Binder Systems And Rheology For Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Binder Systems Evolution and Research Objectives
Binder systems for room-temperature sodium-sulfur (RT-Na/S) batteries have evolved significantly over the past decade, transitioning from simple polymer matrices to sophisticated multi-functional composites. Initially, conventional polyvinylidene fluoride (PVDF) binders dominated the field, borrowed directly from lithium-ion battery technology without specific optimization for sulfur electrochemistry. These early systems suffered from poor ionic conductivity and inadequate mechanical stability during the substantial volume changes inherent to sulfur conversion reactions.
The mid-2010s marked a pivotal shift toward water-soluble binders such as carboxymethyl cellulose (CMC) and polyacrylic acid (PAA), which offered improved environmental compatibility and enhanced adhesion properties. This transition coincided with growing recognition of the unique challenges posed by sodium-sulfur chemistry, particularly the shuttle effect of polysulfides and the larger ionic radius of sodium compared to lithium.
Recent developments have focused on functional binders specifically engineered for RT-Na/S battery environments. These advanced systems incorporate polar functional groups capable of chemical interaction with polysulfides, effectively suppressing their dissolution and migration. Notable examples include modified polyacrylonitrile (PAN) with cyano groups and polyimide derivatives featuring nitrogen-rich structures that demonstrate strong affinity for sodium polysulfides.
The rheological properties of binder systems have emerged as a critical factor in electrode performance. Optimal viscoelastic characteristics enable uniform slurry distribution during manufacturing while maintaining structural integrity during cycling. Research has revealed that precisely controlled rheology can significantly impact electrolyte wetting, ionic transport pathways, and the formation of solid-electrolyte interphase (SEI) layers.
Current research objectives center on developing multi-functional binder systems that simultaneously address several challenges: (1) enhancing mechanical stability to accommodate the 170% volume expansion during sodium-sulfur conversion; (2) improving ionic conductivity to facilitate sodium transport; (3) creating chemical traps for polysulfide intermediates; and (4) optimizing electrode manufacturing processes through tailored rheological properties.
The ultimate goal is to design binder systems that enable RT-Na/S batteries to achieve energy densities exceeding 500 Wh/kg with cycle life greater than 1000 cycles, positioning them as viable alternatives to lithium-ion technology. This requires interdisciplinary approaches combining polymer chemistry, electrochemistry, and materials science to create binder architectures that fundamentally transform electrode-electrolyte interfaces and reaction kinetics in these promising energy storage systems.
The mid-2010s marked a pivotal shift toward water-soluble binders such as carboxymethyl cellulose (CMC) and polyacrylic acid (PAA), which offered improved environmental compatibility and enhanced adhesion properties. This transition coincided with growing recognition of the unique challenges posed by sodium-sulfur chemistry, particularly the shuttle effect of polysulfides and the larger ionic radius of sodium compared to lithium.
Recent developments have focused on functional binders specifically engineered for RT-Na/S battery environments. These advanced systems incorporate polar functional groups capable of chemical interaction with polysulfides, effectively suppressing their dissolution and migration. Notable examples include modified polyacrylonitrile (PAN) with cyano groups and polyimide derivatives featuring nitrogen-rich structures that demonstrate strong affinity for sodium polysulfides.
The rheological properties of binder systems have emerged as a critical factor in electrode performance. Optimal viscoelastic characteristics enable uniform slurry distribution during manufacturing while maintaining structural integrity during cycling. Research has revealed that precisely controlled rheology can significantly impact electrolyte wetting, ionic transport pathways, and the formation of solid-electrolyte interphase (SEI) layers.
Current research objectives center on developing multi-functional binder systems that simultaneously address several challenges: (1) enhancing mechanical stability to accommodate the 170% volume expansion during sodium-sulfur conversion; (2) improving ionic conductivity to facilitate sodium transport; (3) creating chemical traps for polysulfide intermediates; and (4) optimizing electrode manufacturing processes through tailored rheological properties.
The ultimate goal is to design binder systems that enable RT-Na/S batteries to achieve energy densities exceeding 500 Wh/kg with cycle life greater than 1000 cycles, positioning them as viable alternatives to lithium-ion technology. This requires interdisciplinary approaches combining polymer chemistry, electrochemistry, and materials science to create binder architectures that fundamentally transform electrode-electrolyte interfaces and reaction kinetics in these promising energy storage systems.
