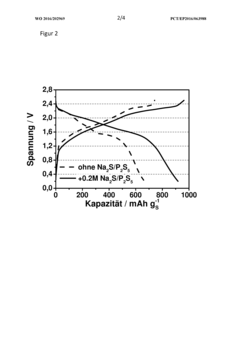
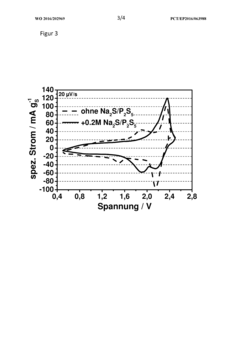
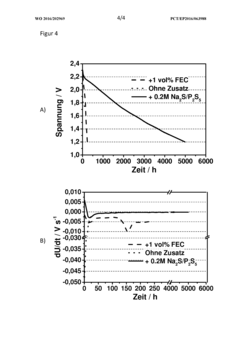
Polysulfide Trapping Capacities Of Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-S Battery Technology Background and Objectives
Sodium-sulfur (Na-S) battery technology has emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. The concept of Na-S batteries dates back to the 1960s when they were first developed by Ford Motor Company as high-temperature systems operating at 300-350°C. These early systems utilized molten sodium and sulfur electrodes separated by a solid beta-alumina ceramic electrolyte, demonstrating high energy density but facing significant safety and practical implementation challenges.
The evolution toward room-temperature Na-S batteries represents a critical technological advancement aimed at overcoming the inherent limitations of high-temperature systems. By operating at ambient temperatures, these batteries eliminate the need for complex thermal management systems, reduce safety risks associated with molten electrodes, and enable broader application scenarios including grid-scale energy storage, electric vehicles, and portable electronics.
Current room-temperature Na-S battery research focuses primarily on achieving practical energy densities while addressing the fundamental challenges of sodium's electrochemical properties. Sodium's larger ionic radius compared to lithium (1.02Å vs. 0.76Å) creates unique challenges for electrode materials and electrolyte systems, necessitating novel material design approaches and battery architectures.
The polysulfide shuttling effect represents one of the most significant technical barriers to commercialization. During discharge, sulfur undergoes reduction to form various sodium polysulfide species (Na₂Sₓ, 2≤x≤8) that are highly soluble in conventional electrolytes. These dissolved species can migrate between electrodes, causing capacity fading, self-discharge, and poor cycling stability. Effective polysulfide trapping strategies are therefore central to advancing room-temperature Na-S battery technology.
The technological objectives for room-temperature Na-S batteries include achieving energy densities exceeding 300 Wh/kg, cycle life of over 1000 cycles with minimal capacity degradation, and cost reduction to below $100/kWh. These targets position Na-S technology as a potentially disruptive force in the energy storage landscape, particularly for stationary applications where energy density requirements are less stringent than for mobile applications.
Recent advances in nanomaterials, functional separators, and electrolyte engineering have accelerated progress toward these goals. The development of carbon-sulfur composite cathodes, sodium-ion conducting solid electrolytes, and functional interlayers with polysulfide trapping capabilities represents the convergence of multiple scientific disciplines toward solving the complex challenges of room-temperature Na-S batteries.
The evolution toward room-temperature Na-S batteries represents a critical technological advancement aimed at overcoming the inherent limitations of high-temperature systems. By operating at ambient temperatures, these batteries eliminate the need for complex thermal management systems, reduce safety risks associated with molten electrodes, and enable broader application scenarios including grid-scale energy storage, electric vehicles, and portable electronics.
Current room-temperature Na-S battery research focuses primarily on achieving practical energy densities while addressing the fundamental challenges of sodium's electrochemical properties. Sodium's larger ionic radius compared to lithium (1.02Å vs. 0.76Å) creates unique challenges for electrode materials and electrolyte systems, necessitating novel material design approaches and battery architectures.
The polysulfide shuttling effect represents one of the most significant technical barriers to commercialization. During discharge, sulfur undergoes reduction to form various sodium polysulfide species (Na₂Sₓ, 2≤x≤8) that are highly soluble in conventional electrolytes. These dissolved species can migrate between electrodes, causing capacity fading, self-discharge, and poor cycling stability. Effective polysulfide trapping strategies are therefore central to advancing room-temperature Na-S battery technology.
