Degradation Mapping And Repair For Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-S Battery Degradation Background and Research Objectives
Room-temperature sodium-sulfur (RT Na-S) batteries have emerged as a promising energy storage technology due to their high theoretical energy density, cost-effectiveness, and abundant raw material resources. Unlike traditional high-temperature Na-S batteries operating at 300-350°C, RT Na-S batteries function at ambient temperatures, making them potentially safer and more practical for widespread applications. The development of these batteries traces back to the early 2000s, with significant acceleration in research over the past decade as the demand for sustainable energy storage solutions has grown.
The evolution of RT Na-S battery technology has been marked by several key advancements in electrode materials, electrolyte formulations, and cell architectures. Initially, these batteries faced severe challenges including rapid capacity fading, poor cycle life, and safety concerns related to sodium polysulfide dissolution. Recent years have witnessed substantial progress in addressing these limitations through innovative approaches in materials science and electrochemistry.
Despite these advancements, degradation mechanisms remain a critical bottleneck for the commercial viability of RT Na-S batteries. The complex electrochemical reactions between sodium and sulfur lead to multiple degradation pathways that significantly impact battery performance and longevity. These include the formation of insulating layers on electrodes, shuttle effects of polysulfides, volume expansion during cycling, and structural deterioration of active materials.
The primary technical objective of this research is to develop comprehensive methodologies for mapping degradation mechanisms in RT Na-S batteries with high spatial and temporal resolution. This involves advanced characterization techniques to identify and quantify degradation processes at different scales—from atomic-level interactions to macroscopic structural changes. Understanding these mechanisms is fundamental to designing effective mitigation strategies.
Furthermore, this research aims to establish innovative repair strategies that can extend battery lifetime and enhance performance metrics. These may include self-healing materials, protective coatings, electrolyte additives, and advanced cell designs that inherently resist degradation. The goal is to push RT Na-S batteries toward practical energy densities exceeding 300 Wh/kg with cycle lives of 1000+ cycles at high capacity retention.
The technological trajectory suggests that RT Na-S batteries could potentially disrupt the energy storage market by offering a balance of high energy density, sustainability, and cost-effectiveness. As global efforts intensify to transition away from fossil fuels, the development of reliable, high-performance RT Na-S batteries aligns with broader objectives of energy security, environmental sustainability, and economic competitiveness in the renewable energy sector.
The evolution of RT Na-S battery technology has been marked by several key advancements in electrode materials, electrolyte formulations, and cell architectures. Initially, these batteries faced severe challenges including rapid capacity fading, poor cycle life, and safety concerns related to sodium polysulfide dissolution. Recent years have witnessed substantial progress in addressing these limitations through innovative approaches in materials science and electrochemistry.
Despite these advancements, degradation mechanisms remain a critical bottleneck for the commercial viability of RT Na-S batteries. The complex electrochemical reactions between sodium and sulfur lead to multiple degradation pathways that significantly impact battery performance and longevity. These include the formation of insulating layers on electrodes, shuttle effects of polysulfides, volume expansion during cycling, and structural deterioration of active materials.
The primary technical objective of this research is to develop comprehensive methodologies for mapping degradation mechanisms in RT Na-S batteries with high spatial and temporal resolution. This involves advanced characterization techniques to identify and quantify degradation processes at different scales—from atomic-level interactions to macroscopic structural changes. Understanding these mechanisms is fundamental to designing effective mitigation strategies.
Furthermore, this research aims to establish innovative repair strategies that can extend battery lifetime and enhance performance metrics. These may include self-healing materials, protective coatings, electrolyte additives, and advanced cell designs that inherently resist degradation. The goal is to push RT Na-S batteries toward practical energy densities exceeding 300 Wh/kg with cycle lives of 1000+ cycles at high capacity retention.
The technological trajectory suggests that RT Na-S batteries could potentially disrupt the energy storage market by offering a balance of high energy density, sustainability, and cost-effectiveness. As global efforts intensify to transition away from fossil fuels, the development of reliable, high-performance RT Na-S batteries aligns with broader objectives of energy security, environmental sustainability, and economic competitiveness in the renewable energy sector.
