Conductive Adhesives in Wearable Health Tech: Regulatory Overview
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Conductive Adhesives Evolution and Objectives
Conductive adhesives have evolved significantly over the past decades, transforming from simple electrical connection materials to sophisticated components critical for wearable health technology. The journey began in the 1950s with basic silver-filled epoxies primarily used in electronic circuits. By the 1970s, these adhesives had advanced to incorporate various conductive fillers including carbon, copper, and nickel, expanding their application potential.
The 1990s marked a pivotal shift with the development of anisotropic conductive adhesives (ACAs), which allowed for directional conductivity—a breakthrough for miniaturized electronics. This innovation paved the way for the integration of conductive adhesives in medical applications, particularly as biocompatible interfaces between electronic components and human skin.
In the early 2000s, the emergence of stretchable conductive adhesives represented another significant milestone, addressing the flexibility requirements essential for wearable devices. These materials could maintain electrical conductivity while accommodating the natural movement of the human body, a critical feature for continuous health monitoring applications.
Recent advancements have focused on enhancing biocompatibility, reducing skin irritation, and improving moisture resistance—all crucial factors for extended wear in health monitoring scenarios. The development of hypoallergenic formulations has expanded the potential user base for wearable health technologies, while innovations in adhesive chemistry have improved durability under various environmental conditions.
The primary objective of current conductive adhesive research for wearable health technology centers on achieving an optimal balance between electrical performance, mechanical properties, and biocompatibility. Researchers aim to develop adhesives that maintain stable electrical connections during physical activity while minimizing skin irritation during prolonged contact.
Additional technical goals include reducing signal interference in biometric measurements, enhancing adhesion in humid conditions (such as during perspiration), and developing formulations that comply with increasingly stringent international regulatory standards for medical devices.
Looking forward, the field is moving toward smart conductive adhesives with integrated sensing capabilities, self-healing properties to extend product lifespan, and environmentally sustainable formulations that reduce ecological impact while maintaining performance standards. These objectives align with the broader trend toward personalized, continuous health monitoring through unobtrusive wearable technology.
The 1990s marked a pivotal shift with the development of anisotropic conductive adhesives (ACAs), which allowed for directional conductivity—a breakthrough for miniaturized electronics. This innovation paved the way for the integration of conductive adhesives in medical applications, particularly as biocompatible interfaces between electronic components and human skin.
In the early 2000s, the emergence of stretchable conductive adhesives represented another significant milestone, addressing the flexibility requirements essential for wearable devices. These materials could maintain electrical conductivity while accommodating the natural movement of the human body, a critical feature for continuous health monitoring applications.
Recent advancements have focused on enhancing biocompatibility, reducing skin irritation, and improving moisture resistance—all crucial factors for extended wear in health monitoring scenarios. The development of hypoallergenic formulations has expanded the potential user base for wearable health technologies, while innovations in adhesive chemistry have improved durability under various environmental conditions.
The primary objective of current conductive adhesive research for wearable health technology centers on achieving an optimal balance between electrical performance, mechanical properties, and biocompatibility. Researchers aim to develop adhesives that maintain stable electrical connections during physical activity while minimizing skin irritation during prolonged contact.
Additional technical goals include reducing signal interference in biometric measurements, enhancing adhesion in humid conditions (such as during perspiration), and developing formulations that comply with increasingly stringent international regulatory standards for medical devices.
Looking forward, the field is moving toward smart conductive adhesives with integrated sensing capabilities, self-healing properties to extend product lifespan, and environmentally sustainable formulations that reduce ecological impact while maintaining performance standards. These objectives align with the broader trend toward personalized, continuous health monitoring through unobtrusive wearable technology.
Market Analysis for Wearable Health Monitoring Adhesives
The global market for conductive adhesives in wearable health monitoring devices is experiencing robust growth, driven by increasing consumer awareness of personal health management and the rising prevalence of chronic diseases requiring continuous monitoring. Current market valuations indicate that the wearable health technology sector is expanding at a compound annual growth rate of approximately 25%, with conductive adhesives representing a critical component segment valued at over 3 billion USD in 2023.
