Current Collector Innovations For Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-S Battery Technology Background and Objectives
Sodium-sulfur (Na-S) batteries have emerged as a promising energy storage technology since their initial development in the 1960s by Ford Motor Company. These batteries were originally designed to operate at high temperatures (300-350°C), which limited their practical applications despite their theoretical advantages of high energy density, abundant raw materials, and low cost. The evolution of Na-S battery technology has been marked by significant research efforts to overcome temperature constraints and safety concerns.
The fundamental principle of Na-S batteries involves sodium as the anode and sulfur as the cathode, with a solid electrolyte separator. Traditional high-temperature Na-S batteries utilize beta-alumina as the solid electrolyte, which requires elevated temperatures to achieve sufficient ionic conductivity. The technological trajectory has gradually shifted toward developing room-temperature sodium-sulfur (RT Na-S) batteries, which represent a critical advancement in making this technology commercially viable for widespread applications.
Room-temperature Na-S batteries offer theoretical energy densities of approximately 760 Wh/kg, significantly higher than current lithium-ion technologies. This potential, combined with the natural abundance of sodium and sulfur (respectively the 6th and 10th most abundant elements in Earth's crust), positions RT Na-S batteries as an economically attractive alternative to lithium-based systems, particularly for grid-scale energy storage applications.
The primary technical objectives for current collector innovations in RT Na-S batteries include addressing the shuttle effect of polysulfides, enhancing the electrical conductivity of sulfur cathodes, and improving the stability of the sodium metal anode. Current collectors play a crucial role in battery performance by facilitating electron transfer between active materials and external circuits while maintaining structural integrity throughout charge-discharge cycles.
Recent research has focused on developing advanced current collector materials and architectures that can effectively contain polysulfides within the cathode compartment, provide sufficient electronic pathways for sulfur utilization, and accommodate the volume changes during cycling. These innovations aim to extend cycle life, increase capacity retention, and improve the overall energy efficiency of RT Na-S batteries.
The technological roadmap for RT Na-S batteries envisions achieving energy densities exceeding 500 Wh/kg at the cell level, cycle life of over 1,000 cycles with minimal capacity degradation, and cost reduction to below $100/kWh. These targets would position Na-S technology as a viable competitor to lithium-ion batteries for both stationary storage and potentially electric vehicle applications.
As global energy demands continue to rise and the transition toward renewable energy sources accelerates, the development of cost-effective, high-performance energy storage solutions becomes increasingly critical. RT Na-S batteries, with appropriate current collector innovations, have the potential to address this need while reducing dependence on critical materials required for conventional battery technologies.
The fundamental principle of Na-S batteries involves sodium as the anode and sulfur as the cathode, with a solid electrolyte separator. Traditional high-temperature Na-S batteries utilize beta-alumina as the solid electrolyte, which requires elevated temperatures to achieve sufficient ionic conductivity. The technological trajectory has gradually shifted toward developing room-temperature sodium-sulfur (RT Na-S) batteries, which represent a critical advancement in making this technology commercially viable for widespread applications.
Room-temperature Na-S batteries offer theoretical energy densities of approximately 760 Wh/kg, significantly higher than current lithium-ion technologies. This potential, combined with the natural abundance of sodium and sulfur (respectively the 6th and 10th most abundant elements in Earth's crust), positions RT Na-S batteries as an economically attractive alternative to lithium-based systems, particularly for grid-scale energy storage applications.
The primary technical objectives for current collector innovations in RT Na-S batteries include addressing the shuttle effect of polysulfides, enhancing the electrical conductivity of sulfur cathodes, and improving the stability of the sodium metal anode. Current collectors play a crucial role in battery performance by facilitating electron transfer between active materials and external circuits while maintaining structural integrity throughout charge-discharge cycles.
Recent research has focused on developing advanced current collector materials and architectures that can effectively contain polysulfides within the cathode compartment, provide sufficient electronic pathways for sulfur utilization, and accommodate the volume changes during cycling. These innovations aim to extend cycle life, increase capacity retention, and improve the overall energy efficiency of RT Na-S batteries.