Market Analysis for Room-Temperature Na-S Battery Applications
The room-temperature sodium-sulfur (RT Na-S) battery market is experiencing significant growth potential due to the increasing demand for cost-effective and sustainable energy storage solutions. Unlike traditional high-temperature Na-S batteries that operate at 300-350°C, RT Na-S batteries function at ambient temperatures, eliminating the need for complex thermal management systems and reducing safety concerns.
The global energy storage market, valued at approximately $211 billion in 2022, is projected to grow at a CAGR of 10-12% through 2030, with grid-scale storage representing the largest segment. Within this landscape, RT Na-S batteries are positioned to capture market share from lithium-ion technologies due to their cost advantages, with sodium being approximately 1000 times more abundant than lithium in the Earth's crust.
Key market drivers for RT Na-S battery adoption include the expanding renewable energy sector, which requires efficient storage solutions to address intermittency issues, and the growing electric vehicle market, particularly in price-sensitive segments where cost advantages outweigh energy density limitations. Additionally, government policies promoting sustainable energy technologies through subsidies and research funding are accelerating market development.
Market segmentation reveals diverse application potential across utility-scale storage, residential and commercial energy systems, and specialized industrial applications. The Asia-Pacific region, led by China, Japan, and South Korea, dominates the current market landscape due to substantial investments in manufacturing infrastructure and supportive government policies.
Despite promising growth projections, market penetration faces challenges including competition from established lithium-ion technologies, technical hurdles related to cycle life and energy density, and the need for scaled manufacturing processes to achieve cost competitiveness. The current market size for RT Na-S batteries remains relatively small at under $500 million but is expected to expand significantly as degradation issues are resolved and commercial viability improves.
Consumer adoption trends indicate increasing interest in sustainable battery technologies, with surveys showing that 65% of commercial energy users would consider sodium-based alternatives if performance metrics approach those of lithium-ion systems while offering cost savings of at least 20%. This price sensitivity highlights the importance of addressing degradation issues to improve cycle life and overall value proposition.
The global energy storage market, valued at approximately $211 billion in 2022, is projected to grow at a CAGR of 10-12% through 2030, with grid-scale storage representing the largest segment. Within this landscape, RT Na-S batteries are positioned to capture market share from lithium-ion technologies due to their cost advantages, with sodium being approximately 1000 times more abundant than lithium in the Earth's crust.
Key market drivers for RT Na-S battery adoption include the expanding renewable energy sector, which requires efficient storage solutions to address intermittency issues, and the growing electric vehicle market, particularly in price-sensitive segments where cost advantages outweigh energy density limitations. Additionally, government policies promoting sustainable energy technologies through subsidies and research funding are accelerating market development.
Market segmentation reveals diverse application potential across utility-scale storage, residential and commercial energy systems, and specialized industrial applications. The Asia-Pacific region, led by China, Japan, and South Korea, dominates the current market landscape due to substantial investments in manufacturing infrastructure and supportive government policies.
Despite promising growth projections, market penetration faces challenges including competition from established lithium-ion technologies, technical hurdles related to cycle life and energy density, and the need for scaled manufacturing processes to achieve cost competitiveness. The current market size for RT Na-S batteries remains relatively small at under $500 million but is expected to expand significantly as degradation issues are resolved and commercial viability improves.
Consumer adoption trends indicate increasing interest in sustainable battery technologies, with surveys showing that 65% of commercial energy users would consider sodium-based alternatives if performance metrics approach those of lithium-ion systems while offering cost savings of at least 20%. This price sensitivity highlights the importance of addressing degradation issues to improve cycle life and overall value proposition.
Technical Challenges in RT Na-S Battery Development
Room-temperature sodium-sulfur (RT Na-S) batteries face significant technical challenges that have hindered their widespread commercialization despite their promising theoretical energy density of 760 Wh/kg. The primary obstacle stems from the complex degradation mechanisms occurring at multiple levels within the battery structure, which severely impact cycle life and performance stability.
The shuttle effect of soluble polysulfide intermediates represents one of the most critical challenges. During discharge, sodium polysulfides (Na2Sx, 4≤x≤8) form and dissolve in the electrolyte, migrating to the anode where they react with sodium, creating a parasitic redox shuttle. This phenomenon leads to active material loss, self-discharge, and rapid capacity fading, typically reducing capacity to below 50% within just 100 cycles.