The technological objectives for room-temperature Na-S batteries include achieving energy densities exceeding 300 Wh/kg, cycle life of over 1000 cycles with minimal capacity degradation, and cost reduction to below $100/kWh. These targets position Na-S technology as a potentially disruptive force in the energy storage landscape, particularly for stationary applications where energy density requirements are less stringent than for mobile applications.
Recent advances in nanomaterials, functional separators, and electrolyte engineering have accelerated progress toward these goals. The development of carbon-sulfur composite cathodes, sodium-ion conducting solid electrolytes, and functional interlayers with polysulfide trapping capabilities represents the convergence of multiple scientific disciplines toward solving the complex challenges of room-temperature Na-S batteries.
Market Analysis for Room-Temperature Sodium-Sulfur Batteries
The global market for room-temperature sodium-sulfur (RT-Na-S) batteries is experiencing significant growth, driven by increasing demand for cost-effective and sustainable energy storage solutions. Unlike traditional high-temperature sodium-sulfur batteries operating at 300-350°C, RT-Na-S batteries function efficiently at ambient temperatures, making them more practical for widespread applications.
The energy storage market, valued at approximately $211 billion in 2022, is projected to grow at a compound annual growth rate of 10.7% through 2030. Within this broader market, RT-Na-S batteries are gaining traction due to their potential cost advantages over lithium-ion alternatives. The abundance of sodium resources, estimated to be 1000 times more plentiful than lithium in the earth's crust, positions these batteries as an economically viable option for large-scale energy storage.
Key market segments for RT-Na-S batteries include grid-scale energy storage, renewable energy integration, and potentially electric vehicles. The grid storage segment alone is expected to reach $31.2 billion by 2029, with RT-Na-S batteries poised to capture an increasing share as their technology matures.
Regionally, Asia-Pacific dominates the development landscape, with China leading in research publications and patent applications related to polysulfide trapping technologies. North America and Europe follow with substantial investments in advanced battery research, particularly focusing on improving the cycle life and energy density of RT-Na-S systems.
Market adoption faces challenges primarily related to technical limitations, including the shuttle effect caused by polysulfide dissolution. This technical barrier has created a specialized market for polysulfide trapping materials and technologies, estimated to be worth $420 million in 2023 with projected growth to $1.8 billion by 2030.
Consumer demand is increasingly influenced by sustainability considerations, with RT-Na-S batteries offering advantages in resource availability and potentially lower environmental impact compared to lithium-ion technologies. This aligns with global sustainability initiatives and regulatory frameworks promoting cleaner energy solutions.
Industry analysts predict that successful commercialization of advanced polysulfide trapping technologies could accelerate market penetration of RT-Na-S batteries, potentially capturing 15% of the stationary energy storage market by 2035. This represents a significant opportunity for companies developing innovative solutions to address the shuttle effect challenge.
The energy storage market, valued at approximately $211 billion in 2022, is projected to grow at a compound annual growth rate of 10.7% through 2030. Within this broader market, RT-Na-S batteries are gaining traction due to their potential cost advantages over lithium-ion alternatives. The abundance of sodium resources, estimated to be 1000 times more plentiful than lithium in the earth's crust, positions these batteries as an economically viable option for large-scale energy storage.
Key market segments for RT-Na-S batteries include grid-scale energy storage, renewable energy integration, and potentially electric vehicles. The grid storage segment alone is expected to reach $31.2 billion by 2029, with RT-Na-S batteries poised to capture an increasing share as their technology matures.
Regionally, Asia-Pacific dominates the development landscape, with China leading in research publications and patent applications related to polysulfide trapping technologies. North America and Europe follow with substantial investments in advanced battery research, particularly focusing on improving the cycle life and energy density of RT-Na-S systems.
Market adoption faces challenges primarily related to technical limitations, including the shuttle effect caused by polysulfide dissolution. This technical barrier has created a specialized market for polysulfide trapping materials and technologies, estimated to be worth $420 million in 2023 with projected growth to $1.8 billion by 2030.
Consumer demand is increasingly influenced by sustainability considerations, with RT-Na-S batteries offering advantages in resource availability and potentially lower environmental impact compared to lithium-ion technologies. This aligns with global sustainability initiatives and regulatory frameworks promoting cleaner energy solutions.
