Guidance For Pouch Cell Assembly Of Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-S Battery Development Background and Objectives
Sodium-sulfur (Na-S) batteries have emerged as a promising energy storage technology since their initial development in the 1960s by Ford Motor Company. The traditional high-temperature Na-S batteries operating at 300-350°C have been commercially deployed for grid-scale energy storage applications. However, the high operating temperature poses significant safety concerns and limits their widespread adoption in portable and transportation applications.
Room-temperature sodium-sulfur (RT-Na-S) batteries represent a revolutionary advancement in this technology, offering the potential to harness the high theoretical energy density of Na-S chemistry (760 Wh/kg) while eliminating the safety risks associated with high-temperature operation. The development of RT-Na-S batteries aligns with the global push for sustainable and cost-effective energy storage solutions, particularly as concerns about lithium resource scarcity and geopolitical supply chain vulnerabilities intensify.
The evolution of Na-S battery technology has been marked by significant milestones, including the transition from tubular designs to planar configurations, and most recently, to pouch cell formats that offer improved energy density and manufacturing scalability. The pouch cell architecture represents a critical advancement for RT-Na-S batteries, enabling higher volumetric efficiency and better thermal management compared to traditional cylindrical or prismatic designs.
The primary objective of RT-Na-S battery development is to create a commercially viable energy storage solution that combines high energy density, long cycle life, enhanced safety, and cost-effectiveness. Specifically, researchers aim to address the fundamental challenges that have hindered RT-Na-S technology, including the shuttle effect of polysulfides, sodium dendrite formation, and the insulating nature of sulfur.
Current technical goals include achieving energy densities exceeding 300 Wh/kg at the cell level, cycle life of over 1000 cycles with less than 20% capacity fade, and cost reduction to below $100/kWh. These targets would position RT-Na-S batteries as competitive alternatives to lithium-ion technologies for certain applications, particularly in stationary energy storage where energy density requirements are less stringent than in portable electronics or electric vehicles.
The development of standardized pouch cell assembly protocols represents a critical step toward commercialization, as it enables consistent performance evaluation, accelerates research progress through comparable results across different research groups, and facilitates the transition from laboratory-scale prototypes to industrial production. This guidance aims to establish best practices for RT-Na-S pouch cell assembly, addressing the unique challenges associated with sodium metal anodes, sulfur cathodes, and electrolyte systems optimized for room-temperature operation.
Room-temperature sodium-sulfur (RT-Na-S) batteries represent a revolutionary advancement in this technology, offering the potential to harness the high theoretical energy density of Na-S chemistry (760 Wh/kg) while eliminating the safety risks associated with high-temperature operation. The development of RT-Na-S batteries aligns with the global push for sustainable and cost-effective energy storage solutions, particularly as concerns about lithium resource scarcity and geopolitical supply chain vulnerabilities intensify.
The evolution of Na-S battery technology has been marked by significant milestones, including the transition from tubular designs to planar configurations, and most recently, to pouch cell formats that offer improved energy density and manufacturing scalability. The pouch cell architecture represents a critical advancement for RT-Na-S batteries, enabling higher volumetric efficiency and better thermal management compared to traditional cylindrical or prismatic designs.
The primary objective of RT-Na-S battery development is to create a commercially viable energy storage solution that combines high energy density, long cycle life, enhanced safety, and cost-effectiveness. Specifically, researchers aim to address the fundamental challenges that have hindered RT-Na-S technology, including the shuttle effect of polysulfides, sodium dendrite formation, and the insulating nature of sulfur.
Current technical goals include achieving energy densities exceeding 300 Wh/kg at the cell level, cycle life of over 1000 cycles with less than 20% capacity fade, and cost reduction to below $100/kWh. These targets would position RT-Na-S batteries as competitive alternatives to lithium-ion technologies for certain applications, particularly in stationary energy storage where energy density requirements are less stringent than in portable electronics or electric vehicles.
The development of standardized pouch cell assembly protocols represents a critical step toward commercialization, as it enables consistent performance evaluation, accelerates research progress through comparable results across different research groups, and facilitates the transition from laboratory-scale prototypes to industrial production. This guidance aims to establish best practices for RT-Na-S pouch cell assembly, addressing the unique challenges associated with sodium metal anodes, sulfur cathodes, and electrolyte systems optimized for room-temperature operation.