Electrode structural degradation presents another significant hurdle. The sulfur cathode undergoes substantial volume changes (up to 170%) during cycling as it converts between S8 and Na2S, causing mechanical stress that leads to particle pulverization and electrode delamination. Similarly, the sodium metal anode suffers from dendrite formation and unstable solid-electrolyte interphase (SEI) layers, further compromising battery integrity and safety.
Electrolyte decomposition accelerates degradation through side reactions with both electrodes. The highly reactive sodium metal anode and the various polysulfide species continuously consume electrolyte components, leading to increased cell impedance and eventual electrolyte depletion. Current electrolyte formulations struggle to maintain stability over extended cycling periods.
Interface instability between electrodes and electrolytes creates additional complications. The dynamic nature of these interfaces during cycling leads to continuous reformation of passivation layers, consuming active materials and increasing internal resistance. The heterogeneous distribution of reaction products further exacerbates local current density variations and uneven degradation patterns.
Temperature sensitivity compounds these issues, as RT Na-S batteries exhibit significantly different degradation behaviors across their operational temperature range (10-60°C). At higher temperatures, polysulfide shuttle effects intensify, while at lower temperatures, sodium plating becomes more irregular, accelerating dendrite formation.
Advanced characterization techniques have revealed that degradation is rarely uniform throughout the cell, creating "hot spots" of accelerated failure that can trigger cascading deterioration. This spatial heterogeneity in degradation patterns makes systematic repair strategies particularly challenging to implement effectively.
The shuttle effect of soluble polysulfide intermediates represents one of the most critical challenges. During discharge, sodium polysulfides (Na2Sx, 4≤x≤8) form and dissolve in the electrolyte, migrating to the anode where they react with sodium, creating a parasitic redox shuttle. This phenomenon leads to active material loss, self-discharge, and rapid capacity fading, typically reducing capacity to below 50% within just 100 cycles.
Electrode structural degradation presents another significant hurdle. The sulfur cathode undergoes substantial volume changes (up to 170%) during cycling as it converts between S8 and Na2S, causing mechanical stress that leads to particle pulverization and electrode delamination. Similarly, the sodium metal anode suffers from dendrite formation and unstable solid-electrolyte interphase (SEI) layers, further compromising battery integrity and safety.
Electrolyte decomposition accelerates degradation through side reactions with both electrodes. The highly reactive sodium metal anode and the various polysulfide species continuously consume electrolyte components, leading to increased cell impedance and eventual electrolyte depletion. Current electrolyte formulations struggle to maintain stability over extended cycling periods.
Interface instability between electrodes and electrolytes creates additional complications. The dynamic nature of these interfaces during cycling leads to continuous reformation of passivation layers, consuming active materials and increasing internal resistance. The heterogeneous distribution of reaction products further exacerbates local current density variations and uneven degradation patterns.
Temperature sensitivity compounds these issues, as RT Na-S batteries exhibit significantly different degradation behaviors across their operational temperature range (10-60°C). At higher temperatures, polysulfide shuttle effects intensify, while at lower temperatures, sodium plating becomes more irregular, accelerating dendrite formation.
Advanced characterization techniques have revealed that degradation is rarely uniform throughout the cell, creating "hot spots" of accelerated failure that can trigger cascading deterioration. This spatial heterogeneity in degradation patterns makes systematic repair strategies particularly challenging to implement effectively.
Current Degradation Mapping Methodologies
01 Electrolyte modifications to prevent degradation
Various electrolyte modifications can be implemented to prevent degradation in room-temperature sodium-sulfur batteries. These include using solid electrolytes, polymer electrolytes, or adding specific additives to liquid electrolytes. These modifications help to suppress the shuttle effect of polysulfides, prevent dendrite formation, and enhance the overall stability of the battery system during cycling, thereby reducing capacity fade and extending battery life.- Electrolyte modifications to prevent degradation: Various electrolyte modifications can be implemented to prevent degradation in room-temperature sodium-sulfur batteries. These include using polymer electrolytes, solid electrolytes, or adding specific additives to liquid electrolytes that form stable interfaces with the electrodes. These modifications help to suppress the shuttle effect of polysulfides and prevent side reactions between sodium and electrolyte components, thereby extending battery life and maintaining capacity over multiple cycles.