The technological roadmap for RT Na-S batteries envisions achieving energy densities exceeding 500 Wh/kg at the cell level, cycle life of over 1,000 cycles with minimal capacity degradation, and cost reduction to below $100/kWh. These targets would position Na-S technology as a viable competitor to lithium-ion batteries for both stationary storage and potentially electric vehicle applications.
As global energy demands continue to rise and the transition toward renewable energy sources accelerates, the development of cost-effective, high-performance energy storage solutions becomes increasingly critical. RT Na-S batteries, with appropriate current collector innovations, have the potential to address this need while reducing dependence on critical materials required for conventional battery technologies.
Market Analysis for Room-Temperature Na-S Batteries
The global market for room-temperature sodium-sulfur (RT Na-S) batteries is experiencing significant growth, driven by increasing demand for cost-effective energy storage solutions. Unlike traditional high-temperature Na-S batteries operating at 300-350°C, room-temperature variants offer safer operation and simplified thermal management systems, making them attractive for grid-scale storage applications.
Market projections indicate that the RT Na-S battery segment is expected to grow at a compound annual growth rate of 18-20% between 2023 and 2030. This growth is primarily fueled by the expanding renewable energy sector, which requires efficient and economical energy storage systems to address intermittency issues associated with solar and wind power generation.
The cost advantage of sodium-sulfur technology represents a major market driver. Sodium is approximately 1000 times more abundant than lithium in the Earth's crust, resulting in significantly lower raw material costs. Current estimates suggest that fully developed RT Na-S batteries could achieve costs below $100/kWh, compared to $130-150/kWh for lithium-ion alternatives, creating a compelling economic case for large-scale adoption.
Regional analysis reveals that Asia-Pacific currently dominates the RT Na-S battery market, with China, Japan, and South Korea leading research and commercialization efforts. North America and Europe are rapidly expanding their market presence through increased R&D investments and supportive government policies promoting clean energy technologies.
The utility sector represents the largest market segment for RT Na-S batteries, accounting for approximately 65% of current demand. This is followed by the commercial and industrial sectors at 25%, with the remaining 10% distributed across residential and specialized applications. The utility-scale storage market is particularly promising due to the technology's potential for long-duration energy storage capabilities exceeding 6-8 hours.
Key market challenges include competition from established lithium-ion technology and emerging alternatives such as flow batteries and solid-state batteries. Additionally, technical hurdles related to current collector degradation, sulfur shuttling, and limited cycle life must be overcome to achieve widespread commercial adoption.
Consumer demand patterns indicate growing interest in sustainable energy storage solutions with minimal environmental impact. RT Na-S batteries align well with this trend, offering reduced reliance on critical minerals and potentially lower lifecycle carbon footprints compared to conventional battery technologies.
Market projections indicate that the RT Na-S battery segment is expected to grow at a compound annual growth rate of 18-20% between 2023 and 2030. This growth is primarily fueled by the expanding renewable energy sector, which requires efficient and economical energy storage systems to address intermittency issues associated with solar and wind power generation.
The cost advantage of sodium-sulfur technology represents a major market driver. Sodium is approximately 1000 times more abundant than lithium in the Earth's crust, resulting in significantly lower raw material costs. Current estimates suggest that fully developed RT Na-S batteries could achieve costs below $100/kWh, compared to $130-150/kWh for lithium-ion alternatives, creating a compelling economic case for large-scale adoption.
Regional analysis reveals that Asia-Pacific currently dominates the RT Na-S battery market, with China, Japan, and South Korea leading research and commercialization efforts. North America and Europe are rapidly expanding their market presence through increased R&D investments and supportive government policies promoting clean energy technologies.
The utility sector represents the largest market segment for RT Na-S batteries, accounting for approximately 65% of current demand. This is followed by the commercial and industrial sectors at 25%, with the remaining 10% distributed across residential and specialized applications. The utility-scale storage market is particularly promising due to the technology's potential for long-duration energy storage capabilities exceeding 6-8 hours.
Key market challenges include competition from established lithium-ion technology and emerging alternatives such as flow batteries and solid-state batteries. Additionally, technical hurdles related to current collector degradation, sulfur shuttling, and limited cycle life must be overcome to achieve widespread commercial adoption.