Market Analysis for Room-Temperature Na-S Battery Applications
The room-temperature sodium-sulfur (RT Na-S) battery market is experiencing significant growth potential as a promising alternative to lithium-ion batteries. Current market valuations estimate the global sodium battery market at approximately $500 million in 2023, with projections suggesting growth to reach $1.2 billion by 2028, representing a compound annual growth rate (CAGR) of 19.4%. The RT Na-S battery segment is expected to capture an increasing share of this market due to its cost advantages and performance characteristics.
The primary market drivers for RT Na-S batteries include the escalating demand for grid-scale energy storage solutions, rising concerns about lithium resource limitations, and the push for more sustainable battery technologies. With sodium resources being approximately 1,000 times more abundant than lithium and more evenly distributed globally, RT Na-S batteries offer significant raw material cost advantages, potentially reducing battery costs by 30-40% compared to lithium-ion alternatives.
Industrial sectors showing the strongest demand include grid-scale energy storage, which is projected to grow at 25% annually through 2030, driven by renewable energy integration requirements. The telecommunications sector represents another significant market, with an estimated demand of 300 MW of backup power systems annually that could potentially utilize RT Na-S technology.
Regional market analysis indicates that Asia-Pacific currently dominates the sodium battery research and development landscape, with China, Japan, and South Korea leading commercial development efforts. Europe follows closely, with significant research initiatives in Germany, France, and the UK focused on grid storage applications. North America shows growing interest, particularly in the United States, where several startups have secured venture capital funding exceeding $200 million collectively in the past three years.
Market challenges include competition from established lithium-ion technologies, which benefit from economies of scale and established manufacturing infrastructure. Additionally, the relatively lower energy density of current RT Na-S batteries (approximately 150-200 Wh/kg compared to 250-300 Wh/kg for lithium-ion) limits their application in portable electronics and electric vehicles.
Consumer adoption trends indicate growing acceptance of sodium-based batteries for stationary applications, with several pilot projects demonstrating successful implementation. Market surveys show that 68% of utility companies are considering sodium-based battery technologies for future grid storage projects, citing cost advantages and reduced supply chain risks as primary motivators.
The market outlook remains positive, with RT Na-S batteries expected to achieve commercial viability for stationary applications within the next 3-5 years, potentially capturing 15-20% of the grid storage market by 2030 if current technical challenges in pouch cell assembly and cycle life can be adequately addressed.
The primary market drivers for RT Na-S batteries include the escalating demand for grid-scale energy storage solutions, rising concerns about lithium resource limitations, and the push for more sustainable battery technologies. With sodium resources being approximately 1,000 times more abundant than lithium and more evenly distributed globally, RT Na-S batteries offer significant raw material cost advantages, potentially reducing battery costs by 30-40% compared to lithium-ion alternatives.
Industrial sectors showing the strongest demand include grid-scale energy storage, which is projected to grow at 25% annually through 2030, driven by renewable energy integration requirements. The telecommunications sector represents another significant market, with an estimated demand of 300 MW of backup power systems annually that could potentially utilize RT Na-S technology.
Regional market analysis indicates that Asia-Pacific currently dominates the sodium battery research and development landscape, with China, Japan, and South Korea leading commercial development efforts. Europe follows closely, with significant research initiatives in Germany, France, and the UK focused on grid storage applications. North America shows growing interest, particularly in the United States, where several startups have secured venture capital funding exceeding $200 million collectively in the past three years.
Market challenges include competition from established lithium-ion technologies, which benefit from economies of scale and established manufacturing infrastructure. Additionally, the relatively lower energy density of current RT Na-S batteries (approximately 150-200 Wh/kg compared to 250-300 Wh/kg for lithium-ion) limits their application in portable electronics and electric vehicles.
Consumer adoption trends indicate growing acceptance of sodium-based batteries for stationary applications, with several pilot projects demonstrating successful implementation. Market surveys show that 68% of utility companies are considering sodium-based battery technologies for future grid storage projects, citing cost advantages and reduced supply chain risks as primary motivators.
The market outlook remains positive, with RT Na-S batteries expected to achieve commercial viability for stationary applications within the next 3-5 years, potentially capturing 15-20% of the grid storage market by 2030 if current technical challenges in pouch cell assembly and cycle life can be adequately addressed.