Market Analysis for Room-Temperature Na-S Battery Applications
The room-temperature sodium-sulfur (RT Na-S) battery market is experiencing significant growth potential as energy storage demands increase globally. Current market projections indicate that the global stationary energy storage market, where RT Na-S batteries could play a crucial role, is expected to reach $40 billion by 2030, with a compound annual growth rate of approximately 20% from 2023 to 2030.
The primary market drivers for RT Na-S battery technology include the growing renewable energy sector, grid stabilization needs, and the push for sustainable energy solutions. As intermittent renewable energy sources like solar and wind continue to expand their market share, the demand for efficient, cost-effective energy storage solutions grows proportionally. RT Na-S batteries are particularly well-positioned in this landscape due to their theoretical high energy density (760 Wh/kg) and the abundant, low-cost nature of their core materials.
Industrial sectors showing the strongest interest in RT Na-S technology include utility companies seeking grid-scale storage solutions, renewable energy developers requiring storage integration, and telecommunications companies needing reliable backup power systems. The electric vehicle sector represents another potential application area, though this market segment faces more significant competition from lithium-ion and solid-state battery technologies.
Geographically, the market for RT Na-S batteries shows promising growth in regions with aggressive renewable energy targets and substantial investments in grid modernization. China, the European Union, and the United States lead in potential adoption, with emerging markets in India and Southeast Asia showing increasing interest as they expand their renewable energy infrastructure.
Consumer demand patterns indicate a growing preference for sustainable energy storage solutions with lower environmental impacts. RT Na-S batteries, with their use of abundant materials and potential for recyclability, align well with these market preferences. The technology's safety improvements at room temperature operation also address critical consumer concerns about battery safety.
Market barriers include the current dominance of lithium-ion technology, which benefits from established manufacturing infrastructure and economies of scale. For RT Na-S batteries to gain significant market share, they must demonstrate competitive performance metrics, particularly in cycle life and energy density, while maintaining their cost advantage. The current market price point for grid-scale energy storage ranges from $150-300/kWh, a target that RT Na-S technology must meet to achieve widespread adoption.
Regulatory trends favor technologies like RT Na-S batteries, with increasing government incentives for renewable energy storage solutions and stricter regulations on critical materials sourcing. These regulatory tailwinds could accelerate market penetration for sodium-based battery technologies in the coming decade.
The primary market drivers for RT Na-S battery technology include the growing renewable energy sector, grid stabilization needs, and the push for sustainable energy solutions. As intermittent renewable energy sources like solar and wind continue to expand their market share, the demand for efficient, cost-effective energy storage solutions grows proportionally. RT Na-S batteries are particularly well-positioned in this landscape due to their theoretical high energy density (760 Wh/kg) and the abundant, low-cost nature of their core materials.
Industrial sectors showing the strongest interest in RT Na-S technology include utility companies seeking grid-scale storage solutions, renewable energy developers requiring storage integration, and telecommunications companies needing reliable backup power systems. The electric vehicle sector represents another potential application area, though this market segment faces more significant competition from lithium-ion and solid-state battery technologies.
Geographically, the market for RT Na-S batteries shows promising growth in regions with aggressive renewable energy targets and substantial investments in grid modernization. China, the European Union, and the United States lead in potential adoption, with emerging markets in India and Southeast Asia showing increasing interest as they expand their renewable energy infrastructure.
Consumer demand patterns indicate a growing preference for sustainable energy storage solutions with lower environmental impacts. RT Na-S batteries, with their use of abundant materials and potential for recyclability, align well with these market preferences. The technology's safety improvements at room temperature operation also address critical consumer concerns about battery safety.
Market barriers include the current dominance of lithium-ion technology, which benefits from established manufacturing infrastructure and economies of scale. For RT Na-S batteries to gain significant market share, they must demonstrate competitive performance metrics, particularly in cycle life and energy density, while maintaining their cost advantage. The current market price point for grid-scale energy storage ranges from $150-300/kWh, a target that RT Na-S technology must meet to achieve widespread adoption.
Regulatory trends favor technologies like RT Na-S batteries, with increasing government incentives for renewable energy storage solutions and stricter regulations on critical materials sourcing. These regulatory tailwinds could accelerate market penetration for sodium-based battery technologies in the coming decade.