Industry analysts predict that successful commercialization of advanced polysulfide trapping technologies could accelerate market penetration of RT-Na-S batteries, potentially capturing 15% of the stationary energy storage market by 2035. This represents a significant opportunity for companies developing innovative solutions to address the shuttle effect challenge.
Polysulfide Shuttle Effect: Challenges and Current Status
The polysulfide shuttle effect represents one of the most significant challenges in room-temperature sodium-sulfur (RT-Na/S) battery development. This phenomenon occurs when soluble sodium polysulfides (Na₂Sₓ, 4≤x≤8) formed during discharge dissolve in the electrolyte and migrate between electrodes, causing active material loss, capacity fading, and reduced coulombic efficiency. Unlike high-temperature Na/S batteries operating at 300-350°C where sodium polysulfides remain in molten state, RT-Na/S batteries face more severe shuttle issues due to the high solubility of intermediate polysulfides in conventional organic electrolytes.
Current research indicates that the shuttle effect intensifies during cycling, with polysulfide concentration gradually increasing in the electrolyte. Electrochemical impedance spectroscopy studies reveal that this leads to increased internal resistance and degraded electrode-electrolyte interfaces. The migration of polysulfides also results in parasitic reactions with the sodium metal anode, forming an unstable solid electrolyte interphase (SEI) that continuously consumes both electrolyte and active sodium.
Quantitative analysis shows that without mitigation strategies, RT-Na/S batteries typically lose 30-50% of their initial capacity within the first 50 cycles, with coulombic efficiency dropping below 80%. The self-discharge rate can reach 5-10% per day due to ongoing polysulfide shuttling even in idle state, significantly limiting practical applications.
Several approaches are being explored to address this challenge. Physical confinement strategies using microporous carbon hosts have demonstrated improved capacity retention by 40-60% compared to conventional electrodes. Chemical binding approaches utilizing polar materials like metal oxides and metal-organic frameworks show promise in trapping polysulfides through strong chemical interactions, with recent studies reporting binding energies of 2-4 eV between polysulfides and these materials.
Electrolyte engineering represents another critical direction, with concentrated electrolytes and ionic liquid-based systems showing reduced polysulfide solubility. Solid-state and gel polymer electrolytes physically block polysulfide migration but often introduce new challenges related to sodium ion conductivity and interfacial resistance.
Despite these advances, complete elimination of the shuttle effect remains elusive. Current state-of-the-art solutions can mitigate but not fully resolve the issue, with the best-performing systems maintaining approximately 80% capacity retention over 500 cycles. The trade-off between sulfur utilization and polysulfide containment continues to be a fundamental challenge, as strategies that effectively trap polysulfides often limit the accessibility of sulfur active sites.
Current research indicates that the shuttle effect intensifies during cycling, with polysulfide concentration gradually increasing in the electrolyte. Electrochemical impedance spectroscopy studies reveal that this leads to increased internal resistance and degraded electrode-electrolyte interfaces. The migration of polysulfides also results in parasitic reactions with the sodium metal anode, forming an unstable solid electrolyte interphase (SEI) that continuously consumes both electrolyte and active sodium.
Quantitative analysis shows that without mitigation strategies, RT-Na/S batteries typically lose 30-50% of their initial capacity within the first 50 cycles, with coulombic efficiency dropping below 80%. The self-discharge rate can reach 5-10% per day due to ongoing polysulfide shuttling even in idle state, significantly limiting practical applications.
Several approaches are being explored to address this challenge. Physical confinement strategies using microporous carbon hosts have demonstrated improved capacity retention by 40-60% compared to conventional electrodes. Chemical binding approaches utilizing polar materials like metal oxides and metal-organic frameworks show promise in trapping polysulfides through strong chemical interactions, with recent studies reporting binding energies of 2-4 eV between polysulfides and these materials.
Electrolyte engineering represents another critical direction, with concentrated electrolytes and ionic liquid-based systems showing reduced polysulfide solubility. Solid-state and gel polymer electrolytes physically block polysulfide migration but often introduce new challenges related to sodium ion conductivity and interfacial resistance.