Consumer demand patterns reveal a strong preference for non-invasive, comfortable, and reliable health monitoring solutions that can be worn for extended periods. This has created significant market opportunities for advanced conductive adhesives that offer superior skin compatibility while maintaining consistent electrical conductivity. Market research indicates that approximately 65% of wearable device users cite comfort and adhesive reliability as primary factors influencing purchasing decisions.
The market segmentation shows distinct categories based on application requirements. Medical-grade monitoring devices, including continuous glucose monitors and cardiac event monitors, command premium pricing due to stringent regulatory requirements and performance specifications. Consumer fitness and wellness trackers represent the largest volume segment, with price sensitivity driving innovation in cost-effective adhesive solutions.
Regional market analysis reveals North America as the dominant market, accounting for approximately 40% of global revenue, followed by Europe and Asia-Pacific. However, the Asia-Pacific region demonstrates the fastest growth trajectory, with emerging economies increasingly adopting wearable health technologies as healthcare infrastructure develops.
Key market drivers include the aging global population, increasing healthcare costs prompting preventive health monitoring, and technological advancements enabling more sophisticated and miniaturized wearable devices. The COVID-19 pandemic has accelerated market growth by heightening health consciousness and normalizing remote patient monitoring practices.
Market challenges include price pressures from commoditization of basic wearable technologies, regulatory hurdles for medical-grade applications, and technical challenges in developing adhesives that balance conductivity, biocompatibility, and durability. Additionally, environmental concerns are driving demand for sustainable and biodegradable adhesive formulations.
Future market projections indicate continued strong growth, with particular expansion in specialized segments such as neonatal monitoring, geriatric care, and athletic performance optimization. The integration of artificial intelligence for data analysis and the development of biodegradable conductive adhesives represent emerging trends with significant market potential.
Consumer demand patterns reveal a strong preference for non-invasive, comfortable, and reliable health monitoring solutions that can be worn for extended periods. This has created significant market opportunities for advanced conductive adhesives that offer superior skin compatibility while maintaining consistent electrical conductivity. Market research indicates that approximately 65% of wearable device users cite comfort and adhesive reliability as primary factors influencing purchasing decisions.
The market segmentation shows distinct categories based on application requirements. Medical-grade monitoring devices, including continuous glucose monitors and cardiac event monitors, command premium pricing due to stringent regulatory requirements and performance specifications. Consumer fitness and wellness trackers represent the largest volume segment, with price sensitivity driving innovation in cost-effective adhesive solutions.
Regional market analysis reveals North America as the dominant market, accounting for approximately 40% of global revenue, followed by Europe and Asia-Pacific. However, the Asia-Pacific region demonstrates the fastest growth trajectory, with emerging economies increasingly adopting wearable health technologies as healthcare infrastructure develops.
Key market drivers include the aging global population, increasing healthcare costs prompting preventive health monitoring, and technological advancements enabling more sophisticated and miniaturized wearable devices. The COVID-19 pandemic has accelerated market growth by heightening health consciousness and normalizing remote patient monitoring practices.
Market challenges include price pressures from commoditization of basic wearable technologies, regulatory hurdles for medical-grade applications, and technical challenges in developing adhesives that balance conductivity, biocompatibility, and durability. Additionally, environmental concerns are driving demand for sustainable and biodegradable adhesive formulations.
Future market projections indicate continued strong growth, with particular expansion in specialized segments such as neonatal monitoring, geriatric care, and athletic performance optimization. The integration of artificial intelligence for data analysis and the development of biodegradable conductive adhesives represent emerging trends with significant market potential.
Global Regulatory Landscape and Technical Barriers
The regulatory landscape for conductive adhesives in wearable health technology varies significantly across global markets, creating a complex environment for manufacturers and developers. In the United States, the FDA classifies most wearable health devices incorporating conductive adhesives as Class II medical devices, requiring 510(k) clearance with specific biocompatibility testing under ISO 10993 standards. The FDA has recently increased scrutiny on skin-contact materials, particularly focusing on potential leaching of conductive particles and long-term dermal compatibility.
