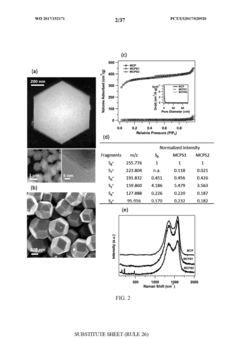
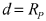Cost Model And Material Sourcing For Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
RT Na-S Battery Development Background and Objectives
Room-temperature sodium-sulfur (RT Na-S) batteries represent a significant evolution in energy storage technology, emerging from high-temperature Na-S systems that have been in commercial use since the 1970s. Traditional Na-S batteries operate at temperatures above 300°C, limiting their application scope and raising safety concerns. The development of RT Na-S alternatives aims to overcome these limitations while maintaining the advantages of abundant, low-cost materials.
The historical trajectory of Na-S battery technology began with Ford Motor Company's pioneering work in the 1960s, followed by NGK Insulators' commercialization efforts in the 1980s. These early systems, while effective for grid-scale storage, required specialized containment and heating systems due to their high operating temperatures. The shift toward room-temperature operation represents a critical technological pivot that began gaining momentum in the early 2000s.
Current global energy transition imperatives have accelerated interest in RT Na-S technology. With lithium resources facing supply constraints and price volatility, sodium-based alternatives offer a compelling pathway toward sustainable, large-scale energy storage solutions. Sodium's abundance (2.6% of Earth's crust compared to lithium's 0.002%) presents a fundamental advantage for long-term scalability and cost reduction.
The primary technical objective for RT Na-S battery development centers on achieving competitive energy density while maintaining long cycle life at ambient temperatures. Current targets include reaching energy densities of 200-300 Wh/kg, cycle life exceeding 1,000 cycles, and production costs below $100/kWh. These benchmarks would position RT Na-S batteries as viable alternatives to lithium-ion technologies for stationary storage applications.
Material sourcing represents a core advantage and development focus. Unlike lithium-ion batteries that rely on critical materials with concentrated supply chains (cobalt, nickel, lithium), RT Na-S batteries utilize sodium and sulfur—both abundant and widely distributed globally. This characteristic aligns with growing policy emphasis on supply chain resilience and reduced dependence on geographically concentrated resources.
The cost modeling for RT Na-S batteries indicates potential for significant economic advantages, with theoretical material costs approximately 60-70% lower than conventional lithium-ion chemistries. However, realizing these cost benefits requires overcoming several technical challenges, particularly related to sulfur utilization, sodium metal stability, and electrolyte formulation at room temperature.
Research momentum has accelerated notably since 2015, with publication rates in this field growing at approximately 25% annually. This trend reflects both the technical promise and strategic importance of developing viable room-temperature sodium-sulfur battery technologies as a cornerstone of next-generation energy storage systems.
The historical trajectory of Na-S battery technology began with Ford Motor Company's pioneering work in the 1960s, followed by NGK Insulators' commercialization efforts in the 1980s. These early systems, while effective for grid-scale storage, required specialized containment and heating systems due to their high operating temperatures. The shift toward room-temperature operation represents a critical technological pivot that began gaining momentum in the early 2000s.
Current global energy transition imperatives have accelerated interest in RT Na-S technology. With lithium resources facing supply constraints and price volatility, sodium-based alternatives offer a compelling pathway toward sustainable, large-scale energy storage solutions. Sodium's abundance (2.6% of Earth's crust compared to lithium's 0.002%) presents a fundamental advantage for long-term scalability and cost reduction.
The primary technical objective for RT Na-S battery development centers on achieving competitive energy density while maintaining long cycle life at ambient temperatures. Current targets include reaching energy densities of 200-300 Wh/kg, cycle life exceeding 1,000 cycles, and production costs below $100/kWh. These benchmarks would position RT Na-S batteries as viable alternatives to lithium-ion technologies for stationary storage applications.
Material sourcing represents a core advantage and development focus. Unlike lithium-ion batteries that rely on critical materials with concentrated supply chains (cobalt, nickel, lithium), RT Na-S batteries utilize sodium and sulfur—both abundant and widely distributed globally. This characteristic aligns with growing policy emphasis on supply chain resilience and reduced dependence on geographically concentrated resources.