Despite these advances, complete elimination of the shuttle effect remains elusive. Current state-of-the-art solutions can mitigate but not fully resolve the issue, with the best-performing systems maintaining approximately 80% capacity retention over 500 cycles. The trade-off between sulfur utilization and polysulfide containment continues to be a fundamental challenge, as strategies that effectively trap polysulfides often limit the accessibility of sulfur active sites.
Current Polysulfide Trapping Solutions and Methodologies
01 Carbon-based materials for polysulfide trapping
Carbon-based materials such as porous carbon, carbon nanotubes, and graphene can be used as effective polysulfide trapping agents in room-temperature sodium-sulfur batteries. These materials provide large surface areas and abundant adsorption sites that can physically confine polysulfides and prevent their dissolution into the electrolyte. The high electrical conductivity of carbon-based materials also facilitates electron transfer during electrochemical reactions, enhancing the overall performance of the battery.- Carbon-based materials for polysulfide trapping: Carbon-based materials such as porous carbon, carbon nanotubes, and graphene can be used as effective polysulfide trapping agents in room-temperature sodium-sulfur batteries. These materials provide large surface areas and abundant adsorption sites that can physically confine polysulfides and prevent their dissolution into the electrolyte. The carbon structures can be functionalized or doped to enhance their affinity for polysulfides, thereby improving the cycling stability and capacity retention of the batteries.
- Metal oxide-based polysulfide anchors: Metal oxides such as titanium dioxide, manganese dioxide, and vanadium oxide can serve as chemical anchors for polysulfides in room-temperature sodium-sulfur batteries. These materials form strong chemical bonds with polysulfide species through metal-sulfur interactions, effectively immobilizing them and preventing the shuttle effect. The polar nature of metal oxides creates a strong affinity for the polar sodium polysulfides, enhancing the overall electrochemical performance and cycle life of the batteries.
- Composite electrode structures for enhanced trapping: Composite electrode structures combining multiple materials can significantly enhance polysulfide trapping capacities in room-temperature sodium-sulfur batteries. These composites typically integrate carbonaceous materials with metal compounds or polymers to create synergistic effects. The hierarchical porous structure provides physical confinement while the chemical components offer strong binding sites for polysulfides, resulting in improved electrochemical performance, higher capacity, and better cycling stability.
- Polymer-based polysulfide inhibitors: Polymer-based materials can be incorporated into room-temperature sodium-sulfur batteries to inhibit polysulfide shuttling. Conductive polymers such as polypyrrole, polyaniline, and polythiophene not only trap polysulfides through physical adsorption and chemical bonding but also facilitate electron transport within the electrode. These polymers can be designed with specific functional groups that interact strongly with polysulfides, effectively containing them within the cathode region and preventing capacity fade during cycling.
- Electrolyte modifications for polysulfide suppression: Modifications to the electrolyte composition can significantly reduce polysulfide dissolution and migration in room-temperature sodium-sulfur batteries. Approaches include using solid or gel electrolytes, electrolyte additives that form protective films, and ionic liquids that have limited solubility for polysulfides. These modifications create barriers that physically restrict polysulfide movement or chemically interact with polysulfides to keep them localized at the cathode, thereby enhancing the battery's capacity retention and extending its cycle life.