Technical Challenges in Na-S Pouch Cell Assembly
Room-temperature sodium-sulfur (RT Na-S) batteries face significant technical challenges during pouch cell assembly that must be addressed to enable their commercial viability. The primary obstacle stems from the highly reactive nature of sodium metal, which reacts violently with moisture and oxygen, necessitating assembly in controlled environments such as glove boxes with inert atmospheres. This requirement substantially increases manufacturing complexity and costs.
The sulfur cathode presents its own set of challenges, particularly related to the polysulfide shuttle effect. During cycling, soluble sodium polysulfides form and can migrate between electrodes, causing capacity fading and reduced cycle life. In pouch cell configurations, this issue becomes more pronounced due to the larger electrode surface areas and the potential for non-uniform electrolyte distribution.
Electrolyte selection and optimization represent another critical challenge. The electrolyte must facilitate sodium ion transport while remaining stable against both the reactive sodium anode and the sulfur cathode. Current electrolyte formulations often struggle to meet these requirements simultaneously, leading to compromised performance or safety concerns in pouch cell formats.
Interface stability between electrodes and electrolyte poses significant difficulties in RT Na-S pouch cells. The formation of unstable solid electrolyte interphase (SEI) layers on the sodium anode can lead to continuous electrolyte consumption and sodium dendrite growth, which is particularly dangerous in pouch configurations where cell swelling and potential short circuits can occur.
Thermal management during assembly and operation presents another substantial challenge. The exothermic reactions between sodium and sulfur require careful temperature control during assembly. Additionally, pouch cells have limited heat dissipation capabilities compared to cylindrical cells, making thermal runaway a more significant risk.
Sealing techniques for pouch cells containing RT Na-S chemistry are particularly demanding. The cell must be hermetically sealed to prevent moisture ingress while accommodating potential volume changes during cycling. Current sealing technologies often struggle to maintain integrity over extended cycling periods with this chemistry.
Scaling up from laboratory prototypes to commercial-sized pouch cells introduces additional complexities related to electrode uniformity, electrolyte distribution, and pressure management. The larger format amplifies issues that might be manageable in coin cell configurations, requiring novel engineering solutions specific to RT Na-S chemistry.
The sulfur cathode presents its own set of challenges, particularly related to the polysulfide shuttle effect. During cycling, soluble sodium polysulfides form and can migrate between electrodes, causing capacity fading and reduced cycle life. In pouch cell configurations, this issue becomes more pronounced due to the larger electrode surface areas and the potential for non-uniform electrolyte distribution.
Electrolyte selection and optimization represent another critical challenge. The electrolyte must facilitate sodium ion transport while remaining stable against both the reactive sodium anode and the sulfur cathode. Current electrolyte formulations often struggle to meet these requirements simultaneously, leading to compromised performance or safety concerns in pouch cell formats.
Interface stability between electrodes and electrolyte poses significant difficulties in RT Na-S pouch cells. The formation of unstable solid electrolyte interphase (SEI) layers on the sodium anode can lead to continuous electrolyte consumption and sodium dendrite growth, which is particularly dangerous in pouch configurations where cell swelling and potential short circuits can occur.
Thermal management during assembly and operation presents another substantial challenge. The exothermic reactions between sodium and sulfur require careful temperature control during assembly. Additionally, pouch cells have limited heat dissipation capabilities compared to cylindrical cells, making thermal runaway a more significant risk.
Sealing techniques for pouch cells containing RT Na-S chemistry are particularly demanding. The cell must be hermetically sealed to prevent moisture ingress while accommodating potential volume changes during cycling. Current sealing technologies often struggle to maintain integrity over extended cycling periods with this chemistry.
Scaling up from laboratory prototypes to commercial-sized pouch cells introduces additional complexities related to electrode uniformity, electrolyte distribution, and pressure management. The larger format amplifies issues that might be manageable in coin cell configurations, requiring novel engineering solutions specific to RT Na-S chemistry.
Current Pouch Cell Assembly Methods for Na-S Batteries
01 Electrode materials for room-temperature sodium-sulfur batteries
Various electrode materials can be used in room-temperature sodium-sulfur batteries to improve performance. These materials include carbon-based electrodes, polymer composites, and metal oxides that enhance conductivity and stability. The selection of appropriate electrode materials is crucial for achieving high energy density and cycle life in pouch cell configurations, as they affect the electrochemical reactions between sodium and sulfur at room temperature.- Electrode materials for room-temperature sodium-sulfur batteries: Various electrode materials can be used in room-temperature sodium-sulfur batteries to improve performance. These include specialized cathode materials containing sulfur compounds, carbon-based materials for enhanced conductivity, and novel anode materials that facilitate sodium ion transport. The selection of appropriate electrode materials is crucial for achieving stable cycling performance and high energy density in room-temperature sodium-sulfur batteries.