The European Union implements more stringent requirements through the Medical Device Regulation (MDR) and the General Product Safety Directive (GPSD). Conductive adhesives must comply with RoHS and REACH regulations, with particular attention to heavy metals content and potential allergens. The EU's emphasis on the precautionary principle has resulted in stricter limitations on certain conductive fillers, including silver nanoparticles, which are commonly used in medical-grade conductive adhesives.
In Asia, regulatory frameworks show significant variation. Japan's PMDA requires extensive biocompatibility documentation similar to FDA standards but adds specific aging tests for adhesives in variable humidity environments. China's NMPA has implemented new regulations specifically addressing wearable medical technologies, with mandatory testing for electromagnetic interference and skin sensitization for conductive materials used in continuous-wear applications.
Technical barriers to global compliance include the challenge of meeting divergent material composition requirements across jurisdictions. For instance, certain conductive fillers permitted in the US market may be restricted in the EU or Japan. This necessitates either market-specific formulations or the development of universally compliant adhesive systems, significantly increasing R&D costs and time-to-market.
Another significant barrier is the lack of harmonized testing protocols for novel conductive materials. While traditional medical adhesives have established testing frameworks, hybrid conductive adhesives combining electrical and adhesive properties fall into regulatory gray areas. This has led to inconsistent approval processes and unpredictable timelines for innovative products.
Emerging markets present additional challenges, with countries like India and Brazil developing their own regulatory frameworks for wearable health technologies. These evolving regulations often incorporate elements from established systems but may include unique requirements reflecting local environmental conditions or healthcare priorities, further complicating global compliance strategies for manufacturers.
The European Union implements more stringent requirements through the Medical Device Regulation (MDR) and the General Product Safety Directive (GPSD). Conductive adhesives must comply with RoHS and REACH regulations, with particular attention to heavy metals content and potential allergens. The EU's emphasis on the precautionary principle has resulted in stricter limitations on certain conductive fillers, including silver nanoparticles, which are commonly used in medical-grade conductive adhesives.
In Asia, regulatory frameworks show significant variation. Japan's PMDA requires extensive biocompatibility documentation similar to FDA standards but adds specific aging tests for adhesives in variable humidity environments. China's NMPA has implemented new regulations specifically addressing wearable medical technologies, with mandatory testing for electromagnetic interference and skin sensitization for conductive materials used in continuous-wear applications.
Technical barriers to global compliance include the challenge of meeting divergent material composition requirements across jurisdictions. For instance, certain conductive fillers permitted in the US market may be restricted in the EU or Japan. This necessitates either market-specific formulations or the development of universally compliant adhesive systems, significantly increasing R&D costs and time-to-market.
Another significant barrier is the lack of harmonized testing protocols for novel conductive materials. While traditional medical adhesives have established testing frameworks, hybrid conductive adhesives combining electrical and adhesive properties fall into regulatory gray areas. This has led to inconsistent approval processes and unpredictable timelines for innovative products.
Emerging markets present additional challenges, with countries like India and Brazil developing their own regulatory frameworks for wearable health technologies. These evolving regulations often incorporate elements from established systems but may include unique requirements reflecting local environmental conditions or healthcare priorities, further complicating global compliance strategies for manufacturers.
Current Conductive Adhesive Solutions for Wearables
01 Metal-filled conductive adhesives
Metal-filled conductive adhesives incorporate metallic particles such as silver, gold, copper, or nickel to create electrical conductivity. These adhesives typically consist of a polymer matrix loaded with metal fillers that form conductive pathways when cured. The concentration and distribution of metal particles directly affect the conductivity level. These adhesives are widely used in electronics assembly, providing both mechanical bonding and electrical connectivity between components.- Metal-filled conductive adhesives: Metal-filled conductive adhesives incorporate metallic particles such as silver, gold, copper, or nickel to create electrical conductivity. These particles form conductive pathways when the adhesive is cured. The concentration and type of metal filler significantly affect the conductivity level, with silver typically providing the highest conductivity. These adhesives are widely used in electronics assembly, semiconductor packaging, and circuit board manufacturing where electrical connections need to be maintained while providing mechanical bonding.