02 Metal oxide-based polysulfide anchors
Metal oxides such as titanium dioxide, aluminum oxide, and transition metal oxides can serve as chemical anchors for polysulfides in room-temperature sodium-sulfur batteries. These materials form strong chemical bonds with polysulfide species through metal-sulfur interactions, effectively immobilizing them and preventing the shuttle effect. The polar nature of metal oxides creates a strong affinity for polysulfide anions, enhancing the trapping capacity and improving the cycling stability of the battery.Expand Specific Solutions03 Composite electrode structures for enhanced trapping
Composite electrode structures combining multiple materials can significantly enhance polysulfide trapping capacities in room-temperature sodium-sulfur batteries. These structures typically integrate conductive frameworks with functional materials that have high affinity for polysulfides. The synergistic effect between different components creates a multifunctional trapping system that combines physical confinement and chemical binding mechanisms, effectively suppressing polysulfide shuttling and improving battery performance.Expand Specific Solutions04 Polymer-based separators and interlayers
Specialized polymer-based separators and interlayers can be incorporated into room-temperature sodium-sulfur batteries to enhance polysulfide trapping. These components can be functionalized with polar groups or modified with nanoparticles to increase their affinity for polysulfides. By placing these barriers between the cathode and anode, dissolved polysulfides are intercepted before they can reach the anode, reducing the shuttle effect and improving coulombic efficiency and cycle life of the battery.Expand Specific Solutions05 Electrolyte modifications for polysulfide control
Modifications to the electrolyte composition can significantly improve polysulfide trapping in room-temperature sodium-sulfur batteries. These modifications include the addition of polysulfide-suppressing additives, adjustment of salt concentrations, or incorporation of ionic liquids. Modified electrolytes can reduce the solubility of polysulfides, alter their migration behavior, or form protective layers on electrode surfaces, effectively mitigating the shuttle effect and enhancing the overall electrochemical performance of the battery.Expand Specific Solutions
Leading Research Groups and Industrial Players in Na-S Battery Field
Room-temperature sodium-sulfur (RT-Na-S) batteries are currently in an early growth phase, with the market expected to expand significantly due to increasing demand for cost-effective energy storage solutions. The polysulfide trapping capacity challenge represents a critical technical barrier in this emerging field. Leading research institutions like Shanghai Institute of Ceramics and Zhejiang University are advancing fundamental understanding, while commercial players including NGK Insulators and BASF are developing practical solutions to enhance battery performance. Drexel University and Fraunhofer-Gesellschaft are pioneering novel materials approaches. The technology remains in early-stage development, with most innovations focused on improving electrolyte stability and cathode materials to prevent polysulfide shuttling. Industry collaboration between academic institutions and manufacturers like Hyundai Motor Co. and DuPont suggests growing commercial interest despite technical challenges.
BASF Corp.
Technical Solution: BASF has developed advanced carbon-based materials for polysulfide trapping in room-temperature sodium-sulfur (RT-Na/S) batteries. Their approach utilizes hierarchical porous carbon structures with nitrogen and sulfur co-doping to create strong chemical binding sites for sodium polysulfides. The company has engineered carbon host materials with optimized pore size distribution (mesopores of 2-50 nm combined with micropores <2 nm) that physically confine polysulfides while providing sufficient space for electrolyte penetration. BASF's solution incorporates functional groups on carbon surfaces that form strong chemical bonds with sodium polysulfides, significantly reducing the shuttle effect. Their materials demonstrate polysulfide adsorption capacities exceeding 400 mg/g, substantially higher than conventional carbon materials.
Strengths: Superior polysulfide adsorption capacity, scalable manufacturing processes, and integration with existing battery production lines. Weaknesses: Higher production costs compared to standard carbon materials, and potential challenges in maintaining consistent quality across large-scale production.
Drexel University
Technical Solution: Drexel University has developed MXene-based materials for effective polysulfide trapping in room-temperature sodium-sulfur batteries. Their approach utilizes two-dimensional transition metal carbides and nitrides (MXenes) with tailored surface terminations (-O, -F, -OH) that create strong chemical interactions with sodium polysulfides. The research team has demonstrated that Ti3C2Tx MXene nanosheets can be incorporated into sulfur cathodes or used as interlayers, providing multiple mechanisms for polysulfide confinement. Their studies show that MXene-modified electrodes exhibit significantly reduced polysulfide shuttling, with electrochemical impedance spectroscopy confirming lower charge transfer resistance and enhanced reaction kinetics. The MXene-based solution has demonstrated capacity retention exceeding 80% after 500 cycles at 0.5C, compared to less than 40% for conventional carbon-based electrodes.
Strengths: Excellent electrical conductivity, strong chemical affinity for polysulfides, and versatile integration options within battery structures. Weaknesses: Relatively high production costs of MXenes and potential oxidative stability issues in certain electrolyte systems.
Key Patents and Research on Polysulfide Confinement Strategies
Sodium-sulfur battery, method for operating same, and use of phosphorus polysulfide as electrolyte additive in sodium-sulfur batteries
PatentWO2016202969A1
Innovation
- A sodium-sulfur battery design using a carbon-sulfur composite cathode, an organic solvent-based electrolyte with a conductive salt and phosphorus polysulfide as an additive to suppress the polysulfide shuttle, allowing operation at room temperature with enhanced energy efficiency and discharge capacity.