The cost modeling for RT Na-S batteries indicates potential for significant economic advantages, with theoretical material costs approximately 60-70% lower than conventional lithium-ion chemistries. However, realizing these cost benefits requires overcoming several technical challenges, particularly related to sulfur utilization, sodium metal stability, and electrolyte formulation at room temperature.
Research momentum has accelerated notably since 2015, with publication rates in this field growing at approximately 25% annually. This trend reflects both the technical promise and strategic importance of developing viable room-temperature sodium-sulfur battery technologies as a cornerstone of next-generation energy storage systems.
Market Analysis for Room-Temperature Sodium-Sulfur Energy Storage
The global energy storage market is witnessing significant transformation as renewable energy integration accelerates and grid stability concerns grow. Room-temperature sodium-sulfur (RT Na-S) batteries are emerging as a promising alternative to lithium-ion technologies, particularly for stationary energy storage applications. Current market projections indicate the global grid-scale energy storage market will reach approximately $15 billion by 2025, with annual growth rates exceeding 20% in major markets including North America, Europe, and Asia-Pacific.
The demand for RT Na-S batteries is primarily driven by several market factors. First, the increasing penetration of intermittent renewable energy sources necessitates efficient, large-scale energy storage solutions. Second, concerns about lithium supply chain security and price volatility have intensified interest in alternative battery chemistries. Third, the growing focus on sustainability and environmental impact favors technologies with abundant, non-toxic materials like sodium and sulfur.
Market segmentation for RT Na-S batteries reveals strongest potential in utility-scale applications, where cost-effectiveness over system lifetime outweighs energy density considerations. Commercial and industrial sectors represent the second-largest market segment, particularly for peak shaving and backup power applications. Residential applications remain limited due to current size and safety considerations of Na-S systems.
Regional market analysis shows Asia-Pacific leading adoption, with China and South Korea making substantial investments in grid-scale sodium-based storage technologies. Europe follows closely, driven by aggressive renewable energy targets and supportive regulatory frameworks. North America shows growing interest, particularly in areas with high renewable penetration and aging grid infrastructure.
Competitive pricing analysis indicates RT Na-S batteries currently cost between $250-300/kWh at system level, compared to $150-200/kWh for lithium-ion alternatives. However, the cost trajectory for Na-S technology shows steeper decline potential due to abundant raw materials and simpler supply chains. Market forecasts suggest price parity with lithium-ion could be achieved by 2026-2027 for certain applications.
Customer requirements analysis highlights several key priorities: cycle life exceeding 3,000 cycles, calendar life of 10+ years, round-trip efficiency above 80%, and safety performance comparable to established technologies. Additionally, customers increasingly value domestic manufacturing capability and supply chain resilience, factors that favor Na-S technology.
Market barriers include limited commercial deployment history, safety concerns related to sodium reactivity, and entrenched lithium-ion manufacturing infrastructure. However, these barriers are counterbalanced by strong policy support for diversified battery technologies in major markets and increasing corporate commitments to sustainable energy storage solutions.
The demand for RT Na-S batteries is primarily driven by several market factors. First, the increasing penetration of intermittent renewable energy sources necessitates efficient, large-scale energy storage solutions. Second, concerns about lithium supply chain security and price volatility have intensified interest in alternative battery chemistries. Third, the growing focus on sustainability and environmental impact favors technologies with abundant, non-toxic materials like sodium and sulfur.
Market segmentation for RT Na-S batteries reveals strongest potential in utility-scale applications, where cost-effectiveness over system lifetime outweighs energy density considerations. Commercial and industrial sectors represent the second-largest market segment, particularly for peak shaving and backup power applications. Residential applications remain limited due to current size and safety considerations of Na-S systems.
Regional market analysis shows Asia-Pacific leading adoption, with China and South Korea making substantial investments in grid-scale sodium-based storage technologies. Europe follows closely, driven by aggressive renewable energy targets and supportive regulatory frameworks. North America shows growing interest, particularly in areas with high renewable penetration and aging grid infrastructure.