Consumer demand patterns indicate growing interest in sustainable energy storage solutions with minimal environmental impact. RT Na-S batteries align well with this trend, offering reduced reliance on critical minerals and potentially lower lifecycle carbon footprints compared to conventional battery technologies.
Current Collector Technical Challenges
Current collector design for room-temperature sodium-sulfur (RT-Na/S) batteries faces significant technical challenges that impede their widespread commercial adoption. The primary issue stems from the highly corrosive nature of polysulfide intermediates generated during battery operation. These polysulfides readily attack conventional current collectors, particularly aluminum, leading to severe corrosion and formation of an insulating passivation layer at the interface. This phenomenon drastically increases internal resistance, resulting in capacity fading and shortened battery lifespan.
Material selection presents another critical challenge. While aluminum is lightweight and cost-effective, its poor chemical stability in sulfur-containing electrolytes limits its application. Stainless steel offers better corrosion resistance but adds substantial weight and cost to the battery system. Carbon-based materials show promising chemical stability but suffer from inadequate mechanical strength and electrical conductivity when used alone as current collectors.
The interface between the current collector and active material represents a significant bottleneck. Poor adhesion between sulfur cathodes and current collectors leads to electrical contact loss during cycling, especially under the volume changes that sulfur electrodes undergo. This detachment accelerates capacity decay and reduces the battery's cycle life. Additionally, the high interfacial resistance at this junction hampers electron transfer efficiency, negatively impacting rate capability and energy density.
Manufacturing scalability poses substantial challenges for advanced current collector designs. Novel approaches such as 3D structured collectors or composite materials often involve complex fabrication processes that are difficult to scale up economically. The trade-off between enhanced performance and manufacturing complexity remains a significant barrier to commercialization.
Temperature sensitivity further complicates current collector design. Even at room temperature, the reactivity of sodium with sulfur species creates a highly aggressive chemical environment. Current collectors must maintain structural integrity and conductivity across varying temperature conditions during operation, as thermal fluctuations can accelerate corrosion processes and interface degradation.
Cost considerations represent perhaps the most practical challenge. While noble metals like platinum or gold would offer excellent corrosion resistance, their prohibitive cost makes them impractical for large-scale battery production. The industry requires cost-effective solutions that balance performance, durability, and economic viability to enable widespread adoption of RT-Na/S battery technology.
Material selection presents another critical challenge. While aluminum is lightweight and cost-effective, its poor chemical stability in sulfur-containing electrolytes limits its application. Stainless steel offers better corrosion resistance but adds substantial weight and cost to the battery system. Carbon-based materials show promising chemical stability but suffer from inadequate mechanical strength and electrical conductivity when used alone as current collectors.
The interface between the current collector and active material represents a significant bottleneck. Poor adhesion between sulfur cathodes and current collectors leads to electrical contact loss during cycling, especially under the volume changes that sulfur electrodes undergo. This detachment accelerates capacity decay and reduces the battery's cycle life. Additionally, the high interfacial resistance at this junction hampers electron transfer efficiency, negatively impacting rate capability and energy density.
Manufacturing scalability poses substantial challenges for advanced current collector designs. Novel approaches such as 3D structured collectors or composite materials often involve complex fabrication processes that are difficult to scale up economically. The trade-off between enhanced performance and manufacturing complexity remains a significant barrier to commercialization.
Temperature sensitivity further complicates current collector design. Even at room temperature, the reactivity of sodium with sulfur species creates a highly aggressive chemical environment. Current collectors must maintain structural integrity and conductivity across varying temperature conditions during operation, as thermal fluctuations can accelerate corrosion processes and interface degradation.
Cost considerations represent perhaps the most practical challenge. While noble metals like platinum or gold would offer excellent corrosion resistance, their prohibitive cost makes them impractical for large-scale battery production. The industry requires cost-effective solutions that balance performance, durability, and economic viability to enable widespread adoption of RT-Na/S battery technology.