- Electrode material engineering: Engineering electrode materials is crucial for mitigating degradation in room-temperature sodium-sulfur batteries. This includes developing sulfur hosts with strong adsorption capabilities for polysulfides, creating protective layers on sodium anodes, and designing composite cathode materials that can accommodate volume changes during cycling. These approaches help to maintain structural integrity, prevent capacity fading, and enhance the overall electrochemical performance of the battery system.
- Interface stabilization strategies: Interface stabilization is essential for preventing degradation in room-temperature sodium-sulfur batteries. This involves creating artificial solid electrolyte interphases (SEI), using functional separators, and implementing interlayers between electrodes and electrolytes. These strategies help to suppress the shuttle effect of polysulfides, prevent dendrite formation on the sodium anode, and maintain stable interfaces during long-term cycling, thereby improving battery performance and longevity.
- Advanced cell design and architecture: Advanced cell designs and architectures can significantly reduce degradation in room-temperature sodium-sulfur batteries. This includes optimizing electrode thickness and porosity, implementing novel cell configurations, and designing specialized battery components. These approaches help to improve ion transport, reduce internal resistance, and enhance the mechanical stability of the battery system, leading to better cycling performance and reduced degradation over time.
- Thermal management and operating conditions: Proper thermal management and control of operating conditions are critical for preventing degradation in room-temperature sodium-sulfur batteries. This includes implementing temperature control systems, optimizing charge-discharge protocols, and establishing appropriate voltage windows for operation. These measures help to prevent thermal runaway, reduce side reactions, and maintain stable electrochemical performance, thereby extending the cycle life and improving the safety of the battery system.
02 Electrode material engineering
Engineering electrode materials is crucial for mitigating degradation in room-temperature sodium-sulfur batteries. This includes developing advanced sulfur cathodes with carbon hosts, designing sodium anodes with protective layers, and creating novel composite electrodes. These approaches aim to accommodate volume changes during cycling, improve electronic conductivity, and enhance the structural stability of electrodes, thereby reducing degradation mechanisms such as pulverization and active material loss.Expand Specific Solutions03 Interface stabilization strategies
Interface stabilization between electrodes and electrolytes is essential for preventing degradation in room-temperature sodium-sulfur batteries. Strategies include forming artificial solid electrolyte interphases (SEI), applying functional coatings on electrodes, and introducing interlayers. These approaches help to suppress side reactions, prevent polysulfide shuttling, and maintain stable interfaces during cycling, which significantly improves the cycling stability and coulombic efficiency of the batteries.Expand Specific Solutions04 Advanced cell design and architecture
Innovative cell designs and architectures can effectively address degradation issues in room-temperature sodium-sulfur batteries. These include developing specialized separators, optimizing electrode configurations, and implementing novel cell structures. Such designs help to physically contain polysulfides, manage thermal issues, and improve ion transport pathways, thereby enhancing the overall performance and longevity of the battery system under room temperature operation conditions.Expand Specific Solutions05 Degradation monitoring and analysis techniques
Advanced monitoring and analysis techniques are essential for understanding and mitigating degradation mechanisms in room-temperature sodium-sulfur batteries. These include in-situ characterization methods, post-mortem analysis, and computational modeling approaches. By identifying specific degradation pathways and failure modes, researchers can develop targeted strategies to improve battery performance, enhance cycle life, and reduce capacity fading under various operating conditions.Expand Specific Solutions
Leading Organizations in Na-S Battery Research
Room-temperature sodium-sulfur (RT-Na-S) battery technology is currently in the early growth phase, with the market expected to expand significantly due to increasing demand for cost-effective energy storage solutions. The global market size for this technology is projected to grow substantially as it offers a promising alternative to lithium-ion batteries with lower material costs. From a technical maturity perspective, the industry is still evolving, with key players focusing on solving degradation mapping and repair challenges. Toyota Motor Corp. and NGK Insulators lead with established research programs, while Samsung SDI, LG Energy Solution, and Toshiba are leveraging their battery expertise to advance this technology. Academic institutions like Ulsan National Institute of Science & Technology and Shanghai Institute of Ceramics are contributing fundamental research, creating a competitive landscape where industrial-academic partnerships are accelerating development toward commercial viability.
Toyota Motor Corp.