Competitive pricing analysis indicates RT Na-S batteries currently cost between $250-300/kWh at system level, compared to $150-200/kWh for lithium-ion alternatives. However, the cost trajectory for Na-S technology shows steeper decline potential due to abundant raw materials and simpler supply chains. Market forecasts suggest price parity with lithium-ion could be achieved by 2026-2027 for certain applications.
Customer requirements analysis highlights several key priorities: cycle life exceeding 3,000 cycles, calendar life of 10+ years, round-trip efficiency above 80%, and safety performance comparable to established technologies. Additionally, customers increasingly value domestic manufacturing capability and supply chain resilience, factors that favor Na-S technology.
Market barriers include limited commercial deployment history, safety concerns related to sodium reactivity, and entrenched lithium-ion manufacturing infrastructure. However, these barriers are counterbalanced by strong policy support for diversified battery technologies in major markets and increasing corporate commitments to sustainable energy storage solutions.
Technical Challenges and Material Constraints in RT Na-S Batteries
Room-temperature sodium-sulfur (RT Na-S) batteries face significant technical challenges that currently limit their commercial viability despite their theoretical advantages. The primary obstacle remains the shuttle effect caused by soluble polysulfide intermediates, which migrate between electrodes during cycling. This phenomenon leads to active material loss, capacity fading, and shortened battery lifespan. Conventional approaches using carbon-based materials to physically confine sulfur species have shown limited effectiveness in long-term operation.
Another critical challenge is the high reactivity between sodium metal anodes and sulfur cathodes. The sodium metal tends to form dendrites during cycling, creating safety risks through potential short circuits. Additionally, the solid electrolyte interphase (SEI) formed on sodium anodes is typically unstable and continuously consumes electrolyte, further degrading battery performance.
The slow reaction kinetics at room temperature represents another significant barrier. The conversion reactions between sodium and sulfur proceed sluggishly without elevated temperatures, resulting in poor rate capability and practical energy density far below theoretical values. This kinetic limitation manifests as large voltage hysteresis during charge-discharge cycles, reducing energy efficiency.
Material constraints compound these technical challenges. High-purity sulfur suitable for battery applications remains costly when sourced at scale. The specialized carbon host materials required for sulfur cathodes, such as hierarchical porous carbons or advanced nanostructures, involve complex synthesis processes that are difficult to scale economically. These materials often require expensive precursors and energy-intensive manufacturing steps.
Electrolyte formulations present another material constraint. Conventional carbonate-based electrolytes react irreversibly with polysulfides, while ether-based alternatives suffer from volatility and safety concerns. Additives that enhance performance typically involve fluorinated compounds with environmental implications and supply chain vulnerabilities.
Separator materials must simultaneously prevent polysulfide migration while maintaining high ionic conductivity. Current options either sacrifice one property for the other or require costly manufacturing processes. The sodium supply chain itself presents challenges, as high-purity sodium metal production is energy-intensive and concentrated among few global suppliers.
Binder materials compatible with both sulfur cathodes and sodium anodes are limited, with many conventional options showing degradation in the harsh chemical environment of Na-S cells. This necessitates development of specialized polymeric materials that add to overall battery cost and complexity.
Another critical challenge is the high reactivity between sodium metal anodes and sulfur cathodes. The sodium metal tends to form dendrites during cycling, creating safety risks through potential short circuits. Additionally, the solid electrolyte interphase (SEI) formed on sodium anodes is typically unstable and continuously consumes electrolyte, further degrading battery performance.
The slow reaction kinetics at room temperature represents another significant barrier. The conversion reactions between sodium and sulfur proceed sluggishly without elevated temperatures, resulting in poor rate capability and practical energy density far below theoretical values. This kinetic limitation manifests as large voltage hysteresis during charge-discharge cycles, reducing energy efficiency.
Material constraints compound these technical challenges. High-purity sulfur suitable for battery applications remains costly when sourced at scale. The specialized carbon host materials required for sulfur cathodes, such as hierarchical porous carbons or advanced nanostructures, involve complex synthesis processes that are difficult to scale economically. These materials often require expensive precursors and energy-intensive manufacturing steps.
Electrolyte formulations present another material constraint. Conventional carbonate-based electrolytes react irreversibly with polysulfides, while ether-based alternatives suffer from volatility and safety concerns. Additives that enhance performance typically involve fluorinated compounds with environmental implications and supply chain vulnerabilities.