Current Challenges in Na-S Battery Binder Technology
Despite significant advancements in room-temperature sodium-sulfur (RT Na-S) battery technology, binder systems remain a critical bottleneck in commercialization efforts. Current binder technologies face several interconnected challenges that impact battery performance, durability, and manufacturability. The conventional polyvinylidene fluoride (PVDF) binder, widely used in lithium-ion batteries, exhibits limited compatibility with sulfur cathodes in Na-S systems due to the volume expansion issues and the polysulfide shuttle effect.
The primary challenge lies in managing the substantial volume changes (up to 170%) that occur during sodium-sulfur conversion reactions. Existing binders struggle to maintain structural integrity throughout multiple charge-discharge cycles, leading to electrode pulverization, active material detachment, and rapid capacity fading. This mechanical instability significantly reduces cycle life and practical energy density of RT Na-S batteries.
Polysulfide dissolution and shuttle effect present another major hurdle. Current binder systems provide insufficient barriers against soluble sodium polysulfide intermediates, which can migrate between electrodes, causing irreversible capacity loss and self-discharge. While some functional binders show promise in mitigating this issue through chemical interactions, they often compromise ionic conductivity or increase internal resistance.
The rheological properties of binder systems directly impact electrode manufacturing processes. Many experimental binders that demonstrate excellent electrochemical performance exhibit poor processability in large-scale production environments. The viscosity, shear thinning behavior, and adhesion properties of slurries must be precisely controlled to ensure uniform electrode coating and adequate adhesion to current collectors.
Ionic conductivity limitations represent another significant challenge. Many binders that effectively address mechanical stability or polysulfide retention create barriers to sodium ion transport, increasing internal resistance and reducing rate capability. The development of binders that simultaneously provide mechanical support while facilitating rapid ion transport remains elusive.
Environmental and cost considerations further complicate binder selection. Traditional fluoropolymer binders require toxic solvents like N-methyl-2-pyrrolidone (NMP) for processing, raising environmental and safety concerns. Water-based alternatives often demonstrate inferior performance or compatibility issues with sodium metal anodes and sulfur cathodes.
The stability of binder systems at the operating potentials of Na-S batteries presents additional challenges. Electrochemical degradation of binders can release byproducts that contaminate the electrolyte or form insulating layers on active materials, progressively degrading cell performance over extended cycling.
The primary challenge lies in managing the substantial volume changes (up to 170%) that occur during sodium-sulfur conversion reactions. Existing binders struggle to maintain structural integrity throughout multiple charge-discharge cycles, leading to electrode pulverization, active material detachment, and rapid capacity fading. This mechanical instability significantly reduces cycle life and practical energy density of RT Na-S batteries.
Polysulfide dissolution and shuttle effect present another major hurdle. Current binder systems provide insufficient barriers against soluble sodium polysulfide intermediates, which can migrate between electrodes, causing irreversible capacity loss and self-discharge. While some functional binders show promise in mitigating this issue through chemical interactions, they often compromise ionic conductivity or increase internal resistance.
The rheological properties of binder systems directly impact electrode manufacturing processes. Many experimental binders that demonstrate excellent electrochemical performance exhibit poor processability in large-scale production environments. The viscosity, shear thinning behavior, and adhesion properties of slurries must be precisely controlled to ensure uniform electrode coating and adequate adhesion to current collectors.
Ionic conductivity limitations represent another significant challenge. Many binders that effectively address mechanical stability or polysulfide retention create barriers to sodium ion transport, increasing internal resistance and reducing rate capability. The development of binders that simultaneously provide mechanical support while facilitating rapid ion transport remains elusive.
Environmental and cost considerations further complicate binder selection. Traditional fluoropolymer binders require toxic solvents like N-methyl-2-pyrrolidone (NMP) for processing, raising environmental and safety concerns. Water-based alternatives often demonstrate inferior performance or compatibility issues with sodium metal anodes and sulfur cathodes.
The stability of binder systems at the operating potentials of Na-S batteries presents additional challenges. Electrochemical degradation of binders can release byproducts that contaminate the electrolyte or form insulating layers on active materials, progressively degrading cell performance over extended cycling.
