Adhesive Solutions for Implants
01 Metal-filled conductive adhesives
Metal-filled conductive adhesives incorporate metallic particles such as silver, gold, copper, or nickel to create electrical conductivity. These adhesives typically consist of a polymer matrix loaded with metal fillers that form conductive pathways when cured. The concentration and distribution of metal particles significantly affect the conductivity, with higher metal loading generally providing better electrical performance. These adhesives are widely used in electronics assembly, particularly for applications requiring both mechanical bonding and electrical connectivity.- Metal-filled conductive adhesives: Metal-filled conductive adhesives incorporate metallic particles such as silver, gold, copper, or nickel to create electrical conductivity. These particles form conductive pathways when the adhesive cures, allowing for electrical connections between components. The metal fillers can be in various forms including flakes, spheres, or nanowires, with the concentration and distribution affecting the overall conductivity. These adhesives are widely used in electronics assembly, particularly in applications where traditional soldering is not suitable.
- Carbon-based conductive adhesives: Carbon-based conductive adhesives utilize carbon materials such as graphite, carbon black, carbon nanotubes, or graphene as conductive fillers. These materials provide electrical conductivity while often being more cost-effective than metal-filled alternatives. Carbon-based adhesives typically offer moderate conductivity suitable for applications like EMI shielding, static dissipation, and some electronic interconnects. The unique properties of carbon nanomaterials can also provide additional benefits such as thermal conductivity and mechanical reinforcement to the adhesive matrix.
- Anisotropic conductive adhesives: Anisotropic conductive adhesives (ACAs) provide electrical conductivity in one direction while maintaining insulation in others. These specialized adhesives contain conductive particles suspended in an insulating adhesive matrix. When compressed between electrical contacts, the particles form conductive paths only in the direction of compression. This unique property makes ACAs ideal for fine-pitch electronics connections, display technologies, and flexible circuit applications where controlled, directional conductivity is required without the risk of short circuits.
- Thermally conductive adhesives: Thermally conductive adhesives are designed to transfer heat efficiently while providing bonding strength. These adhesives incorporate fillers such as ceramic particles, metal oxides, or boron nitride to enhance thermal conductivity. They are crucial in electronics applications where heat dissipation is essential for device performance and reliability. These adhesives create thermal pathways between heat-generating components and heat sinks or other cooling structures, while simultaneously providing mechanical attachment and sometimes electrical insulation or conductivity depending on the specific formulation.
- Environmentally friendly conductive adhesives: Environmentally friendly conductive adhesives are formulated to reduce or eliminate hazardous substances while maintaining electrical performance. These adhesives often replace lead-based solders and other toxic components with more sustainable alternatives. Innovations include bio-based resins, reduced VOC content, and recyclable formulations. These green alternatives address increasing regulatory requirements and market demand for environmentally responsible electronics manufacturing while providing comparable electrical conductivity, adhesion strength, and reliability to conventional conductive adhesives.
02 Carbon-based conductive adhesives
Carbon-based conductive adhesives utilize carbon materials such as graphene, carbon nanotubes, or carbon black as conductive fillers. These materials offer advantages including lighter weight, lower cost compared to precious metals, and resistance to oxidation. Carbon-based fillers can provide both electrical and thermal conductivity while maintaining flexibility in the cured adhesive. These formulations are particularly valuable in applications where weight considerations are important or where metal migration might cause reliability issues.Expand Specific Solutions03 Anisotropic conductive adhesives
Anisotropic conductive adhesives (ACAs) provide electrical conductivity in one direction while maintaining insulation in others. These specialized adhesives typically contain conductive particles dispersed in an insulating adhesive matrix. When compressed between electrical contacts, the particles form conductive pathways in the z-direction while remaining electrically isolated in the x and y directions. This technology is particularly valuable for fine-pitch electronics connections, display technologies, and flexible circuit applications where directional conductivity is required.Expand Specific Solutions04 Thermally conductive adhesives
Thermally conductive adhesives are designed to transfer heat while providing adhesive properties. These formulations typically incorporate thermally conductive fillers such as ceramic particles, metal oxides, or specialized materials like boron nitride. While some thermally conductive adhesives also provide electrical conductivity, others are specifically engineered to be electrically insulating while maximizing thermal transfer. These materials are crucial for heat management in electronic devices, LED applications, and power electronics where efficient heat dissipation is essential for reliability and performance.Expand Specific Solutions05 Environmentally friendly conductive adhesives
Environmentally friendly conductive adhesives address concerns about toxicity and environmental impact by eliminating or reducing hazardous materials like lead and certain solvents. These formulations often use bio-based polymers, water-based systems, or other sustainable materials as the adhesive matrix. Conductive fillers may include alternatives to traditional metals or use reduced amounts of precious metals through innovative dispersion techniques. These adhesives are designed to meet increasingly stringent environmental regulations while maintaining the necessary electrical, thermal, and mechanical properties for electronic applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The conductive adhesives market in biomedical implants is currently in a growth phase, with increasing demand driven by miniaturization trends and biocompatibility requirements. The global market is estimated to reach $4-5 billion by 2027, expanding at a CAGR of approximately 6-8%. Leading players include established medical technology companies like Medtronic and Cochlear, alongside materials specialists such as 3M, Henkel, and Sekisui Chemical. Academic institutions including MIT, California Institute of Technology, and the University of California are advancing fundamental research, while companies like DuPont Electronics and Dexerials are developing specialized formulations with enhanced conductivity and biocompatibility. The technology is maturing but still faces challenges in long-term stability and regulatory approval for implantable applications.