Current Collector Material Solutions
01 Metal-based current collectors for room-temperature sodium-sulfur batteries
Metal-based materials are commonly used as current collectors in room-temperature sodium-sulfur batteries due to their excellent electrical conductivity and mechanical stability. These materials include aluminum, copper, stainless steel, and nickel. The choice of metal affects the battery's performance, including its cycle life, energy density, and rate capability. Metal current collectors provide a stable substrate for active materials while facilitating efficient electron transfer during charge and discharge processes.- Metal-based current collectors for room-temperature sodium-sulfur batteries: Metal-based materials are commonly used as current collectors in room-temperature sodium-sulfur batteries due to their excellent electrical conductivity and mechanical stability. These materials include aluminum, copper, stainless steel, and nickel. The choice of metal affects the battery's performance, including its cycle life, energy density, and rate capability. Some metals may form protective layers that prevent corrosion from the sulfur and sodium polysulfides, enhancing the battery's durability.
- Carbon-based current collectors for enhanced performance: Carbon-based materials such as carbon paper, carbon cloth, and carbon nanotubes are increasingly being used as current collectors in room-temperature sodium-sulfur batteries. These materials offer advantages including lightweight properties, high surface area, and good electrical conductivity. Carbon-based current collectors can also help contain the sulfur active material and mitigate the shuttle effect of polysulfides, leading to improved cycling stability and coulombic efficiency of the batteries.
- Composite and coated current collectors for sodium-sulfur batteries: Composite and coated current collectors combine different materials to achieve enhanced properties for room-temperature sodium-sulfur batteries. These may include metal substrates coated with carbon materials, conductive polymers, or metal oxides. Such composite structures can provide better adhesion to active materials, improved corrosion resistance, and enhanced electrical contact. These innovations help address challenges like polysulfide shuttling and interface degradation, resulting in batteries with longer cycle life and higher capacity retention.
- 3D structured current collectors for increased surface area: Three-dimensional structured current collectors offer increased surface area and improved electrolyte penetration in room-temperature sodium-sulfur batteries. These structures include foams, meshes, and hierarchical porous architectures that provide better accommodation for volume changes during cycling and facilitate more efficient ion transport. The 3D structures also help in containing sulfur and its discharge products, reducing capacity fading and improving the overall electrochemical performance of the battery.
- Surface-modified current collectors for improved interface stability: Surface modification of current collectors is an effective approach to enhance the interface stability in room-temperature sodium-sulfur batteries. Techniques include chemical treatment, plasma processing, and functional layer deposition to create surfaces with improved wettability, reduced interfacial resistance, and enhanced chemical stability against the corrosive sulfur species. These modifications help in forming stable solid-electrolyte interphases, preventing unwanted side reactions, and maintaining good electrical contact throughout battery cycling.
02 Carbon-based current collectors for enhanced performance
Carbon-based materials serve as effective current collectors in room-temperature sodium-sulfur batteries due to their lightweight nature, good conductivity, and chemical stability. These materials include carbon paper, carbon cloth, carbon nanotubes, and graphene. Carbon-based current collectors can improve the sulfur utilization and mitigate the shuttle effect of polysulfides, leading to enhanced cycling stability and rate performance. Their porous structure also facilitates electrolyte penetration and ion transport.Expand Specific Solutions03 Composite current collectors with protective coatings
Composite current collectors with protective coatings are designed to address the corrosion issues in room-temperature sodium-sulfur batteries. These collectors typically consist of a conductive substrate coated with corrosion-resistant materials such as carbon, polymers, or metal oxides. The protective layer prevents direct contact between the current collector and the reactive sodium or sulfur species, thereby extending the battery's lifespan and improving its safety characteristics.Expand Specific Solutions04 3D structured current collectors for high-performance batteries
Three-dimensional structured current collectors offer advantages for room-temperature sodium-sulfur batteries by providing increased surface area, improved electrolyte accessibility, and enhanced mechanical stability. These structures include foam-like materials, hierarchical porous frameworks, and interconnected networks. The 3D architecture accommodates volume changes during cycling, facilitates faster ion transport, and enables higher sulfur loading, resulting in batteries with improved energy density and rate capability.Expand Specific Solutions05 Current collector surface modifications and treatments