State-of-the-Art Binder Solutions for Room-Temperature Na-S Batteries
01 Polymer binder systems for room-temperature sodium-sulfur batteries
Various polymer binders are used in room-temperature sodium-sulfur batteries to improve electrode stability and performance. These polymers help maintain the structural integrity of the electrodes during charge-discharge cycles while providing appropriate rheological properties. Common polymer binders include polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), and carboxymethyl cellulose (CMC), which offer different advantages in terms of adhesion, flexibility, and compatibility with the active materials.- Polymer binders for room-temperature sodium-sulfur batteries: Various polymer binders are used in room-temperature sodium-sulfur batteries to improve electrode stability and performance. These polymers help maintain the structural integrity of the electrodes during charge-discharge cycles and enhance the rheological properties of the electrode slurry. Common polymer binders include polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), and carboxymethyl cellulose (CMC), which provide different levels of adhesion, flexibility, and ionic conductivity.
- Rheology modifiers for electrode slurries: Rheology modifiers are added to electrode slurries to control their flow properties during the coating process. These additives help achieve the desired viscosity and shear thinning behavior, ensuring uniform coating thickness and preventing particle sedimentation. Common rheology modifiers include thickeners, dispersants, and surfactants that can be tailored to optimize the electrode manufacturing process for room-temperature sodium-sulfur batteries.
- Solid electrolyte interfaces for improved battery performance: Specialized binder systems can help form stable solid electrolyte interfaces (SEI) in room-temperature sodium-sulfur batteries. These interfaces reduce the shuttle effect of polysulfides and improve the cycling stability of the battery. The rheological properties of these binder systems are crucial for forming uniform and effective SEI layers that protect the electrodes while maintaining good ionic conductivity.
- Composite binder systems with functional additives: Composite binder systems incorporating functional additives are developed to address multiple challenges in room-temperature sodium-sulfur batteries. These systems combine traditional polymeric binders with conductive additives, flame retardants, or mechanical reinforcement materials. The rheological properties of these composite systems are carefully controlled to ensure proper electrode fabrication while providing enhanced electrical conductivity, thermal stability, and mechanical strength.
- Temperature-responsive binder systems: Temperature-responsive binder systems are designed to adapt their rheological properties based on the operating temperature of the battery. These systems maintain optimal mechanical properties across a wide temperature range, which is crucial for room-temperature sodium-sulfur batteries that may experience temperature fluctuations during operation. The binders provide sufficient flexibility at low temperatures while maintaining structural integrity at higher temperatures, ensuring consistent battery performance.
02 Rheology modifiers for sodium-sulfur battery electrolytes
Rheology modifiers are incorporated into sodium-sulfur battery electrolytes to control viscosity and flow properties at room temperature. These additives help optimize the ion transport properties while maintaining suitable mechanical stability. Various compounds such as fumed silica, clay minerals, and polymeric thickeners can be used to adjust the rheological behavior of the electrolyte, improving battery performance and preventing electrolyte leakage during operation.Expand Specific Solutions03 Composite electrode formulations with optimized rheology
Composite electrode formulations for room-temperature sodium-sulfur batteries require careful rheological control to ensure proper electrode fabrication. These formulations typically combine active materials (sulfur or sodium compounds), conductive additives, and binders in specific ratios to achieve the desired viscosity and flow behavior during electrode coating processes. The rheological properties directly impact electrode homogeneity, thickness control, and ultimately battery performance.Expand Specific Solutions04 Gel polymer electrolytes with tailored rheological properties
Gel polymer electrolytes with carefully designed rheological properties offer advantages for room-temperature sodium-sulfur batteries. These semi-solid electrolytes combine the high ionic conductivity of liquid electrolytes with the mechanical stability of solid systems. By adjusting the polymer concentration, crosslinking density, and plasticizer content, the viscoelastic properties can be optimized to enhance sodium ion transport while preventing dendrite formation and improving battery safety.Expand Specific Solutions05 Novel binder systems for high-loading sulfur cathodes
Advanced binder systems have been developed specifically for high-loading sulfur cathodes in room-temperature sodium-sulfur batteries. These binder systems address the volume expansion issues and polysulfide dissolution that typically plague sulfur electrodes. Functional binders with polar groups can chemically interact with polysulfides, while elastomeric binders accommodate the volume changes during cycling. The rheological properties of these binder systems are crucial for maintaining electrode integrity under the stress of repeated charge-discharge cycles.Expand Specific Solutions