Henkel AG & Co. KGaA
Technical Solution: Henkel has developed an advanced portfolio of biomedical-grade conductive adhesives specifically engineered for implantable medical devices. Their LOCTITE® Medical series incorporates biocompatible silver-filled epoxy systems that provide reliable electrical conductivity while meeting stringent biocompatibility requirements. Henkel's technology utilizes proprietary surface modification of conductive particles to enhance dispersion stability and prevent agglomeration, resulting in consistent electrical properties throughout the adhesive matrix. Their formulations feature controlled curing profiles that minimize thermal stress on sensitive electronic components and biological tissues during assembly[3]. The company has pioneered hybrid adhesive systems that combine conductive and non-conductive regions within a single application, allowing for precise control of signal pathways in complex implantable devices. Henkel's materials undergo comprehensive biocompatibility testing according to ISO 10993 standards, with documented performance in long-term implantation studies showing minimal tissue reaction and stable electrical properties over 7+ years of simulated use[4].
Strengths: Extensive portfolio covering various conductivity ranges and mechanical properties; excellent manufacturing scalability with consistent batch-to-batch performance; comprehensive regulatory documentation supporting medical device approvals. Weaknesses: Some formulations exhibit higher moisture sensitivity than competing technologies; relatively rigid after curing which may limit applications in highly flexible implant designs.
Massachusetts Institute of Technology
Technical Solution: MIT has developed groundbreaking conductive adhesive technologies for biomedical implants through their Institute for Medical Engineering and Science. Their approach centers on novel nanocomposite materials that combine carbon nanotubes with biocompatible hydrogels to create soft, stretchable conductive interfaces that closely match the mechanical properties of biological tissues. MIT researchers have pioneered stimulus-responsive conductive adhesives that can modulate their properties in response to physiological signals, enabling "smart" interfaces that adapt to changing biological conditions[7]. Their materials incorporate specialized surface chemistry that enhances both tissue integration and electrode adhesion, creating stable interfaces that resist delamination under mechanical stress. MIT's conductive adhesives feature self-healing capabilities that can restore electrical pathways after mechanical damage, significantly enhancing the longevity of implanted electronic systems. Their research has demonstrated biocompatible conductive materials with unprecedented stretchability (up to 600% strain while maintaining conductivity) and tissue-matching elastic moduli (5-100 kPa), properties that are critical for interfaces with dynamic tissues such as cardiac and neural applications[8]. MIT's technology incorporates biodegradable components that allow for controlled remodeling of the electrode-tissue interface over time, potentially reducing long-term foreign body responses.
Strengths: Exceptional mechanical compatibility with soft tissues; innovative self-healing and adaptive properties; potential for reduced long-term foreign body response through controlled biodegradation. Weaknesses: Early-stage technology with limited long-term in vivo validation; complex manufacturing processes that may challenge commercial scalability; current formulations may have lower conductivity than traditional metal-based systems.
Key Patents and Innovations in Biocompatible Conductives
Conductive adhesives and biomedical articles including same
PatentActiveEP1917318A1
Innovation
- A conductive adhesive composition comprising a pressure-sensitive adhesive, a water-soluble or water-dispersible organic chloride electrolyte, and a humectant, which forms a bicontinuous structure to maintain electrical conductivity even under low humidity conditions, using a microemulsion process to create a polymerized adhesive with improved moisture retention and stability.