- Carbon-based conductive adhesives: Carbon-based conductive adhesives utilize carbon materials such as graphite, carbon black, carbon nanotubes, or graphene as conductive fillers. These materials provide moderate conductivity at lower cost compared to metal fillers. Carbon nanotubes and graphene offer excellent electrical properties while requiring lower loading levels. These adhesives are particularly useful in applications requiring static dissipation, electromagnetic interference (EMI) shielding, or moderate conductivity with enhanced mechanical properties.
- Anisotropic conductive adhesives: Anisotropic conductive adhesives (ACAs) provide electrical conductivity in one direction while maintaining insulation in others. They typically contain conductive particles dispersed in an insulating adhesive matrix. When compressed between electrical contacts, the particles form conductive paths only in the direction of compression. This technology is particularly valuable for fine-pitch connections in display technologies, flexible electronics, and high-density interconnects where preventing short circuits between adjacent connections is critical.
- Thermally conductive adhesives: Thermally conductive adhesives are designed to transfer heat while providing bonding strength. They incorporate thermally conductive fillers such as aluminum oxide, boron nitride, or aluminum nitride in a polymer matrix. These adhesives are crucial in electronic device assembly where heat dissipation is essential for component reliability and performance. They provide an alternative to mechanical fasteners and can reduce thermal interface resistance between heat-generating components and heat sinks.
- Environmentally friendly conductive adhesives: Environmentally friendly conductive adhesives are formulated to reduce or eliminate hazardous substances while maintaining electrical performance. These formulations often replace lead-based solders or adhesives containing volatile organic compounds with more sustainable alternatives. Bio-based polymers, water-based systems, and lead-free conductive fillers are incorporated to meet increasingly stringent environmental regulations while providing reliable electrical connections. These adhesives are particularly important in consumer electronics, medical devices, and automotive applications subject to RoHS and REACH compliance.
02 Carbon-based conductive adhesives
Carbon-based conductive adhesives utilize carbon materials such as graphite, carbon black, carbon nanotubes, or graphene as conductive fillers. These materials offer advantages including lower cost compared to precious metals, lighter weight, and corrosion resistance. Carbon-based adhesives provide moderate conductivity suitable for applications requiring electromagnetic interference (EMI) shielding, static dissipation, or moderate current flow. The unique properties of carbon nanomaterials can enhance both electrical and mechanical performance of the adhesive.Expand Specific Solutions03 Anisotropic conductive adhesives
Anisotropic conductive adhesives (ACAs) provide electrical conductivity in a specific direction while maintaining insulation in other directions. These specialized adhesives contain conductive particles suspended in an insulating adhesive matrix. When compressed between electrical contacts, the particles form conductive pathways only in the direction of compression. This technology is particularly valuable for fine-pitch electronics connections, display technologies, and flexible circuit applications where directional conductivity is required.Expand Specific Solutions04 Thermally conductive adhesives
Thermally conductive adhesives are designed to transfer heat while providing adhesive properties. These formulations incorporate thermally conductive fillers such as aluminum oxide, boron nitride, or metal particles within an adhesive matrix. They serve dual functions by creating mechanical bonds between components while efficiently dissipating heat. These adhesives are crucial in electronics cooling applications, LED assemblies, power electronics, and other heat-generating devices where thermal management is essential for performance and reliability.Expand Specific Solutions05 Environmentally friendly conductive adhesives
Environmentally friendly conductive adhesives address sustainability concerns by eliminating toxic components like lead while maintaining electrical performance. These formulations use bio-based polymers, water-based systems, or renewable materials as alternatives to traditional solvent-based adhesives. They may incorporate novel conductive fillers that reduce environmental impact while providing necessary conductivity. These green alternatives are increasingly important as electronics manufacturing faces stricter environmental regulations and sustainability requirements.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The conductive adhesives market in wearable health technology is currently in a growth phase, with increasing regulatory scrutiny as the sector matures. Market size is expanding rapidly due to rising demand for non-invasive monitoring devices, with projections indicating substantial growth over the next five years. Technologically, the field shows varying maturity levels across applications, with companies demonstrating different specialization areas. Industry leaders like Henkel AG, 3M Innovative Properties, and Dexerials Corp. have established strong positions through advanced formulations meeting biocompatibility requirements, while medical device manufacturers such as Medtronic, DexCom, and Abbott Diabetes Care are driving innovation through integration of these adhesives in FDA-approved wearable health monitoring systems. Academic institutions like MIT and Sichuan University are contributing fundamental research to address persistent challenges in skin-compatible conductive interfaces.