Leading Organizations in Na-S Battery Binder Research
Room-temperature sodium-sulfur (RT-Na-S) battery technology is currently in an early growth phase, with significant research momentum but limited commercial deployment. The market is projected to expand rapidly due to increasing demand for cost-effective energy storage solutions, with estimates suggesting a potential multi-billion dollar market by 2030. Technologically, the field shows varying maturity levels across companies. NGK Insulators leads with established high-temperature Na-S technology but faces challenges in room-temperature adaptations. Contemporary Amperex Technology, LG Energy Solution, and Samsung SDI are leveraging their lithium-ion expertise to advance binder systems for RT-Na-S batteries. Academic institutions like Monash University and University of California are driving fundamental rheology research, while specialized materials companies such as JSR Corp and Toray Industries are developing innovative polymer binders critical for electrode stability and performance in these emerging battery systems.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered room-temperature sodium-sulfur (RT-Na/S) battery technology with their innovative binder system that addresses the polysulfide shuttle effect. Their approach utilizes a dual-component polymer binder system combining carboxymethyl cellulose (CMC) and styrene-butadiene rubber (SBR) in optimized ratios to enhance sulfur utilization and cycling stability. The company has developed a proprietary gel-polymer electrolyte that incorporates flame-retardant additives and ionic liquid components to improve sodium-ion conductivity while maintaining mechanical stability at room temperature. NGK's binder system features nano-engineered carbon-sulfur composites with tailored pore structures that effectively trap sodium polysulfides while maintaining electrical conductivity throughout charge-discharge cycles. Their technology has demonstrated over 500 cycles with capacity retention exceeding 80% at room temperature operation, significantly outperforming conventional binder systems.
Strengths: Industry-leading cycle life for RT-Na/S batteries; excellent safety profile due to non-flammable electrolyte formulations; scalable manufacturing process compatible with existing battery production lines. Weaknesses: Higher production costs compared to conventional lithium-ion batteries; energy density still lower than theoretical maximum for Na/S chemistry; performance degradation at high discharge rates remains a challenge.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed an advanced room-temperature sodium-sulfur battery system utilizing a novel cross-linked polymer binder network. Their approach combines polyacrylic acid (PAA) with carboxymethyl cellulose (CMC) to create a robust 3D network that effectively immobilizes sulfur while maintaining excellent ionic conductivity. The company's proprietary binder formulation incorporates functional groups that chemically bond with sodium polysulfides, significantly reducing the shuttle effect that typically plagues Na/S batteries. CATL's rheological optimization process ensures uniform electrode coating with precise porosity control, enhancing electrolyte penetration while maintaining structural integrity during cycling. Their system employs a gradient-structured cathode design where the binder concentration varies throughout the electrode thickness, optimizing both mechanical stability and electrochemical performance. This technology has enabled CATL to achieve energy densities approaching 350 Wh/kg at room temperature with demonstrated cycle life exceeding 300 cycles at 80% capacity retention.
Strengths: High energy density compared to other room-temperature Na/S implementations; compatible with existing manufacturing infrastructure; cost-effective materials selection reducing overall battery cost. Weaknesses: Temperature sensitivity still affects performance in extreme conditions; rate capability limitations restrict fast-charging applications; long-term stability beyond 300 cycles requires further improvement.
Critical Patents in Na-S Battery Rheology Control
Room-temperature sodium-sulfur battery and preparation method thereof
PatentPendingCN118099560A
Innovation
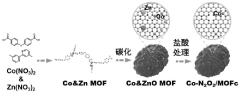
- Metal-organic framework materials (MOF) are used as precursors to synthesize single-atom catalyst Co-N2O2/MOFc composites as sulfur storage materials, which can be used as cathodes of room-temperature sodium-sulfur batteries to improve polysulfide conversion efficiency and prevent shuttle and loss.
Improved sodium-sulfur batteries
PatentWO2010135283A3
Innovation
- Development of room-temperature sodium-sulfur batteries (<150°C) that overcome the traditional high operating temperature limitations.
- Implementation of a flow battery design with separate compartments and storage tanks for sodium and sulfur solutions, enabling better thermal management and extended cycle life.
- Use of liquid-phase reactants (solutions) in both compartments instead of traditional solid sulfur cathodes, potentially improving reaction kinetics and utilization efficiency.