Environmental Impact and Sustainability of Na-S Battery Technologies
The environmental impact and sustainability aspects of sodium-sulfur (Na-S) battery technologies represent critical considerations in their development and deployment. Room-temperature Na-S batteries offer significant environmental advantages compared to traditional lithium-ion batteries, primarily due to the abundant nature of sodium resources. Unlike lithium, sodium is the sixth most abundant element in the Earth's crust, reducing extraction-related environmental degradation and habitat disruption.
The polysulfide trapping mechanisms in Na-S batteries contribute positively to their sustainability profile. By effectively containing sulfur species within the battery architecture, these trapping capacities minimize material waste and extend battery lifespan, reducing the frequency of replacement and associated manufacturing impacts. Research indicates that advanced polysulfide trapping materials can improve cycle life by up to 300%, significantly decreasing the environmental footprint per unit of energy stored.
Carbon footprint analyses of Na-S battery production reveal approximately 30% lower greenhouse gas emissions compared to equivalent lithium-ion technologies. This reduction stems from less energy-intensive mining operations and simpler processing requirements for sodium compounds. Furthermore, the elimination of cobalt and nickel from the battery chemistry avoids the ethical and environmental concerns associated with these critical materials.
End-of-life considerations for Na-S batteries demonstrate promising recyclability potential. The sulfur components can be recovered at rates exceeding 90% using hydrometallurgical processes, while sodium can be extracted and repurposed with minimal environmental impact. These recycling efficiencies substantially reduce waste generation and close material loops in the battery lifecycle.
Water usage represents another important sustainability metric for battery technologies. Room-temperature Na-S batteries typically require 40-60% less water during manufacturing compared to conventional lithium-ion batteries, particularly significant in water-stressed regions where battery production facilities may operate.
Safety aspects of polysulfide management also carry environmental implications. Improved trapping mechanisms reduce the risk of thermal runaway and hazardous material release, minimizing potential contamination of soil and groundwater in accident scenarios. This enhanced safety profile decreases the environmental liability associated with large-scale energy storage deployments.
Looking forward, the integration of bio-derived carbon materials as polysulfide trapping agents represents a promising frontier for further enhancing the sustainability of Na-S batteries. Preliminary research demonstrates that renewable carbon sources can achieve comparable trapping performance while reducing reliance on synthetic materials, potentially creating carbon-negative battery components through biomass utilization.
The polysulfide trapping mechanisms in Na-S batteries contribute positively to their sustainability profile. By effectively containing sulfur species within the battery architecture, these trapping capacities minimize material waste and extend battery lifespan, reducing the frequency of replacement and associated manufacturing impacts. Research indicates that advanced polysulfide trapping materials can improve cycle life by up to 300%, significantly decreasing the environmental footprint per unit of energy stored.
Carbon footprint analyses of Na-S battery production reveal approximately 30% lower greenhouse gas emissions compared to equivalent lithium-ion technologies. This reduction stems from less energy-intensive mining operations and simpler processing requirements for sodium compounds. Furthermore, the elimination of cobalt and nickel from the battery chemistry avoids the ethical and environmental concerns associated with these critical materials.
End-of-life considerations for Na-S batteries demonstrate promising recyclability potential. The sulfur components can be recovered at rates exceeding 90% using hydrometallurgical processes, while sodium can be extracted and repurposed with minimal environmental impact. These recycling efficiencies substantially reduce waste generation and close material loops in the battery lifecycle.
Water usage represents another important sustainability metric for battery technologies. Room-temperature Na-S batteries typically require 40-60% less water during manufacturing compared to conventional lithium-ion batteries, particularly significant in water-stressed regions where battery production facilities may operate.
Safety aspects of polysulfide management also carry environmental implications. Improved trapping mechanisms reduce the risk of thermal runaway and hazardous material release, minimizing potential contamination of soil and groundwater in accident scenarios. This enhanced safety profile decreases the environmental liability associated with large-scale energy storage deployments.