Henkel AG & Co. KGaA
Technical Solution: Henkel has developed LOCTITE Ablestik ICP conductive adhesives specifically engineered for wearable health monitoring devices. These adhesives utilize silver-filled epoxy technology that maintains electrical conductivity while offering biocompatibility for skin contact applications. Their formulations comply with ISO 10993 standards for biocompatibility and meet FDA requirements for medical devices. Henkel's regulatory approach includes comprehensive documentation packages that address both EU Medical Device Regulation (MDR) and FDA 510(k) pathways, facilitating faster approval processes for their customers. Their conductive adhesives feature controlled ion migration properties to prevent skin irritation while maintaining signal integrity in high-moisture environments typical in health monitoring applications. Henkel also provides specialized low-temperature curing options (below 80°C) to accommodate temperature-sensitive components in wearable devices.
Strengths: Extensive regulatory documentation support across global markets; formulations specifically designed for skin-contact applications with proven biocompatibility. Weaknesses: Higher cost compared to non-medical grade alternatives; some formulations require specialized curing equipment that may increase manufacturing complexity.
3M Innovative Properties Co.
Technical Solution: 3M has pioneered electrically conductive adhesive transfer tapes (ECATTs) specifically designed for wearable health monitoring devices. Their proprietary technology combines anisotropic electrical conductivity (z-direction) with biocompatible adhesive matrices that meet ISO 10993 standards for cytotoxicity, sensitization, and irritation. 3M's regulatory strategy includes master file submissions to the FDA, allowing medical device manufacturers to reference their materials in regulatory submissions. Their conductive adhesives incorporate moisture-resistant properties that maintain electrical performance in high-perspiration environments while adhering to strict leachable and extractable limits required for medical applications. 3M's manufacturing processes comply with ISO 13485 medical device quality management systems, ensuring batch-to-batch consistency critical for regulatory compliance. Their adhesives feature controlled impedance characteristics specifically calibrated for biopotential signal acquisition in ECG and EMG applications.
Strengths: Extensive regulatory expertise with established FDA master files; manufacturing consistency with validated processes that simplify customer qualification. Weaknesses: Limited customization options for specialized applications; higher minimum order quantities that may challenge smaller wearable device manufacturers.
Key Patents and Scientific Breakthroughs
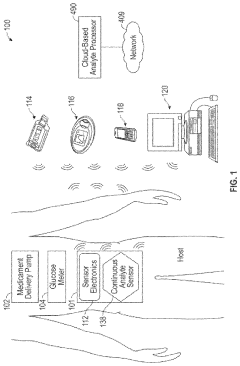
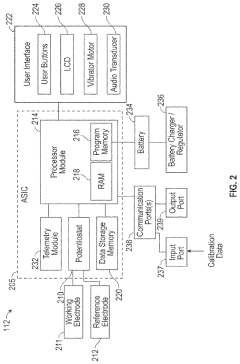
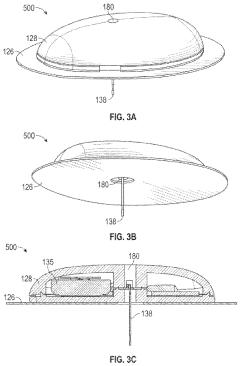
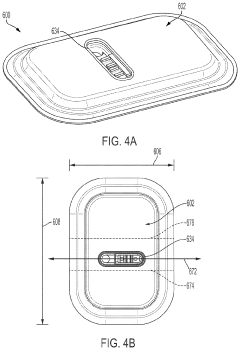
Miniaturized wearable devices for analyte measurement
PatentPendingUS20240215876A1
Innovation
- An on-skin wearable medical device with a transcutaneous analyte sensor and a body made from liquid crystal polymer, featuring a housing with bending sections and anisotropic conductive adhesive electrical conduits, designed to be minimally invasive and provide continuous glucose monitoring.
Patent
Innovation
- Development of stretchable conductive adhesives with maintained electrical conductivity under mechanical deformation for wearable health monitoring devices.