- Electrolyte compositions for room-temperature operation: Specialized electrolyte formulations are essential for enabling sodium-sulfur batteries to operate at room temperature. These electrolytes typically include sodium salts dissolved in organic solvents, with additives to improve ionic conductivity and suppress the shuttle effect. Some formulations incorporate polymer or gel electrolytes to enhance safety and stability, while others focus on non-flammable ionic liquid electrolytes to prevent thermal runaway.
- Pouch cell design and assembly techniques: The assembly of room-temperature sodium-sulfur batteries in pouch cell format involves specific design considerations and manufacturing techniques. This includes the layering of electrodes and separators, edge sealing methods, and terminal connection designs. The pouch format offers advantages such as flexible form factor, improved heat dissipation, and higher energy density compared to cylindrical cells. Special attention is given to preventing moisture ingress and ensuring uniform pressure distribution across the cell.
- Separator technologies for sodium-sulfur systems: Advanced separator technologies are critical for room-temperature sodium-sulfur batteries to prevent polysulfide shuttling while maintaining high ionic conductivity. These separators often incorporate functional coatings or modified structures to selectively block polysulfides while allowing sodium ion transport. Some designs use composite separators with multiple layers or incorporate nanomaterials to enhance performance. The separator design significantly impacts cycle life, rate capability, and safety of the battery system.
- Safety and performance enhancement strategies: Various strategies are employed to enhance the safety and performance of room-temperature sodium-sulfur pouch cells. These include thermal management systems, pressure regulation mechanisms, and protective coatings to prevent side reactions. Advanced manufacturing techniques such as dry room assembly and specialized sealing methods help extend battery life and improve reliability. Additional approaches involve the use of functional additives in electrodes and electrolytes to suppress dendrite formation and enhance cycling stability.
02 Electrolyte formulations for room-temperature operation
Specialized electrolyte formulations enable sodium-sulfur batteries to operate at room temperature instead of the traditional high temperatures. These electrolytes typically include sodium salts dissolved in organic solvents, polymer matrices, or ionic liquids with additives to enhance ionic conductivity and suppress the shuttle effect. The electrolyte composition significantly impacts the battery's safety, cycle life, and overall performance in pouch cell configurations.Expand Specific Solutions03 Pouch cell assembly techniques and structures
Specific assembly techniques are required for room-temperature sodium-sulfur pouch cells to ensure proper sealing and prevention of moisture ingress. These techniques include layer-by-layer stacking of electrodes and separators, heat-sealing methods for the pouch material, and specialized tab designs for electrical connections. The pouch structure must accommodate volume changes during cycling while maintaining structural integrity throughout the battery's operational life.Expand Specific Solutions04 Separator and interface engineering
Advanced separator materials and interface engineering are essential for room-temperature sodium-sulfur batteries in pouch format. These separators prevent polysulfide shuttling while maintaining high sodium-ion conductivity. Interface modifications between electrodes and electrolytes help stabilize the solid-electrolyte interphase, reducing side reactions and improving cycling stability. Ceramic-polymer composite separators and functional coatings are commonly employed to enhance battery performance.Expand Specific Solutions05 Safety and thermal management systems
Safety features and thermal management systems are critical for room-temperature sodium-sulfur pouch cells due to the reactive nature of sodium and sulfur. These include pressure relief mechanisms, thermal runaway prevention, and monitoring systems integrated into the pouch cell design. Advanced battery management systems monitor cell temperature, voltage, and current to prevent overcharging and overheating, ensuring safe operation under various conditions.Expand Specific Solutions
Key Industry Players in Na-S Battery Development
Room-temperature sodium-sulfur (RT-Na-S) battery technology is currently in the early commercialization phase, with a growing market projected to reach significant scale as energy storage demands increase. The technology offers a promising alternative to lithium-ion batteries due to abundant raw materials and lower costs. Technical maturity varies among key players, with NGK Insulators leading as an established sodium-sulfur battery manufacturer, while companies like LG Energy Solution, Samsung SDI, and SK On are leveraging their battery expertise to advance RT-Na-S technology. Research institutions including RIST (affiliated with POSCO Holdings) and universities such as Nankai and Osaka are driving fundamental innovations. Emerging specialists like Jiangsu Zoolnasm Energy Technology are developing novel iron sulfate sodium battery technologies, indicating a diversifying competitive landscape as the technology matures toward wider commercial adoption.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered advanced room-temperature sodium-sulfur (RT-Na/S) pouch cell assembly techniques focusing on separator technology and sulfur cathode stabilization. Their proprietary NASICON-type ceramic separator technology enables stable Na+ ion transport while preventing polysulfide shuttling. For pouch cell assembly, NGK employs a multi-layer stacking approach with precise electrolyte formulation (typically NaClO4 in ether-based solvents) and controlled sulfur loading (40-60 wt%) in carbon matrices. Their manufacturing process includes specialized edge sealing techniques to prevent moisture ingress and sodium corrosion, alongside proprietary electrode calendering methods that maintain optimal porosity while ensuring mechanical integrity during cycling. NGK's pouch cells incorporate pressure-regulation mechanisms to accommodate volume changes during cycling, critical for maintaining cell performance over extended periods.