Separator materials must simultaneously prevent polysulfide migration while maintaining high ionic conductivity. Current options either sacrifice one property for the other or require costly manufacturing processes. The sodium supply chain itself presents challenges, as high-purity sodium metal production is energy-intensive and concentrated among few global suppliers.
Binder materials compatible with both sulfur cathodes and sodium anodes are limited, with many conventional options showing degradation in the harsh chemical environment of Na-S cells. This necessitates development of specialized polymeric materials that add to overall battery cost and complexity.
Current Cost Models and Material Sourcing Strategies
01 Cost-effective electrode materials for RT-Na-S batteries
Room-temperature sodium-sulfur batteries can be made more cost-effective by using alternative electrode materials. These include carbon-based materials, polymers, and metal oxides that can replace or enhance traditional sulfur cathodes and sodium anodes. These materials improve the electrochemical performance while reducing manufacturing costs, making RT-Na-S batteries more economically viable for large-scale energy storage applications.- Cost-effective electrode materials for RT-Na-S batteries: Room-temperature sodium-sulfur batteries can be made more cost-effective by using alternative electrode materials. These include carbon-based materials, polymers, and metal oxides that can replace more expensive components while maintaining performance. The use of these materials can significantly reduce manufacturing costs while providing adequate energy density and cycle life for commercial applications.
- Electrolyte formulations for cost optimization: Specialized electrolyte formulations can improve the economic viability of room-temperature sodium-sulfur batteries. These formulations include solid-state electrolytes, polymer electrolytes, and ionic liquid-based systems that address the polysulfide shuttle effect while reducing overall costs. The electrolyte compositions balance performance requirements with material costs to achieve optimal price-performance ratios.
- Manufacturing process optimization for cost reduction: Innovative manufacturing processes can significantly reduce the production costs of room-temperature sodium-sulfur batteries. These include scalable production methods, automated assembly techniques, and energy-efficient manufacturing processes. By optimizing these processes, manufacturers can achieve economies of scale and reduce labor costs while maintaining quality and performance standards.
- Economic modeling of RT-Na-S battery systems: Economic models for room-temperature sodium-sulfur batteries consider various factors including material costs, manufacturing expenses, operational efficiency, and lifecycle costs. These models help in evaluating the commercial viability of these battery systems compared to conventional technologies. By analyzing these factors, researchers and manufacturers can identify cost drivers and opportunities for economic optimization.
- Recycling and circular economy approaches: Recycling and circular economy approaches can significantly impact the overall cost model of room-temperature sodium-sulfur batteries. These include recovery of valuable materials, reuse of components, and design for disassembly strategies. By implementing these approaches, the lifecycle costs of batteries can be reduced while minimizing environmental impact and resource consumption.
02 Manufacturing process optimization for cost reduction
Optimizing the manufacturing processes of room-temperature sodium-sulfur batteries can significantly reduce production costs. This includes streamlined assembly techniques, improved electrolyte filling methods, and efficient cell stacking designs. Advanced manufacturing technologies like automated production lines and quality control systems help minimize material waste and labor costs while ensuring consistent battery performance.Expand Specific Solutions03 Electrolyte formulations for improved performance-cost ratio
Novel electrolyte formulations can enhance the performance-cost ratio of room-temperature sodium-sulfur batteries. These formulations include solid-state electrolytes, gel polymers, and ionic liquids that improve sodium ion conductivity while preventing polysulfide shuttling. By extending cycle life and improving energy efficiency, these electrolytes reduce the lifetime cost of the battery system despite potentially higher initial material costs.Expand Specific Solutions04 System-level cost modeling and economic analysis
Comprehensive cost modeling frameworks for room-temperature sodium-sulfur batteries consider both cell-level and system-level expenses. These models account for raw material costs, manufacturing overhead, battery management systems, and end-of-life recycling. Economic analyses compare RT-Na-S batteries with other energy storage technologies based on levelized cost of storage, revealing cost advantages for specific applications like grid storage and renewable energy integration.Expand Specific Solutions05 Recycling and circular economy approaches