- Integration of self-healing properties in conductive adhesives to extend the lifespan of wearable health tech devices under repeated mechanical stress.
- Novel multi-functional adhesive systems that combine electrical conductivity with antimicrobial properties to address infection control concerns in medical wearables.
Biocompatibility and Skin Safety Standards
Biocompatibility and skin safety standards represent critical regulatory considerations for conductive adhesives used in wearable health technology. The prolonged skin contact inherent in wearable devices necessitates rigorous testing and compliance with established international standards to ensure user safety and prevent adverse reactions.
ISO 10993 series, particularly ISO 10993-10 for skin irritation and sensitization testing, forms the cornerstone of biocompatibility assessment for these materials. This standard requires manufacturers to evaluate potential cytotoxicity, sensitization, and irritation effects through validated laboratory protocols. For wearable health technologies utilizing conductive adhesives, compliance with these standards is mandatory for market approval in most jurisdictions.
The FDA's guidance document "Use of International Standard ISO 10993-1" provides specific recommendations for biological evaluation of medical devices with skin contact. For conductive adhesives in wearable health tech, the FDA typically requires submission of biocompatibility data as part of the 510(k) or De Novo pathway, depending on the device classification and intended use.
In the European market, the Medical Device Regulation (MDR 2017/745) imposes stringent requirements for skin-contacting materials. Conductive adhesives must demonstrate compliance with General Safety and Performance Requirements (GSPRs), with particular emphasis on chemical, physical, and biological properties that might affect biocompatibility.
Beyond regulatory compliance, industry standards such as AAMI/ANSI/ISO 10993-23:2021 provide specific protocols for assessing irritation in medical devices. This standard introduces in vitro methods that can potentially reduce animal testing while maintaining rigorous safety evaluation.
For wearable devices intended for sensitive populations such as neonates or individuals with compromised skin integrity, additional testing parameters may be required. These often include extended wear testing and evaluation under various environmental conditions to simulate real-world usage scenarios.
Manufacturers must also consider the potential for cumulative irritation from repeated application and removal of adhesives, particularly for devices designed for long-term monitoring. The OECD Test Guideline 439 for in vitro skin irritation provides validated methods for assessing such risks without reliance on animal models.
Recent regulatory trends indicate increasing scrutiny of chemical components in adhesives, particularly regarding potential endocrine disruptors and allergens. The EU's REACH regulation and similar global initiatives have expanded the list of restricted substances that may be present in skin-contacting materials, requiring manufacturers to continuously update formulations and testing protocols.
ISO 10993 series, particularly ISO 10993-10 for skin irritation and sensitization testing, forms the cornerstone of biocompatibility assessment for these materials. This standard requires manufacturers to evaluate potential cytotoxicity, sensitization, and irritation effects through validated laboratory protocols. For wearable health technologies utilizing conductive adhesives, compliance with these standards is mandatory for market approval in most jurisdictions.
The FDA's guidance document "Use of International Standard ISO 10993-1" provides specific recommendations for biological evaluation of medical devices with skin contact. For conductive adhesives in wearable health tech, the FDA typically requires submission of biocompatibility data as part of the 510(k) or De Novo pathway, depending on the device classification and intended use.
In the European market, the Medical Device Regulation (MDR 2017/745) imposes stringent requirements for skin-contacting materials. Conductive adhesives must demonstrate compliance with General Safety and Performance Requirements (GSPRs), with particular emphasis on chemical, physical, and biological properties that might affect biocompatibility.
Beyond regulatory compliance, industry standards such as AAMI/ANSI/ISO 10993-23:2021 provide specific protocols for assessing irritation in medical devices. This standard introduces in vitro methods that can potentially reduce animal testing while maintaining rigorous safety evaluation.
For wearable devices intended for sensitive populations such as neonates or individuals with compromised skin integrity, additional testing parameters may be required. These often include extended wear testing and evaluation under various environmental conditions to simulate real-world usage scenarios.
Manufacturers must also consider the potential for cumulative irritation from repeated application and removal of adhesives, particularly for devices designed for long-term monitoring. The OECD Test Guideline 439 for in vitro skin irritation provides validated methods for assessing such risks without reliance on animal models.