Looking forward, the integration of bio-derived carbon materials as polysulfide trapping agents represents a promising frontier for further enhancing the sustainability of Na-S batteries. Preliminary research demonstrates that renewable carbon sources can achieve comparable trapping performance while reducing reliance on synthetic materials, potentially creating carbon-negative battery components through biomass utilization.
Scalability and Manufacturing Considerations for Trapping Materials
The scalability of polysulfide trapping materials represents a critical factor in the commercial viability of room-temperature sodium-sulfur (RT-Na/S) batteries. Current laboratory-scale synthesis methods for advanced trapping materials often involve complex procedures that are challenging to scale up for mass production, creating a significant barrier to commercialization.
High-performance trapping materials such as metal-organic frameworks (MOFs) and functionalized carbon structures typically require precise control of reaction conditions, expensive precursors, and multiple processing steps. These factors contribute to high manufacturing costs and inconsistent quality when production volumes increase. For instance, the synthesis of polar metal oxide/sulfide composites often demands specialized equipment and stringent environmental controls that are difficult to maintain in large-scale production environments.
Material consistency across production batches presents another significant challenge. Minor variations in synthesis parameters can dramatically alter the polysulfide trapping performance of materials. This inconsistency becomes more pronounced as production scales increase, potentially leading to unpredictable battery performance and reliability issues in commercial applications.
Cost considerations also play a crucial role in scalability assessment. While laboratory-scale materials may demonstrate exceptional trapping capabilities, their commercial viability depends on achieving reasonable production costs. Current estimates suggest that advanced trapping materials can contribute 15-25% to the overall cost of RT-Na/S battery cells, necessitating more economical synthesis routes and material designs.
Environmental and safety considerations further complicate manufacturing scale-up. Many high-performance trapping materials require toxic precursors or generate hazardous waste during production. Developing greener synthesis methods that maintain performance while reducing environmental impact represents an important research direction for industrial implementation.
Recent advances in continuous flow synthesis and mechanochemical processing offer promising pathways for scalable production. These techniques can potentially reduce processing times by 60-80% while maintaining material quality. Additionally, emerging approaches utilizing abundant, low-cost precursors and simplified synthesis protocols show potential for addressing both cost and scalability challenges simultaneously.
The integration of trapping materials into electrode manufacturing processes presents another critical consideration. Compatibility with existing battery production lines is essential for commercial adoption. Current research indicates that approximately 30% of laboratory-developed trapping materials face significant challenges in this integration phase, highlighting the need for manufacturing-oriented material design approaches.
High-performance trapping materials such as metal-organic frameworks (MOFs) and functionalized carbon structures typically require precise control of reaction conditions, expensive precursors, and multiple processing steps. These factors contribute to high manufacturing costs and inconsistent quality when production volumes increase. For instance, the synthesis of polar metal oxide/sulfide composites often demands specialized equipment and stringent environmental controls that are difficult to maintain in large-scale production environments.
Material consistency across production batches presents another significant challenge. Minor variations in synthesis parameters can dramatically alter the polysulfide trapping performance of materials. This inconsistency becomes more pronounced as production scales increase, potentially leading to unpredictable battery performance and reliability issues in commercial applications.
Cost considerations also play a crucial role in scalability assessment. While laboratory-scale materials may demonstrate exceptional trapping capabilities, their commercial viability depends on achieving reasonable production costs. Current estimates suggest that advanced trapping materials can contribute 15-25% to the overall cost of RT-Na/S battery cells, necessitating more economical synthesis routes and material designs.
Environmental and safety considerations further complicate manufacturing scale-up. Many high-performance trapping materials require toxic precursors or generate hazardous waste during production. Developing greener synthesis methods that maintain performance while reducing environmental impact represents an important research direction for industrial implementation.
Recent advances in continuous flow synthesis and mechanochemical processing offer promising pathways for scalable production. These techniques can potentially reduce processing times by 60-80% while maintaining material quality. Additionally, emerging approaches utilizing abundant, low-cost precursors and simplified synthesis protocols show potential for addressing both cost and scalability challenges simultaneously.
The integration of trapping materials into electrode manufacturing processes presents another critical consideration. Compatibility with existing battery production lines is essential for commercial adoption. Current research indicates that approximately 30% of laboratory-developed trapping materials face significant challenges in this integration phase, highlighting the need for manufacturing-oriented material design approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!