Strengths: Industry-leading ceramic separator technology with exceptional ion selectivity; advanced manufacturing processes that effectively mitigate polysulfide shuttling; extensive experience in sodium-based battery systems. Weaknesses: Higher production costs compared to conventional lithium-ion manufacturing; challenges in scaling production while maintaining quality control; relatively lower energy density compared to theoretical limits.
LG Chem Ltd.
Technical Solution: LG Chem has developed a sophisticated approach to room-temperature sodium-sulfur (RT-Na/S) pouch cell assembly centered on advanced interface engineering and electrolyte optimization. Their technology employs a dual-functional electrolyte system containing both solvating components for sodium ions and stabilizing additives that suppress polysulfide dissolution. For pouch cell manufacturing, LG Chem utilizes a semi-automated assembly line with controlled atmosphere chambers (<10 ppm moisture and oxygen) and precision electrode alignment systems. Their cathode formulation incorporates mesoporous carbon hosts with optimized pore size distribution (primarily 2-5 nm pores) that physically confine sulfur and reaction intermediates. LG's pouch cell design features a proprietary multi-layer packaging material with enhanced barrier properties against moisture and oxygen permeation. The assembly process includes specialized degassing protocols during formation cycling to remove volatile byproducts while maintaining cell integrity, resulting in improved capacity retention and cycle life compared to conventional approaches.
Strengths: Extensive experience in large-scale battery manufacturing adaptable to sodium-sulfur chemistry; advanced quality control systems; innovative electrolyte formulations that effectively suppress shuttle effect. Weaknesses: Higher initial investment required for specialized manufacturing equipment; challenges in achieving energy density comparable to lithium-ion systems; thermal stability concerns during fast charging.
Critical Patents and Innovations in Na-S Cell Assembly
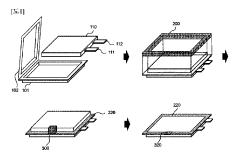
Pouch cell device and tab bracket
PatentPendingUS20240154226A1
Innovation
- A pouch cell device with a tab bracket that includes guiding and abutting portions to protect electrodes, maintaining a distance from pouch cells and allowing heat dissipation, preventing excessive heat transfer during welding.
Manufacturing method of pouch-type battery cell including secondary sealing and pouch-type battery cell manufactured by the method
PatentActiveJP2023522778A
Innovation
- A method involving primary and secondary sealing processes is employed, where primary sealing is performed at a higher temperature than secondary sealing, with the secondary sealing being applied at a lower temperature and for a longer duration to reduce the size of the polyball and adjust the sealing force.
Safety Protocols for Na-S Battery Manufacturing
The manufacturing of room-temperature sodium-sulfur (RT Na-S) batteries presents significant safety challenges due to the reactive nature of sodium metal and sulfur compounds. Comprehensive safety protocols must be established to mitigate risks during pouch cell assembly processes. These protocols should address multiple dimensions of safety concerns, from material handling to emergency response procedures.