Surface modifications and treatments of current collectors can significantly enhance the performance of room-temperature sodium-sulfur batteries. These modifications include surface roughening, chemical etching, plasma treatment, and functional group introduction. Such treatments improve the adhesion between the current collector and active materials, enhance wettability with the electrolyte, and create favorable interfaces for electrochemical reactions, leading to reduced interfacial resistance and improved cycling stability.Expand Specific Solutions
Key Industry Players in Na-S Battery Development
Room-temperature sodium-sulfur (RT-Na-S) battery technology is currently in the early growth stage, with a projected market size expected to reach significant expansion as energy storage demands increase globally. The competitive landscape is characterized by diverse players focusing on current collector innovations to overcome challenges of electrode degradation and enhance battery performance. Leading battery manufacturers like LG Energy Solution, CATV, Samsung SDI, and SK On are investing heavily in R&D to commercialize this technology. Materials specialists including Furukawa Electric, UACJ Corp, and Höganäs AB are developing advanced current collector materials with enhanced corrosion resistance and conductivity. Research institutions such as Shanghai Institute of Ceramics and Huazhong University of Science & Technology are pioneering fundamental breakthroughs, while established energy companies like NGK Insulators and Tokyo Electric Power are leveraging their expertise to accelerate technology maturation from laboratory to commercial applications.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered significant innovations in current collectors for room-temperature sodium-sulfur (RT-Na/S) batteries, focusing on specialized ceramic-based current collectors that address the corrosion issues typical with conventional metal collectors. Their proprietary NASICON (Na Super Ionic CONductor) ceramic material serves as both an electrolyte and current collector interface, providing superior sodium ion conductivity while resisting sulfur corrosion. NGK has developed a unique dual-layer current collector design where a protective ceramic coating is applied to a conductive metal substrate, creating a barrier against polysulfide shuttling while maintaining excellent electrical conductivity. Their latest innovation involves carbon-ceramic composite current collectors that combine the corrosion resistance of ceramics with the conductivity of carbon materials, achieving conductivity values of 10^2-10^3 S/cm while demonstrating stability over 500+ cycles in room temperature conditions.
Strengths: Exceptional corrosion resistance against sulfur and polysulfides; industry-leading expertise in ceramic technologies; proven long-term stability in commercial sodium-sulfur battery systems. Weaknesses: Higher manufacturing costs compared to conventional metal collectors; potential brittleness of ceramic components requiring careful cell design; lower electrical conductivity than pure metal collectors requiring optimization of collector thickness.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: Contemporary Amperex Technology (CATL) has developed advanced current collector solutions for room-temperature sodium-sulfur batteries focusing on nanostructured carbon-metal composite materials. Their proprietary technology utilizes a three-dimensional porous carbon framework infused with nano-dispersed metal particles (primarily aluminum and copper) that significantly enhances both electrical conductivity and electrochemical stability. CATL's current collectors feature a gradient structure with varying porosity levels that facilitates efficient sodium ion transport while effectively containing polysulfides. The company has implemented a specialized surface modification technique using nitrogen-doped carbon coatings that creates strong chemical bonding with sulfur species, reducing the shuttle effect. Their latest innovation incorporates graphene-reinforced aluminum foils with nano-engineered surface textures that increase the effective surface area by 300% compared to conventional collectors, while maintaining structural integrity during repeated charge-discharge cycles at room temperature.
Strengths: Superior electrical conductivity (>10^5 S/cm) combined with excellent corrosion resistance; scalable manufacturing processes compatible with existing production lines; enhanced cycle life exceeding 1000 cycles in prototype cells. Weaknesses: Higher material costs compared to traditional aluminum collectors; complex multi-step manufacturing process requiring precise quality control; potential long-term reliability concerns under extreme temperature fluctuations.
Core Patents in Current Collector Technology
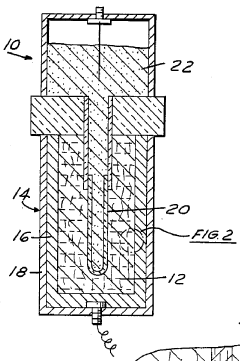
Method of making a current collector for a sodium/sulfur battery
PatentInactiveUS4649022A
Innovation
- A current collector made from a composite material of aluminum filled with graphite or silicon carbide fibers, treated to form an electronically conductive path and a passivating aluminum sulfide surface coating, providing resistance to corrosive attack and thermal cycling.