Technical Solution: Toyota has developed an innovative approach to degradation mapping and repair for room-temperature sodium-sulfur batteries focused on automotive applications. Their system employs a multi-layered carbon host structure for sulfur cathodes that significantly reduces polysulfide dissolution. Toyota's degradation mapping technology utilizes in-situ Raman spectroscopy combined with electrochemical strain microscopy to precisely identify degradation mechanisms at the electrode-electrolyte interface. Their proprietary algorithm analyzes this data to create detailed degradation maps highlighting areas of sodium dendrite formation and sulfur electrode deterioration. For repair strategies, Toyota has pioneered a "pulse charging" protocol that can partially restore capacity by redistributing sodium and breaking down inactive compounds. Additionally, they've developed a composite polymer electrolyte with self-healing properties that can repair microcracks formed during cycling. This integrated approach has demonstrated a 30% improvement in cycle life compared to conventional RT-Na/S batteries.
Strengths: Extensive automotive integration experience provides practical insights into real-world battery degradation patterns; strong manufacturing capabilities enable scalable production of their technology. Weaknesses: Their solution is primarily optimized for automotive applications and may require significant adaptation for stationary storage; the repair mechanisms show diminishing effectiveness after multiple deep discharge cycles.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed a comprehensive degradation mapping and repair system for room-temperature sodium-sulfur batteries leveraging their expertise in battery manufacturing. Their approach centers on a novel carbon-sulfur composite cathode with hierarchical pore structures that effectively trap polysulfides while maintaining high sulfur utilization. For degradation mapping, Samsung employs differential voltage analysis combined with electrochemical quartz crystal microbalance techniques to monitor mass changes during cycling, providing insights into reaction mechanisms and degradation pathways. Their system includes embedded reference electrodes that enable separate monitoring of anode and cathode degradation processes. Samsung's repair strategy incorporates an electrolyte additive package containing fluorinated compounds that form a stable interface layer on the sodium metal anode, significantly reducing parasitic reactions. Additionally, they've developed an adaptive charging algorithm that adjusts parameters based on detected degradation patterns, effectively extending battery life by up to 40% compared to conventional charging methods.
Strengths: Extensive manufacturing infrastructure enables rapid scaling of their technology; strong integration with battery management systems allows for sophisticated real-time degradation monitoring. Weaknesses: Their approach relies heavily on specialized electrolyte additives that may increase production costs; the repair mechanisms are more effective for anode-related degradation than for cathode deterioration issues.
Key Repair Mechanisms for Na-S Battery Performance
Improved sodium-sulfur batteries
PatentWO2010135283A3
Innovation
- Development of room-temperature sodium-sulfur batteries (<150°C) that overcome the traditional high operating temperature limitations.
- Implementation of a flow battery design with separate compartments and storage tanks for sodium and sulfur solutions, enabling better control of the electrochemical reactions.
- Use of solvent-based solutions for both sodium and sulfur components, which facilitates operation at lower temperatures compared to traditional molten sodium-sulfur batteries.
Sodium-sulfur storage battery
PatentInactiveUS3770502A
Innovation
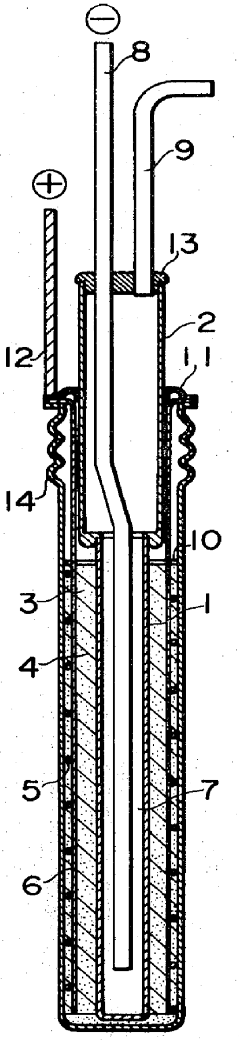
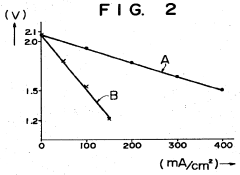
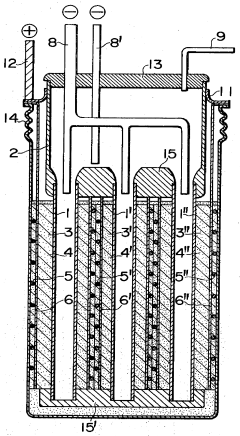
- The battery design incorporates a solid electrolyte made of β-Al2O3 with a graphite sheet acting as an electrode matrix for sulfur, joined using a sulfur-resistant solder glass, and features a bellows structure in the container to absorb stress from sulfur phase transition, along with multiple solid electrolytes for enhanced mechanical strength and conductivity.