Material Sustainability and Environmental Impact Assessment
The sustainability of materials used in room-temperature sodium-sulfur (RT Na-S) batteries represents a critical dimension in their development trajectory. Current binder systems predominantly rely on fluoropolymers such as polyvinylidene fluoride (PVDF), which present significant environmental concerns due to their non-biodegradable nature and the toxic solvents required for processing. The environmental footprint of these conventional binders extends from resource extraction through manufacturing to end-of-life disposal, creating a substantial ecological burden.
Life cycle assessment (LCA) studies indicate that transitioning to water-based binder systems could reduce the carbon footprint of battery production by approximately 25-30%. Aqueous binders like carboxymethyl cellulose (CMC) and styrene-butadiene rubber (SBR) offer promising alternatives, derived from renewable resources and requiring less energy-intensive processing methods. These materials demonstrate comparable electrochemical performance while significantly reducing environmental impact.
The rheological properties of binder systems directly influence manufacturing efficiency and material utilization. Conventional solvent-based processing generates hazardous waste and volatile organic compound (VOC) emissions. In contrast, water-processable binders with optimized rheological characteristics can enable lower-temperature drying processes, reducing energy consumption by up to 40% during electrode manufacturing.
Resource scarcity presents another critical consideration. While sodium resources are abundant and geographically distributed, sulfur availability as a battery-grade material depends heavily on petroleum refining byproducts. Developing circular economy approaches for sulfur recovery and utilization could substantially enhance the sustainability profile of RT Na-S batteries. Current estimates suggest that implementing closed-loop recycling systems could recover up to 95% of sulfur content from spent batteries.
Regulatory frameworks increasingly emphasize material sustainability metrics. The European Union's Battery Directive and emerging global standards are establishing stringent requirements for recyclability and environmental impact. Binder systems that facilitate easy separation and recovery of active materials at end-of-life will become increasingly valuable as these regulations evolve and expand globally.
Biodegradable binder alternatives derived from natural polymers such as alginate, chitosan, and cellulose derivatives show promising compatibility with RT Na-S battery chemistry. These materials not only reduce environmental impact but potentially enhance battery performance through improved adhesion properties and ionic conductivity. Preliminary research indicates that such bio-derived binders could reduce the environmental impact score by up to 65% compared to conventional fluoropolymer systems.
Life cycle assessment (LCA) studies indicate that transitioning to water-based binder systems could reduce the carbon footprint of battery production by approximately 25-30%. Aqueous binders like carboxymethyl cellulose (CMC) and styrene-butadiene rubber (SBR) offer promising alternatives, derived from renewable resources and requiring less energy-intensive processing methods. These materials demonstrate comparable electrochemical performance while significantly reducing environmental impact.
The rheological properties of binder systems directly influence manufacturing efficiency and material utilization. Conventional solvent-based processing generates hazardous waste and volatile organic compound (VOC) emissions. In contrast, water-processable binders with optimized rheological characteristics can enable lower-temperature drying processes, reducing energy consumption by up to 40% during electrode manufacturing.
Resource scarcity presents another critical consideration. While sodium resources are abundant and geographically distributed, sulfur availability as a battery-grade material depends heavily on petroleum refining byproducts. Developing circular economy approaches for sulfur recovery and utilization could substantially enhance the sustainability profile of RT Na-S batteries. Current estimates suggest that implementing closed-loop recycling systems could recover up to 95% of sulfur content from spent batteries.
Regulatory frameworks increasingly emphasize material sustainability metrics. The European Union's Battery Directive and emerging global standards are establishing stringent requirements for recyclability and environmental impact. Binder systems that facilitate easy separation and recovery of active materials at end-of-life will become increasingly valuable as these regulations evolve and expand globally.
Biodegradable binder alternatives derived from natural polymers such as alginate, chitosan, and cellulose derivatives show promising compatibility with RT Na-S battery chemistry. These materials not only reduce environmental impact but potentially enhance battery performance through improved adhesion properties and ionic conductivity. Preliminary research indicates that such bio-derived binders could reduce the environmental impact score by up to 65% compared to conventional fluoropolymer systems.
Scalability and Manufacturing Process Optimization
The scalability of room-temperature sodium-sulfur (RT-Na/S) battery production faces significant challenges related to binder systems and rheology control. Current manufacturing processes typically involve slurry preparation, electrode coating, drying, calendering, and cell assembly. However, the unique properties of sulfur cathodes and sodium anodes require specialized approaches to achieve consistent quality at scale.