Current collector of positive electrode and sodium-sulfur battery using the same
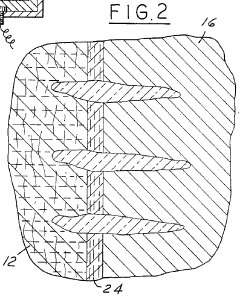
PatentInactiveEP1296392B1
Innovation
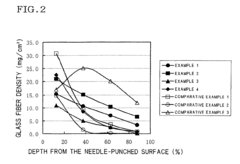
- A current collector for the positive electrode with a high resistance layer formed by needle-punching glass fibers into a felt substrate made of carbon or graphite fibers, where the glass fiber density gradually decreases from the surface to the depth, ensuring the deepest fibers reach 85-100% of the substrate thickness, enhancing sodium polysulfide migration and reducing internal resistance.
Material Compatibility and Interface Engineering
Material compatibility and interface engineering represent critical challenges in the development of room-temperature sodium-sulfur (RT-Na/S) batteries, particularly concerning current collector innovations. The interface between the current collector and active materials significantly influences battery performance, cycle life, and safety characteristics. Traditional current collectors such as aluminum and copper often suffer from severe corrosion when exposed to the highly reactive polysulfide species generated during battery operation.
Recent research has focused on developing protective coatings and surface modifications to enhance compatibility between current collectors and the electrochemical environment of RT-Na/S batteries. Carbon-based coatings, including graphene and carbon nanotubes, have demonstrated promising results by forming a physical barrier against polysulfide attack while maintaining excellent electrical conductivity. These coatings effectively reduce the interfacial resistance and enhance the overall electrochemical performance.
Metal nitrides and carbides have emerged as another class of protective materials for current collectors. Titanium nitride (TiN) and vanadium carbide (VC) coatings exhibit superior chemical stability in the presence of sodium polysulfides while providing adequate electrical conductivity. The atomic layer deposition (ALD) technique has proven particularly effective for creating uniform, pinhole-free protective layers with precisely controlled thickness.
Interface engineering strategies have extended beyond simple protective coatings to include functional interlayers that actively participate in the electrochemical processes. Transition metal oxide interlayers, such as MnO2 and V2O5, not only protect the current collector but also catalyze the conversion reactions of sodium polysulfides, thereby improving the utilization of active materials and mitigating the shuttle effect.
Nano-architectured current collectors represent an innovative approach to interface engineering. Three-dimensional porous structures created through techniques such as template-assisted electrodeposition and selective etching provide increased surface area for active material loading while facilitating efficient ion transport. These structures effectively accommodate the volume changes during cycling and maintain intimate contact between the current collector and active materials.
The development of composite current collectors incorporating both conductive and protective components has shown significant promise. For instance, nickel foam coated with nitrogen-doped carbon demonstrates synergistic effects, combining the mechanical strength and conductivity of nickel with the chemical stability and polysulfide-trapping capability of nitrogen-doped carbon. These composite structures effectively address multiple interface-related challenges simultaneously.
Recent research has focused on developing protective coatings and surface modifications to enhance compatibility between current collectors and the electrochemical environment of RT-Na/S batteries. Carbon-based coatings, including graphene and carbon nanotubes, have demonstrated promising results by forming a physical barrier against polysulfide attack while maintaining excellent electrical conductivity. These coatings effectively reduce the interfacial resistance and enhance the overall electrochemical performance.
Metal nitrides and carbides have emerged as another class of protective materials for current collectors. Titanium nitride (TiN) and vanadium carbide (VC) coatings exhibit superior chemical stability in the presence of sodium polysulfides while providing adequate electrical conductivity. The atomic layer deposition (ALD) technique has proven particularly effective for creating uniform, pinhole-free protective layers with precisely controlled thickness.
Interface engineering strategies have extended beyond simple protective coatings to include functional interlayers that actively participate in the electrochemical processes. Transition metal oxide interlayers, such as MnO2 and V2O5, not only protect the current collector but also catalyze the conversion reactions of sodium polysulfides, thereby improving the utilization of active materials and mitigating the shuttle effect.