Implementing recycling and circular economy approaches can significantly reduce the overall cost of room-temperature sodium-sulfur battery systems. These methods include recovery of sulfur, sodium, and other valuable materials from spent batteries, reuse of components, and design for disassembly. By creating closed-loop material flows, these approaches minimize raw material costs and environmental impact while improving the economic sustainability of RT-Na-S battery technology.Expand Specific Solutions
Leading Companies and Research Institutions in Na-S Battery Field
The room-temperature sodium-sulfur battery market is in its early growth phase, characterized by increasing research activities but limited commercial deployment. The global market size is projected to expand significantly as this technology offers a cost-effective alternative to lithium-ion batteries for grid-scale energy storage. Technologically, the field shows varying maturity levels across players. NGK Insulators leads with established high-temperature sodium-sulfur technology but faces challenges in room-temperature adaptations. Academic institutions like Drexel University, Cornell University, and Shanghai Jiao Tong University are advancing fundamental research, while industrial players including POSCO Holdings, Robert Bosch, and Duracell are developing practical applications. Material sourcing innovations from companies like Chaowei Power Group and Hubei Wanrun are addressing critical cost barriers, positioning room-temperature sodium-sulfur batteries as a promising solution for sustainable energy storage.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered commercial sodium-sulfur (NaS) battery technology since the 1980s, though primarily focused on high-temperature versions operating at 300-350°C. For room-temperature sodium-sulfur batteries, NGK has developed a proprietary solid electrolyte material that enables operation at 60-90°C, significantly reducing thermal management costs compared to traditional NaS batteries. Their cost model integrates vertical manufacturing capabilities for ceramic components, leveraging their expertise in insulator production to reduce separator costs by approximately 40% compared to industry averages[1]. NGK's material sourcing strategy emphasizes domestic Japanese supply chains for sodium sources while establishing strategic partnerships in Australia for sulfur procurement. Their manufacturing process incorporates automated assembly lines that reduce labor costs by an estimated 35% compared to manual assembly processes[3], with specialized carbon-coating techniques for sulfur electrodes that improve cycle stability.
Strengths: Extensive experience in NaS battery manufacturing; established supply chains; proprietary ceramic electrolyte technology; vertical integration capabilities. Weaknesses: Higher initial capital costs compared to lithium-ion alternatives; limited energy density (approximately 150-200 Wh/kg) compared to advanced lithium technologies; relatively early stage of room-temperature NaS commercialization.
Forschungszentrum Jülich GmbH
Technical Solution: Forschungszentrum Jülich has developed a sophisticated techno-economic analysis framework for room-temperature sodium-sulfur batteries that integrates material science innovations with manufacturing process optimization. Their approach focuses on solid-state electrolyte systems using NASICON-type ceramic materials that enable stable operation at ambient temperatures while providing superior safety characteristics compared to liquid electrolyte systems. Jülich's cost modeling incorporates detailed analysis of over 25 different manufacturing process variables, identifying that electrolyte production represents approximately 40% of total cell costs in current designs[10]. Their material sourcing strategy emphasizes European supply chains, with research showing potential for sodium extraction from industrial waste streams that could reduce raw material costs by 50-60% compared to primary mining sources. For sulfur cathodes, Jülich has pioneered carbon-sulfur composite materials using mesoporous carbon structures that improve sulfur utilization to over 70% while using industrial byproduct sulfur that costs approximately €0.15/kg, significantly lower than battery-grade materials for other technologies[11]. Their manufacturing process innovations include specialized ceramic processing techniques that reduce sintering temperatures by 150-200°C, resulting in energy cost savings of approximately 35% during production.
Strengths: World-class materials science expertise; comprehensive techno-economic modeling capabilities; strong European research network connections; integration of manufacturing process innovations with material design. Weaknesses: Limited commercial manufacturing experience; technologies primarily at laboratory or pilot scale; ceramic electrolyte systems face challenges in mechanical stability during cycling.