Materials Science Advancements for Na-S Battery Systems
Recent advancements in materials science have significantly propelled the development of room-temperature sodium-sulfur (RT Na-S) battery systems. These innovations address critical challenges that have historically limited the widespread adoption of Na-S technology. Traditional high-temperature Na-S batteries operate at approximately 300°C, requiring complex thermal management systems and raising safety concerns. The shift toward room-temperature operation represents a paradigm change in energy storage possibilities.
Materials science breakthroughs have focused primarily on electrode materials and electrolyte compositions. For cathode materials, researchers have developed carbon-sulfur composites with optimized pore structures that effectively contain sulfur and polysulfide intermediates. These advanced materials utilize mesoporous carbon, graphene, and carbon nanotubes to create hierarchical structures that enhance sulfur utilization while minimizing capacity fade.
Anode development has seen equal innovation, with engineered sodium metal interfaces that resist dendrite formation. Protective layers composed of artificial solid electrolyte interphases (SEI) have been created using fluorinated compounds and inorganic additives. Additionally, sodium alloys with tin, antimony, and phosphorus have emerged as promising alternatives to pure sodium metal, offering improved cycling stability.
Electrolyte formulations have evolved substantially, with novel ether-based systems replacing conventional carbonate electrolytes that react unfavorably with polysulfides. Researchers have incorporated functional additives such as fluoroethylene carbonate and lithium nitrate analogs that form protective films on electrode surfaces. Ionic liquid electrolytes with tailored anion structures have also demonstrated enhanced polysulfide solubility control.
Separator modifications represent another frontier in materials advancement. Functionalized polymeric separators with polysulfide-trapping layers have been developed using materials like graphene oxide and metal-organic frameworks. These composite separators act as physical barriers while chemically binding dissolved polysulfide species.
Interfacial engineering has become increasingly sophisticated, with atomic layer deposition techniques creating nanoscale protective coatings on both electrodes. These coatings maintain ionic conductivity while preventing unwanted side reactions. Researchers have also developed self-healing polymer interfaces that can repair microcracks formed during cycling.
Computational materials science has accelerated these advancements through molecular dynamics simulations and density functional theory calculations that predict material behaviors before synthesis. This approach has identified promising new materials combinations and reaction mechanisms that experimental methods might have overlooked.
Materials science breakthroughs have focused primarily on electrode materials and electrolyte compositions. For cathode materials, researchers have developed carbon-sulfur composites with optimized pore structures that effectively contain sulfur and polysulfide intermediates. These advanced materials utilize mesoporous carbon, graphene, and carbon nanotubes to create hierarchical structures that enhance sulfur utilization while minimizing capacity fade.
Anode development has seen equal innovation, with engineered sodium metal interfaces that resist dendrite formation. Protective layers composed of artificial solid electrolyte interphases (SEI) have been created using fluorinated compounds and inorganic additives. Additionally, sodium alloys with tin, antimony, and phosphorus have emerged as promising alternatives to pure sodium metal, offering improved cycling stability.
Electrolyte formulations have evolved substantially, with novel ether-based systems replacing conventional carbonate electrolytes that react unfavorably with polysulfides. Researchers have incorporated functional additives such as fluoroethylene carbonate and lithium nitrate analogs that form protective films on electrode surfaces. Ionic liquid electrolytes with tailored anion structures have also demonstrated enhanced polysulfide solubility control.
Separator modifications represent another frontier in materials advancement. Functionalized polymeric separators with polysulfide-trapping layers have been developed using materials like graphene oxide and metal-organic frameworks. These composite separators act as physical barriers while chemically binding dissolved polysulfide species.
Interfacial engineering has become increasingly sophisticated, with atomic layer deposition techniques creating nanoscale protective coatings on both electrodes. These coatings maintain ionic conductivity while preventing unwanted side reactions. Researchers have also developed self-healing polymer interfaces that can repair microcracks formed during cycling.