Nano-architectured current collectors represent an innovative approach to interface engineering. Three-dimensional porous structures created through techniques such as template-assisted electrodeposition and selective etching provide increased surface area for active material loading while facilitating efficient ion transport. These structures effectively accommodate the volume changes during cycling and maintain intimate contact between the current collector and active materials.
The development of composite current collectors incorporating both conductive and protective components has shown significant promise. For instance, nickel foam coated with nitrogen-doped carbon demonstrates synergistic effects, combining the mechanical strength and conductivity of nickel with the chemical stability and polysulfide-trapping capability of nitrogen-doped carbon. These composite structures effectively address multiple interface-related challenges simultaneously.
Scalability and Manufacturing Considerations
The scalability and manufacturing of current collectors for room-temperature sodium-sulfur (RT-Na/S) batteries present significant challenges that must be addressed for commercial viability. Current collector materials must balance conductivity, corrosion resistance, and cost-effectiveness while being compatible with large-scale production methods. Traditional aluminum and copper collectors, widely used in lithium-ion batteries, face limitations in Na/S systems due to sulfur's corrosive nature and the unique electrochemical environment.
Advanced manufacturing techniques such as roll-to-roll processing show promise for continuous production of specialized current collectors. This approach enables the deposition of protective coatings or surface modifications at industrial scales, potentially reducing production costs while maintaining performance integrity. However, the uniformity of these coatings across large surface areas remains challenging, particularly for complex nanostructured collectors that enhance sulfur utilization.
Material selection presents another critical consideration. Carbon-based collectors offer excellent corrosion resistance but may introduce conductivity and mechanical strength concerns at scale. Metal-based collectors modified with protective layers require precise control of coating thickness and composition across large areas. The development of composite collectors combining metals with carbon materials shows promise but introduces additional manufacturing complexity.
Cost analysis indicates that specialized current collectors currently represent a significant portion of RT-Na/S battery expenses. Economies of scale could potentially reduce these costs, but initial capital investment for specialized equipment remains substantial. Material availability assessments suggest that while sodium and sulfur are abundant, some advanced coating materials or rare metals used in composite collectors may face supply constraints at commercial scales.
Quality control processes for large-scale production require sophisticated in-line monitoring systems to detect microscopic defects that could lead to battery failure. Non-destructive testing methods such as optical inspection and electrochemical impedance spectroscopy are being adapted for high-throughput manufacturing environments but require further refinement.
Environmental considerations also impact scalability, as manufacturing processes must comply with increasingly stringent regulations. Water-based processing methods are being developed to replace organic solvent-based approaches, potentially reducing environmental impact while improving worker safety. Additionally, designing current collectors with end-of-life recycling in mind has become essential for sustainable large-scale production.
Advanced manufacturing techniques such as roll-to-roll processing show promise for continuous production of specialized current collectors. This approach enables the deposition of protective coatings or surface modifications at industrial scales, potentially reducing production costs while maintaining performance integrity. However, the uniformity of these coatings across large surface areas remains challenging, particularly for complex nanostructured collectors that enhance sulfur utilization.
Material selection presents another critical consideration. Carbon-based collectors offer excellent corrosion resistance but may introduce conductivity and mechanical strength concerns at scale. Metal-based collectors modified with protective layers require precise control of coating thickness and composition across large areas. The development of composite collectors combining metals with carbon materials shows promise but introduces additional manufacturing complexity.
Cost analysis indicates that specialized current collectors currently represent a significant portion of RT-Na/S battery expenses. Economies of scale could potentially reduce these costs, but initial capital investment for specialized equipment remains substantial. Material availability assessments suggest that while sodium and sulfur are abundant, some advanced coating materials or rare metals used in composite collectors may face supply constraints at commercial scales.
Quality control processes for large-scale production require sophisticated in-line monitoring systems to detect microscopic defects that could lead to battery failure. Non-destructive testing methods such as optical inspection and electrochemical impedance spectroscopy are being adapted for high-throughput manufacturing environments but require further refinement.
Environmental considerations also impact scalability, as manufacturing processes must comply with increasingly stringent regulations. Water-based processing methods are being developed to replace organic solvent-based approaches, potentially reducing environmental impact while improving worker safety. Additionally, designing current collectors with end-of-life recycling in mind has become essential for sustainable large-scale production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!