Personal protective equipment (PPE) requirements for Na-S battery manufacturing include chemical-resistant gloves, safety goggles, lab coats, and in some cases, face shields. When handling sodium metal, which is highly reactive with moisture, specialized non-porous gloves and flame-resistant clothing are mandatory. Respiratory protection may be necessary when working with sulfur compounds to prevent inhalation of potentially harmful particulates.
Environmental controls within manufacturing facilities must be rigorously maintained. Humidity levels should be kept below 1% in dry rooms where sodium handling occurs, as sodium reacts violently with moisture. Specialized ventilation systems with HEPA filtration are essential to remove airborne particulates and sulfur compounds. Temperature monitoring systems should be installed to detect any exothermic reactions that could lead to thermal runaway.
Material handling procedures require particular attention. Sodium metal must be stored under inert atmosphere, typically in mineral oil or argon-filled containers. Transfer of sodium should occur only in controlled environments using specialized tools that minimize exposure to air. Sulfur and sulfur-carbon composites must be handled with precautions to prevent dust formation and static discharge, which could ignite these materials.
Waste management protocols for Na-S battery manufacturing must address the disposal of reactive materials. Unreacted sodium requires controlled oxidation before disposal, while sulfur-containing waste may require specialized treatment to prevent environmental contamination. All waste containers must be clearly labeled and segregated according to chemical compatibility.
Emergency response procedures should include specific protocols for sodium fires, which cannot be extinguished with water. Class D fire extinguishers containing sodium chloride or copper powder should be readily available. Spill containment kits designed specifically for alkali metals must be accessible throughout the facility. Regular emergency drills should be conducted to ensure all personnel are familiar with evacuation routes and response procedures.
Training requirements for personnel involved in RT Na-S battery assembly must be comprehensive and ongoing. Workers should receive specialized training on alkali metal handling, chemical safety, and emergency response. Certification programs should be implemented to ensure competency before allowing independent work with these hazardous materials.
AI-powered monitoring systems can enhance safety by providing real-time detection of abnormal conditions during manufacturing. These systems can track environmental parameters, detect gas emissions, and alert personnel to potential safety issues before they escalate to dangerous levels.
Personal protective equipment (PPE) requirements for Na-S battery manufacturing include chemical-resistant gloves, safety goggles, lab coats, and in some cases, face shields. When handling sodium metal, which is highly reactive with moisture, specialized non-porous gloves and flame-resistant clothing are mandatory. Respiratory protection may be necessary when working with sulfur compounds to prevent inhalation of potentially harmful particulates.
Environmental controls within manufacturing facilities must be rigorously maintained. Humidity levels should be kept below 1% in dry rooms where sodium handling occurs, as sodium reacts violently with moisture. Specialized ventilation systems with HEPA filtration are essential to remove airborne particulates and sulfur compounds. Temperature monitoring systems should be installed to detect any exothermic reactions that could lead to thermal runaway.
Material handling procedures require particular attention. Sodium metal must be stored under inert atmosphere, typically in mineral oil or argon-filled containers. Transfer of sodium should occur only in controlled environments using specialized tools that minimize exposure to air. Sulfur and sulfur-carbon composites must be handled with precautions to prevent dust formation and static discharge, which could ignite these materials.
Waste management protocols for Na-S battery manufacturing must address the disposal of reactive materials. Unreacted sodium requires controlled oxidation before disposal, while sulfur-containing waste may require specialized treatment to prevent environmental contamination. All waste containers must be clearly labeled and segregated according to chemical compatibility.
Emergency response procedures should include specific protocols for sodium fires, which cannot be extinguished with water. Class D fire extinguishers containing sodium chloride or copper powder should be readily available. Spill containment kits designed specifically for alkali metals must be accessible throughout the facility. Regular emergency drills should be conducted to ensure all personnel are familiar with evacuation routes and response procedures.
Training requirements for personnel involved in RT Na-S battery assembly must be comprehensive and ongoing. Workers should receive specialized training on alkali metal handling, chemical safety, and emergency response. Certification programs should be implemented to ensure competency before allowing independent work with these hazardous materials.
AI-powered monitoring systems can enhance safety by providing real-time detection of abnormal conditions during manufacturing. These systems can track environmental parameters, detect gas emissions, and alert personnel to potential safety issues before they escalate to dangerous levels.