Electrically conductive adhesive
PatentActiveEP3940035A1
Innovation
- An electrically conductive adhesive comprising a (meth)acrylate monomer, a biocompatible polymer, a biocompatible metal with a median particle size below 50 µm, and a polymerization initiator, which forms a durable and conductive bond suitable for implantable medical devices under mild conditions.
Biocompatibility and Safety Standards
Biocompatibility and safety standards for conductive adhesives in biomedical implants represent a critical framework that ensures these materials can function effectively while maintaining patient safety. The ISO 10993 series serves as the cornerstone for evaluating biocompatibility of medical devices, with specific attention to ISO 10993-1 for risk assessment and ISO 10993-5 for cytotoxicity testing. These standards require rigorous evaluation of material interactions with biological systems, including tissue response, systemic toxicity, and long-term compatibility.
For conductive adhesives specifically, additional considerations include leaching of conductive particles (often silver or gold) and potential immune responses to polymer matrices. The FDA's guidance document "Use of International Standard ISO 10993-1" provides regulatory pathways for manufacturers seeking approval for implantable devices utilizing conductive adhesives, emphasizing the need for comprehensive biological evaluation plans.
Material safety data must demonstrate minimal cytotoxicity, absence of genotoxicity, and negligible immunogenicity. The ASTM F748 standard for selecting biological tests for materials and devices is frequently applied alongside IEC 60601-1 for electrical medical equipment safety. These standards collectively ensure that conductive adhesives maintain their electrical properties without compromising biological safety.
Recent developments in safety standards have begun addressing nanomaterials in conductive adhesives, as carbon nanotubes and graphene increasingly appear in newer formulations. The ISO/TR 10993-22:2017 provides guidance on nanomaterial considerations, though regulatory frameworks continue to evolve in this area. Manufacturers must demonstrate that nanoparticle migration from adhesives remains below established thresholds throughout the implant's lifetime.
Biocompatibility testing protocols typically include in vitro cytotoxicity assays, sensitization studies, irritation tests, systemic toxicity evaluations, and implantation studies. For long-term implants, additional carcinogenicity and biodegradation assessments become mandatory. The European Union's Medical Device Regulation (MDR) imposes particularly stringent requirements for Class III implantable devices, requiring extensive clinical evidence of safety.
Electrical safety standards intersect with biocompatibility considerations, as leakage currents must remain within physiologically safe limits. The IEC 60601 series establishes these parameters, while ANSI/AAMI VP20:2015 addresses biological evaluation planning specifically for implantable electronic devices. Manufacturers must demonstrate that their conductive adhesives maintain stable electrical properties without generating harmful currents or electromagnetic interference with other medical devices.
For conductive adhesives specifically, additional considerations include leaching of conductive particles (often silver or gold) and potential immune responses to polymer matrices. The FDA's guidance document "Use of International Standard ISO 10993-1" provides regulatory pathways for manufacturers seeking approval for implantable devices utilizing conductive adhesives, emphasizing the need for comprehensive biological evaluation plans.
Material safety data must demonstrate minimal cytotoxicity, absence of genotoxicity, and negligible immunogenicity. The ASTM F748 standard for selecting biological tests for materials and devices is frequently applied alongside IEC 60601-1 for electrical medical equipment safety. These standards collectively ensure that conductive adhesives maintain their electrical properties without compromising biological safety.
Recent developments in safety standards have begun addressing nanomaterials in conductive adhesives, as carbon nanotubes and graphene increasingly appear in newer formulations. The ISO/TR 10993-22:2017 provides guidance on nanomaterial considerations, though regulatory frameworks continue to evolve in this area. Manufacturers must demonstrate that nanoparticle migration from adhesives remains below established thresholds throughout the implant's lifetime.
Biocompatibility testing protocols typically include in vitro cytotoxicity assays, sensitization studies, irritation tests, systemic toxicity evaluations, and implantation studies. For long-term implants, additional carcinogenicity and biodegradation assessments become mandatory. The European Union's Medical Device Regulation (MDR) imposes particularly stringent requirements for Class III implantable devices, requiring extensive clinical evidence of safety.
Electrical safety standards intersect with biocompatibility considerations, as leakage currents must remain within physiologically safe limits. The IEC 60601 series establishes these parameters, while ANSI/AAMI VP20:2015 addresses biological evaluation planning specifically for implantable electronic devices. Manufacturers must demonstrate that their conductive adhesives maintain stable electrical properties without generating harmful currents or electromagnetic interference with other medical devices.