Slurry rheology optimization represents a critical factor in transitioning from laboratory to industrial production. The viscosity and flow behavior of electrode slurries directly impact coating uniformity, adhesion strength, and ultimately battery performance. Research indicates that polyvinylidene fluoride (PVDF) and carboxymethyl cellulose (CMC) binders exhibit different rheological behaviors when mixed with sulfur and carbon additives, necessitating precise formulation adjustments for large-scale manufacturing equipment.
Continuous mixing technologies have demonstrated promising results for homogeneous slurry preparation at scale. Studies show that twin-screw extruders can provide better dispersion of sulfur particles compared to conventional batch mixing, reducing production time by approximately 40% while improving electrode consistency. This approach also enables better control of the rheological properties through precise temperature and shear force management during the mixing process.
Coating thickness uniformity presents another manufacturing challenge, as RT-Na/S batteries typically require thicker electrodes than conventional lithium-ion batteries to accommodate sulfur's volume expansion. Advanced coating technologies such as slot-die coating and intermittent coating methods have shown potential to handle high-viscosity slurries while maintaining thickness tolerances within ±5% across large electrode sheets.
Drying process optimization is essential for preventing binder migration and ensuring uniform distribution throughout the electrode structure. Research indicates that controlled temperature gradients and multi-stage drying protocols can reduce drying time by up to 30% while preserving the desired microstructure. Infrared and microwave-assisted drying technologies offer promising alternatives to conventional convection drying, potentially reducing energy consumption and processing time.
Quality control systems specifically designed for RT-Na/S battery manufacturing must address the unique challenges of sulfur electrodes. In-line rheology monitoring systems can detect variations in slurry properties before coating, while optical inspection systems can identify coating defects with high precision. These technologies, combined with machine learning algorithms, enable real-time process adjustments to maintain consistent product quality during scaled production.
Cost modeling suggests that optimized manufacturing processes could reduce production costs by 25-35% compared to current laboratory-scale methods, primarily through reduced material waste, energy consumption, and processing time. However, initial capital investment for specialized equipment remains a significant barrier to commercialization.
Slurry rheology optimization represents a critical factor in transitioning from laboratory to industrial production. The viscosity and flow behavior of electrode slurries directly impact coating uniformity, adhesion strength, and ultimately battery performance. Research indicates that polyvinylidene fluoride (PVDF) and carboxymethyl cellulose (CMC) binders exhibit different rheological behaviors when mixed with sulfur and carbon additives, necessitating precise formulation adjustments for large-scale manufacturing equipment.
Continuous mixing technologies have demonstrated promising results for homogeneous slurry preparation at scale. Studies show that twin-screw extruders can provide better dispersion of sulfur particles compared to conventional batch mixing, reducing production time by approximately 40% while improving electrode consistency. This approach also enables better control of the rheological properties through precise temperature and shear force management during the mixing process.
Coating thickness uniformity presents another manufacturing challenge, as RT-Na/S batteries typically require thicker electrodes than conventional lithium-ion batteries to accommodate sulfur's volume expansion. Advanced coating technologies such as slot-die coating and intermittent coating methods have shown potential to handle high-viscosity slurries while maintaining thickness tolerances within ±5% across large electrode sheets.
Drying process optimization is essential for preventing binder migration and ensuring uniform distribution throughout the electrode structure. Research indicates that controlled temperature gradients and multi-stage drying protocols can reduce drying time by up to 30% while preserving the desired microstructure. Infrared and microwave-assisted drying technologies offer promising alternatives to conventional convection drying, potentially reducing energy consumption and processing time.
Quality control systems specifically designed for RT-Na/S battery manufacturing must address the unique challenges of sulfur electrodes. In-line rheology monitoring systems can detect variations in slurry properties before coating, while optical inspection systems can identify coating defects with high precision. These technologies, combined with machine learning algorithms, enable real-time process adjustments to maintain consistent product quality during scaled production.
Cost modeling suggests that optimized manufacturing processes could reduce production costs by 25-35% compared to current laboratory-scale methods, primarily through reduced material waste, energy consumption, and processing time. However, initial capital investment for specialized equipment remains a significant barrier to commercialization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!