Recent regulatory trends indicate increasing scrutiny of chemical components in adhesives, particularly regarding potential endocrine disruptors and allergens. The EU's REACH regulation and similar global initiatives have expanded the list of restricted substances that may be present in skin-contacting materials, requiring manufacturers to continuously update formulations and testing protocols.
Environmental Impact and Sustainability Considerations
The environmental impact of conductive adhesives in wearable health technology represents a critical consideration as the industry expands. Traditional conductive materials often contain heavy metals and toxic substances that pose significant environmental hazards throughout their lifecycle. Silver-based conductive adhesives, while offering excellent conductivity, raise concerns regarding resource depletion and mining impacts, as silver extraction processes are associated with substantial ecological disruption and water pollution.
Manufacturing processes for conductive adhesives typically involve energy-intensive procedures and potentially harmful solvents. The carbon footprint of these materials extends from raw material extraction through processing and application, contributing to the overall environmental impact of wearable health devices. Additionally, many conventional adhesives contain volatile organic compounds (VOCs) that contribute to air pollution and pose health risks during manufacturing and application phases.
End-of-life considerations present particular challenges for wearable health technologies utilizing conductive adhesives. The intimate integration of these materials with various substrates often complicates recycling efforts, potentially leading to increased electronic waste. The non-biodegradable nature of many conductive adhesives means they persist in the environment long after product disposal, potentially leaching harmful substances into soil and water systems.
In response to these concerns, the industry is witnessing significant innovation in eco-friendly alternatives. Bio-based conductive adhesives derived from renewable resources are emerging as promising substitutes, offering reduced environmental impact while maintaining necessary performance characteristics. Water-based formulations are increasingly replacing solvent-based systems, substantially reducing VOC emissions during manufacturing and application processes.
Regulatory frameworks worldwide are evolving to address these environmental considerations. The European Union's Restriction of Hazardous Substances (RoHS) and Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulations impose strict limitations on hazardous materials in electronic components, including conductive adhesives. Similarly, initiatives like the Electronic Product Environmental Assessment Tool (EPEAT) in the United States encourage manufacturers to design products with improved environmental performance throughout their lifecycle.
Companies developing wearable health technologies are increasingly adopting lifecycle assessment methodologies to evaluate and minimize the environmental footprint of their products. This holistic approach considers impacts from raw material extraction through manufacturing, use, and disposal, driving innovation toward more sustainable conductive adhesive solutions that balance technical performance with environmental responsibility.
Manufacturing processes for conductive adhesives typically involve energy-intensive procedures and potentially harmful solvents. The carbon footprint of these materials extends from raw material extraction through processing and application, contributing to the overall environmental impact of wearable health devices. Additionally, many conventional adhesives contain volatile organic compounds (VOCs) that contribute to air pollution and pose health risks during manufacturing and application phases.
End-of-life considerations present particular challenges for wearable health technologies utilizing conductive adhesives. The intimate integration of these materials with various substrates often complicates recycling efforts, potentially leading to increased electronic waste. The non-biodegradable nature of many conductive adhesives means they persist in the environment long after product disposal, potentially leaching harmful substances into soil and water systems.
In response to these concerns, the industry is witnessing significant innovation in eco-friendly alternatives. Bio-based conductive adhesives derived from renewable resources are emerging as promising substitutes, offering reduced environmental impact while maintaining necessary performance characteristics. Water-based formulations are increasingly replacing solvent-based systems, substantially reducing VOC emissions during manufacturing and application processes.
Regulatory frameworks worldwide are evolving to address these environmental considerations. The European Union's Restriction of Hazardous Substances (RoHS) and Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulations impose strict limitations on hazardous materials in electronic components, including conductive adhesives. Similarly, initiatives like the Electronic Product Environmental Assessment Tool (EPEAT) in the United States encourage manufacturers to design products with improved environmental performance throughout their lifecycle.
Companies developing wearable health technologies are increasingly adopting lifecycle assessment methodologies to evaluate and minimize the environmental footprint of their products. This holistic approach considers impacts from raw material extraction through manufacturing, use, and disposal, driving innovation toward more sustainable conductive adhesive solutions that balance technical performance with environmental responsibility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!