Long-term Stability and Degradation Analysis
The long-term stability of conductive adhesives in biomedical implants represents a critical concern for ensuring device functionality and patient safety over extended periods. Current research indicates that most conductive adhesives demonstrate initial stability periods ranging from 3-5 years in vivo, though significant variations exist depending on material composition and environmental conditions. The primary degradation mechanisms observed include oxidative stress, hydrolytic degradation, and enzymatic breakdown of polymer matrices.
Environmental factors within the human body present unique challenges to adhesive stability. The constant presence of moisture, varying pH levels (typically 7.2-7.4 but fluctuating in different tissue environments), and elevated temperatures (37°C) accelerate degradation processes compared to standard laboratory testing conditions. Notably, studies by Zhang et al. (2022) demonstrated that silver-filled epoxy adhesives exhibited a 15-30% reduction in conductivity after simulated aging equivalent to two years of implantation.
Ionic interactions between bodily fluids and conductive fillers represent another significant degradation pathway. Silver nanoparticles, commonly used in conductive adhesives, may gradually dissolve and migrate from the adhesive matrix, leading to both decreased conductivity and potential toxicity concerns. Gold-based conductive fillers demonstrate superior stability but at significantly higher cost implications for commercial applications.
Mechanical stresses from surrounding tissues and micro-movements of implanted devices contribute to interfacial failures between adhesive layers and substrate materials. Research by Kang and colleagues (2023) revealed that cyclic loading equivalent to normal body movements resulted in progressive delamination at adhesive interfaces after approximately 18 months of simulated use, particularly in applications involving flexible substrates.
Advanced characterization techniques including electrochemical impedance spectroscopy (EIS) and accelerated aging protocols have enabled more accurate prediction of long-term performance. Recent developments in degradation modeling suggest that incorporating sacrificial antioxidants and moisture barriers can extend functional lifetimes by 40-60% compared to conventional formulations.
The biocompatibility of degradation products remains insufficiently characterized in current literature. While initial adhesive formulations undergo rigorous biocompatibility testing, the long-term effects of degradation byproducts require further investigation. Preliminary studies indicate that certain degradation products from silver-epoxy systems may trigger localized inflammatory responses after extended implantation periods exceeding three years.
Emerging strategies to enhance long-term stability include the development of self-healing adhesive matrices, incorporation of corrosion inhibitors, and novel encapsulation approaches using hydrophobic barrier layers. These innovations show promise for extending functional lifetimes beyond the current 5-year benchmark that represents the industry standard for many implantable electronic devices.
Environmental factors within the human body present unique challenges to adhesive stability. The constant presence of moisture, varying pH levels (typically 7.2-7.4 but fluctuating in different tissue environments), and elevated temperatures (37°C) accelerate degradation processes compared to standard laboratory testing conditions. Notably, studies by Zhang et al. (2022) demonstrated that silver-filled epoxy adhesives exhibited a 15-30% reduction in conductivity after simulated aging equivalent to two years of implantation.
Ionic interactions between bodily fluids and conductive fillers represent another significant degradation pathway. Silver nanoparticles, commonly used in conductive adhesives, may gradually dissolve and migrate from the adhesive matrix, leading to both decreased conductivity and potential toxicity concerns. Gold-based conductive fillers demonstrate superior stability but at significantly higher cost implications for commercial applications.
Mechanical stresses from surrounding tissues and micro-movements of implanted devices contribute to interfacial failures between adhesive layers and substrate materials. Research by Kang and colleagues (2023) revealed that cyclic loading equivalent to normal body movements resulted in progressive delamination at adhesive interfaces after approximately 18 months of simulated use, particularly in applications involving flexible substrates.
Advanced characterization techniques including electrochemical impedance spectroscopy (EIS) and accelerated aging protocols have enabled more accurate prediction of long-term performance. Recent developments in degradation modeling suggest that incorporating sacrificial antioxidants and moisture barriers can extend functional lifetimes by 40-60% compared to conventional formulations.
The biocompatibility of degradation products remains insufficiently characterized in current literature. While initial adhesive formulations undergo rigorous biocompatibility testing, the long-term effects of degradation byproducts require further investigation. Preliminary studies indicate that certain degradation products from silver-epoxy systems may trigger localized inflammatory responses after extended implantation periods exceeding three years.
Emerging strategies to enhance long-term stability include the development of self-healing adhesive matrices, incorporation of corrosion inhibitors, and novel encapsulation approaches using hydrophobic barrier layers. These innovations show promise for extending functional lifetimes beyond the current 5-year benchmark that represents the industry standard for many implantable electronic devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!