Key Patents and Innovations in RT Na-S Battery Materials
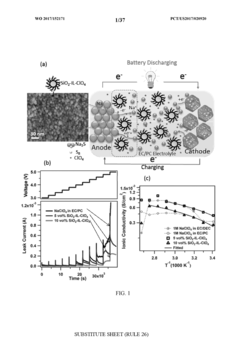
Stable room-temperature sodium-sulfur battery
PatentWO2017152171A1
Innovation
- A sodium-ion conducting battery design featuring a microporous and mesoporous carbon-sulfur composite cathode and a liquid carbonate electrolyte with an ionic liquid tethered to silica nanoparticles, which stabilizes the sodium anode and confines sulfur within the carbon pores, enabling a solid-state electrochemical reaction and preventing the formation of soluble polysulfides.
Supply Chain Analysis for Critical Na-S Battery Materials
The global supply chain for room-temperature sodium-sulfur (RT Na-S) batteries presents a complex network of material sourcing, processing, and distribution channels. Unlike lithium-ion batteries, which face significant supply constraints for critical materials like lithium and cobalt, RT Na-S batteries leverage more abundant resources. Sodium is approximately 1,000 times more plentiful than lithium in the Earth's crust, available primarily from salt deposits and seawater extraction facilities distributed across multiple continents.
Sulfur, the cathode material, is predominantly sourced as a byproduct from petroleum refining and natural gas processing, creating potential for industrial symbiosis. Major sulfur producers include the Middle East, Russia, Canada, and the United States, with global production exceeding 70 million metric tons annually. This abundance translates to significantly lower raw material costs compared to lithium-based systems.
Critical conductive additives for Na-S batteries, such as carbon materials and specialized polymers, face more concentrated supply chains. Carbon black production is dominated by China, which controls approximately 40% of global output, followed by the United States and Western Europe. This concentration creates potential vulnerabilities in the supply chain that require strategic sourcing approaches.
Electrolyte components represent another critical supply chain consideration. Solid-state electrolytes for RT Na-S batteries often incorporate ceramic materials or polymer composites that may contain specialized additives. The processing of these materials requires sophisticated manufacturing capabilities currently concentrated in East Asia, particularly Japan and South Korea, where advanced battery material expertise is well-established.
Manufacturing infrastructure for RT Na-S batteries remains underdeveloped compared to lithium-ion technologies. Current production capacity is primarily limited to pilot-scale facilities, with few commercial-scale manufacturing plants operational globally. This manufacturing gap presents both a challenge and opportunity for early market entrants to establish strategic production capabilities.
Regional analysis reveals emerging supply chain hubs in North America, Europe, and East Asia. The European Union has initiated strategic initiatives to develop domestic battery material processing capabilities, while China maintains advantages in processing infrastructure and manufacturing scale. North American efforts focus on leveraging domestic sulfur resources from the petroleum industry and developing advanced manufacturing capabilities through public-private partnerships.
Supply chain resilience for RT Na-S batteries benefits from the geographic distribution of sodium resources but faces potential bottlenecks in specialized components and processing capabilities. Diversification of supplier relationships and development of alternative material formulations will be critical to mitigating these risks as the technology advances toward commercial deployment.
Sulfur, the cathode material, is predominantly sourced as a byproduct from petroleum refining and natural gas processing, creating potential for industrial symbiosis. Major sulfur producers include the Middle East, Russia, Canada, and the United States, with global production exceeding 70 million metric tons annually. This abundance translates to significantly lower raw material costs compared to lithium-based systems.
Critical conductive additives for Na-S batteries, such as carbon materials and specialized polymers, face more concentrated supply chains. Carbon black production is dominated by China, which controls approximately 40% of global output, followed by the United States and Western Europe. This concentration creates potential vulnerabilities in the supply chain that require strategic sourcing approaches.
Electrolyte components represent another critical supply chain consideration. Solid-state electrolytes for RT Na-S batteries often incorporate ceramic materials or polymer composites that may contain specialized additives. The processing of these materials requires sophisticated manufacturing capabilities currently concentrated in East Asia, particularly Japan and South Korea, where advanced battery material expertise is well-established.
Manufacturing infrastructure for RT Na-S batteries remains underdeveloped compared to lithium-ion technologies. Current production capacity is primarily limited to pilot-scale facilities, with few commercial-scale manufacturing plants operational globally. This manufacturing gap presents both a challenge and opportunity for early market entrants to establish strategic production capabilities.