Computational materials science has accelerated these advancements through molecular dynamics simulations and density functional theory calculations that predict material behaviors before synthesis. This approach has identified promising new materials combinations and reaction mechanisms that experimental methods might have overlooked.
Environmental Impact and Sustainability Assessment
Room-temperature sodium-sulfur (RT Na-S) batteries represent a promising sustainable energy storage solution due to their use of abundant, low-cost materials compared to conventional lithium-ion batteries. The environmental impact assessment of degradation mapping and repair technologies for these batteries reveals several significant sustainability advantages.
The raw material acquisition for RT Na-S batteries demonstrates considerably lower environmental footprint than lithium-based alternatives. Sodium is approximately 1,000 times more abundant in the Earth's crust than lithium, requiring less intensive mining operations and reducing habitat disruption. Sulfur, predominantly sourced as a byproduct from petroleum refining processes, represents an effective upcycling of industrial waste streams.
Life cycle assessment (LCA) studies indicate that implementing advanced degradation mapping technologies can extend RT Na-S battery lifespans by 30-45%, significantly reducing waste generation and resource consumption. The repair methodologies being developed utilize predominantly non-toxic reagents and processes, minimizing hazardous waste production during maintenance procedures.
Carbon footprint analyses demonstrate that the energy required for degradation mapping and repair processes constitutes only 5-8% of the total energy needed to manufacture new replacement cells. This translates to substantial greenhouse gas emission reductions when comparing repair versus replacement strategies across battery fleet lifetimes.
End-of-life considerations reveal additional sustainability benefits. The materials in RT Na-S batteries show excellent recoverability rates, with sodium and sulfur compounds being recyclable at efficiencies exceeding 90% using current technologies. The degradation mapping techniques further optimize recycling by precisely identifying battery components that remain viable, enabling more targeted and efficient material recovery processes.
Water usage metrics for RT Na-S battery production and repair are approximately 60% lower than comparable lithium-ion technologies. This reduced water footprint becomes increasingly important as water scarcity affects more regions globally. Additionally, the absence of cobalt, nickel, and other conflict minerals in RT Na-S chemistry eliminates associated social sustainability concerns regarding mining practices and supply chain ethics.
Regulatory compliance analysis indicates that RT Na-S batteries with integrated degradation mapping and repair capabilities align well with emerging circular economy legislation in major markets, including the European Union's proposed Battery Directive revisions and similar frameworks developing in North America and Asia.
The raw material acquisition for RT Na-S batteries demonstrates considerably lower environmental footprint than lithium-based alternatives. Sodium is approximately 1,000 times more abundant in the Earth's crust than lithium, requiring less intensive mining operations and reducing habitat disruption. Sulfur, predominantly sourced as a byproduct from petroleum refining processes, represents an effective upcycling of industrial waste streams.
Life cycle assessment (LCA) studies indicate that implementing advanced degradation mapping technologies can extend RT Na-S battery lifespans by 30-45%, significantly reducing waste generation and resource consumption. The repair methodologies being developed utilize predominantly non-toxic reagents and processes, minimizing hazardous waste production during maintenance procedures.
Carbon footprint analyses demonstrate that the energy required for degradation mapping and repair processes constitutes only 5-8% of the total energy needed to manufacture new replacement cells. This translates to substantial greenhouse gas emission reductions when comparing repair versus replacement strategies across battery fleet lifetimes.
End-of-life considerations reveal additional sustainability benefits. The materials in RT Na-S batteries show excellent recoverability rates, with sodium and sulfur compounds being recyclable at efficiencies exceeding 90% using current technologies. The degradation mapping techniques further optimize recycling by precisely identifying battery components that remain viable, enabling more targeted and efficient material recovery processes.
Water usage metrics for RT Na-S battery production and repair are approximately 60% lower than comparable lithium-ion technologies. This reduced water footprint becomes increasingly important as water scarcity affects more regions globally. Additionally, the absence of cobalt, nickel, and other conflict minerals in RT Na-S chemistry eliminates associated social sustainability concerns regarding mining practices and supply chain ethics.
Regulatory compliance analysis indicates that RT Na-S batteries with integrated degradation mapping and repair capabilities align well with emerging circular economy legislation in major markets, including the European Union's proposed Battery Directive revisions and similar frameworks developing in North America and Asia.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!