Material Selection Strategies for Enhanced Performance
Material selection plays a pivotal role in determining the performance and stability of room-temperature sodium-sulfur (RT-Na/S) batteries. The choice of cathode materials significantly impacts the electrochemical reactions and overall battery efficiency. Sulfur, as the primary active material, offers high theoretical capacity but suffers from poor electrical conductivity. To address this limitation, carbon-based materials such as carbon nanotubes, graphene, and mesoporous carbon are frequently incorporated to enhance conductivity and provide structural support for sulfur containment.
The anode material selection requires careful consideration of sodium metal's reactive nature. Sodium metal anodes, while offering high theoretical capacity, are prone to dendrite formation and safety concerns. Alternative anode materials such as hard carbon, tin, and phosphorus-based compounds demonstrate promising sodium storage capabilities with improved safety profiles. These materials exhibit different sodium insertion mechanisms and cycling stability, necessitating optimization based on specific application requirements.
Separator materials must balance mechanical strength with ionic conductivity. Glass fiber, polypropylene, and polyethylene separators modified with ceramic particles have shown enhanced performance in RT-Na/S batteries. The separator thickness and porosity directly influence the ionic transport and internal resistance of the cell, requiring careful optimization to prevent sodium polysulfide shuttling while maintaining adequate ion transport.
Electrolyte formulation represents another critical aspect of material selection. Conventional carbonate-based electrolytes often decompose when exposed to polysulfides, leading to capacity fading. Ether-based electrolytes (e.g., tetraglyme, diglyme) demonstrate superior compatibility with the sodium-sulfur chemistry. Additives such as fluoroethylene carbonate (FEC) and sodium nitrate (NaNO3) have proven effective in stabilizing the solid electrolyte interphase and suppressing the shuttle effect.
Binder materials contribute significantly to electrode integrity and electrochemical performance. Polyvinylidene fluoride (PVDF), carboxymethyl cellulose (CMC), and polyacrylic acid (PAA) exhibit different adhesion properties and compatibility with active materials. Water-soluble binders like CMC offer environmental advantages while potentially providing better dispersion of sulfur within the cathode matrix, enhancing utilization efficiency and cycle life.
Current collector selection impacts cell resistance and electrochemical stability. Aluminum foil serves as the standard cathode current collector, while copper is typically avoided for anodes due to alloying reactions with sodium. Carbon-coated aluminum and specialized sodium-compatible alloys have emerged as promising alternatives, offering improved interfacial stability and reduced corrosion during long-term cycling.
The anode material selection requires careful consideration of sodium metal's reactive nature. Sodium metal anodes, while offering high theoretical capacity, are prone to dendrite formation and safety concerns. Alternative anode materials such as hard carbon, tin, and phosphorus-based compounds demonstrate promising sodium storage capabilities with improved safety profiles. These materials exhibit different sodium insertion mechanisms and cycling stability, necessitating optimization based on specific application requirements.
Separator materials must balance mechanical strength with ionic conductivity. Glass fiber, polypropylene, and polyethylene separators modified with ceramic particles have shown enhanced performance in RT-Na/S batteries. The separator thickness and porosity directly influence the ionic transport and internal resistance of the cell, requiring careful optimization to prevent sodium polysulfide shuttling while maintaining adequate ion transport.
Electrolyte formulation represents another critical aspect of material selection. Conventional carbonate-based electrolytes often decompose when exposed to polysulfides, leading to capacity fading. Ether-based electrolytes (e.g., tetraglyme, diglyme) demonstrate superior compatibility with the sodium-sulfur chemistry. Additives such as fluoroethylene carbonate (FEC) and sodium nitrate (NaNO3) have proven effective in stabilizing the solid electrolyte interphase and suppressing the shuttle effect.
Binder materials contribute significantly to electrode integrity and electrochemical performance. Polyvinylidene fluoride (PVDF), carboxymethyl cellulose (CMC), and polyacrylic acid (PAA) exhibit different adhesion properties and compatibility with active materials. Water-soluble binders like CMC offer environmental advantages while potentially providing better dispersion of sulfur within the cathode matrix, enhancing utilization efficiency and cycle life.
Current collector selection impacts cell resistance and electrochemical stability. Aluminum foil serves as the standard cathode current collector, while copper is typically avoided for anodes due to alloying reactions with sodium. Carbon-coated aluminum and specialized sodium-compatible alloys have emerged as promising alternatives, offering improved interfacial stability and reduced corrosion during long-term cycling.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!