Regional analysis reveals emerging supply chain hubs in North America, Europe, and East Asia. The European Union has initiated strategic initiatives to develop domestic battery material processing capabilities, while China maintains advantages in processing infrastructure and manufacturing scale. North American efforts focus on leveraging domestic sulfur resources from the petroleum industry and developing advanced manufacturing capabilities through public-private partnerships.
Supply chain resilience for RT Na-S batteries benefits from the geographic distribution of sodium resources but faces potential bottlenecks in specialized components and processing capabilities. Diversification of supplier relationships and development of alternative material formulations will be critical to mitigating these risks as the technology advances toward commercial deployment.
Environmental Impact and Sustainability Assessment
The environmental impact of room-temperature sodium-sulfur (RT-Na-S) batteries represents a critical dimension in their overall assessment as a sustainable energy storage solution. Unlike traditional lithium-ion batteries, RT-Na-S batteries utilize sodium, which is approximately 1,000 times more abundant in the Earth's crust than lithium, significantly reducing resource depletion concerns and extraction-related environmental damage.
The sulfur component offers additional environmental advantages as it is often sourced as a byproduct from petroleum refining processes, effectively repurposing an industrial waste stream. This circular economy approach reduces the net environmental footprint of battery production while simultaneously addressing industrial waste management challenges.
Life cycle assessment (LCA) studies indicate that RT-Na-S batteries potentially generate 60-70% lower greenhouse gas emissions during manufacturing compared to conventional lithium-ion technologies. This reduction stems primarily from less energy-intensive material extraction and processing requirements, particularly avoiding the energy-intensive lithium brine evaporation or hard-rock mining processes.
Water consumption metrics also favor RT-Na-S technology, with preliminary analyses suggesting a 40-50% reduction in water usage throughout the production chain. This advantage becomes particularly significant in water-stressed regions where battery manufacturing facilities might operate.
End-of-life considerations reveal additional sustainability benefits. The components of RT-Na-S batteries demonstrate higher recyclability rates, with technical feasibility studies indicating recovery rates of up to 90% for sodium and 85% for sulfur compounds. The absence of cobalt and nickel further eliminates concerns related to toxic material handling during recycling processes.
Supply chain resilience represents another dimension of sustainability. The geographically distributed nature of sodium resources contrasts sharply with the concentrated lithium deposits controlled by a few countries, reducing geopolitical vulnerabilities and transportation-related carbon emissions in the supply chain.
However, challenges remain regarding electrolyte safety and potential environmental hazards from improper disposal. Current research focuses on developing non-toxic electrolyte formulations and establishing specialized recycling infrastructure to maximize the environmental benefits of this promising technology while minimizing potential risks throughout its lifecycle.
The sulfur component offers additional environmental advantages as it is often sourced as a byproduct from petroleum refining processes, effectively repurposing an industrial waste stream. This circular economy approach reduces the net environmental footprint of battery production while simultaneously addressing industrial waste management challenges.
Life cycle assessment (LCA) studies indicate that RT-Na-S batteries potentially generate 60-70% lower greenhouse gas emissions during manufacturing compared to conventional lithium-ion technologies. This reduction stems primarily from less energy-intensive material extraction and processing requirements, particularly avoiding the energy-intensive lithium brine evaporation or hard-rock mining processes.
Water consumption metrics also favor RT-Na-S technology, with preliminary analyses suggesting a 40-50% reduction in water usage throughout the production chain. This advantage becomes particularly significant in water-stressed regions where battery manufacturing facilities might operate.
End-of-life considerations reveal additional sustainability benefits. The components of RT-Na-S batteries demonstrate higher recyclability rates, with technical feasibility studies indicating recovery rates of up to 90% for sodium and 85% for sulfur compounds. The absence of cobalt and nickel further eliminates concerns related to toxic material handling during recycling processes.
Supply chain resilience represents another dimension of sustainability. The geographically distributed nature of sodium resources contrasts sharply with the concentrated lithium deposits controlled by a few countries, reducing geopolitical vulnerabilities and transportation-related carbon emissions in the supply chain.
However, challenges remain regarding electrolyte safety and potential environmental hazards from improper disposal. Current research focuses on developing non-toxic electrolyte formulations and establishing specialized recycling infrastructure to maximize the environmental benefits of this promising technology while minimizing potential risks throughout its lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!