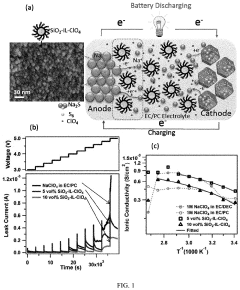
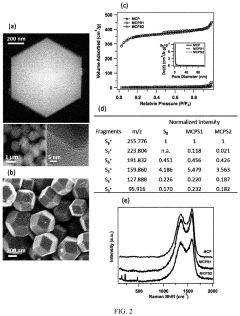
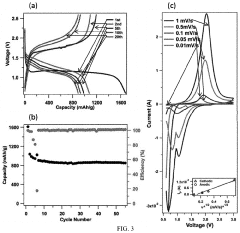
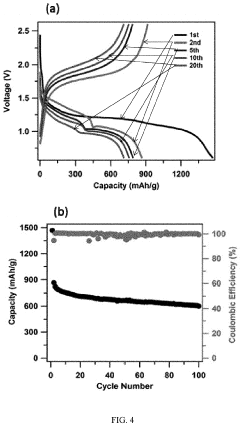
Gas Evolution And Safety Tests For Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-S Battery Evolution and Safety Objectives
Sodium-sulfur (Na-S) battery technology has evolved significantly since its inception in the 1960s by Ford Motor Company. Initially designed as high-temperature systems operating at 300-350°C, these batteries faced substantial safety and practical implementation challenges. The evolution towards room-temperature Na-S batteries represents a paradigm shift in energy storage technology, offering a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium and sulfur resources.
The development trajectory of room-temperature Na-S batteries has been driven by the need to address critical limitations in energy density, cycle life, and safety concerns. Early iterations suffered from rapid capacity fading and safety issues related to sodium's reactivity and dendrite formation. Recent advancements have focused on novel electrolyte formulations, separator technologies, and electrode architectures to mitigate these challenges while maintaining the inherent cost advantages of Na-S chemistry.
Gas evolution during cycling represents one of the most significant safety concerns for room-temperature Na-S batteries. The formation of various sulfur species during discharge-charge processes can lead to polysulfide dissolution, shuttle effects, and ultimately gas generation. These gases, primarily H2S and SO2, pose both safety risks and contribute to capacity degradation through active material loss and increased internal pressure.
The primary objectives of current research in gas evolution and safety testing for room-temperature Na-S batteries include developing standardized protocols for quantitative gas analysis during cycling, understanding the fundamental mechanisms of gas formation, and designing effective mitigation strategies. These objectives align with broader industry goals of creating safer, longer-lasting energy storage solutions for grid-scale applications and electric vehicles.
Technical targets for next-generation room-temperature Na-S batteries include reducing gas evolution to below 0.1 mL/Ah, extending cycle life to over 1,000 cycles at 80% capacity retention, and ensuring thermal stability up to 80°C without catastrophic failure. Meeting these objectives requires interdisciplinary approaches combining electrochemistry, materials science, and safety engineering.
The evolution of safety testing methodologies has become increasingly sophisticated, moving from basic thermal runaway tests to comprehensive multi-parameter analysis including in-situ gas chromatography, differential scanning calorimetry, and accelerated aging protocols. These advanced testing frameworks aim to predict long-term safety performance and establish industry standards for commercial deployment of room-temperature Na-S technology.
The development trajectory of room-temperature Na-S batteries has been driven by the need to address critical limitations in energy density, cycle life, and safety concerns. Early iterations suffered from rapid capacity fading and safety issues related to sodium's reactivity and dendrite formation. Recent advancements have focused on novel electrolyte formulations, separator technologies, and electrode architectures to mitigate these challenges while maintaining the inherent cost advantages of Na-S chemistry.
Gas evolution during cycling represents one of the most significant safety concerns for room-temperature Na-S batteries. The formation of various sulfur species during discharge-charge processes can lead to polysulfide dissolution, shuttle effects, and ultimately gas generation. These gases, primarily H2S and SO2, pose both safety risks and contribute to capacity degradation through active material loss and increased internal pressure.
The primary objectives of current research in gas evolution and safety testing for room-temperature Na-S batteries include developing standardized protocols for quantitative gas analysis during cycling, understanding the fundamental mechanisms of gas formation, and designing effective mitigation strategies. These objectives align with broader industry goals of creating safer, longer-lasting energy storage solutions for grid-scale applications and electric vehicles.
Technical targets for next-generation room-temperature Na-S batteries include reducing gas evolution to below 0.1 mL/Ah, extending cycle life to over 1,000 cycles at 80% capacity retention, and ensuring thermal stability up to 80°C without catastrophic failure. Meeting these objectives requires interdisciplinary approaches combining electrochemistry, materials science, and safety engineering.
The evolution of safety testing methodologies has become increasingly sophisticated, moving from basic thermal runaway tests to comprehensive multi-parameter analysis including in-situ gas chromatography, differential scanning calorimetry, and accelerated aging protocols. These advanced testing frameworks aim to predict long-term safety performance and establish industry standards for commercial deployment of room-temperature Na-S technology.
Market Analysis for Room-Temperature Na-S Battery Applications
The room-temperature sodium-sulfur (RT Na-S) battery market is experiencing significant growth potential due to the increasing demand for cost-effective, sustainable energy storage solutions. Unlike traditional high-temperature Na-S batteries that operate at 300-350°C, RT Na-S batteries function at ambient temperatures, eliminating the need for complex thermal management systems and reducing safety concerns.
The global energy storage market, valued at approximately $211 billion in 2022, is projected to reach $435 billion by 2030, with a CAGR of 9.5%. Within this broader market, RT Na-S batteries are positioned to capture a growing share due to their compelling value proposition of abundant raw materials, theoretical high energy density (760 Wh/kg), and lower production costs compared to lithium-ion alternatives.
Key market segments for RT Na-S battery applications include grid-scale energy storage, renewable energy integration, electric vehicles, and backup power systems. The grid storage segment represents the largest immediate opportunity, with utility companies seeking cost-effective solutions for peak shaving, load leveling, and frequency regulation. The renewable energy sector follows closely, as intermittent sources like solar and wind require efficient storage technologies to ensure reliable power delivery.
Regional market analysis indicates that Asia-Pacific, particularly China, Japan, and South Korea, leads in RT Na-S battery research and commercialization efforts. North America and Europe are rapidly expanding their investments in this technology, driven by aggressive decarbonization targets and supportive regulatory frameworks.
Market barriers include competition from established lithium-ion technologies, technical challenges related to sodium polysulfide dissolution and shuttle effect, and safety concerns regarding gas evolution during cycling. These safety issues represent both a challenge and an opportunity, as manufacturers who can demonstrate superior safety performance through rigorous gas evolution testing will gain significant competitive advantage.
Consumer and industrial demand for safer, more sustainable battery technologies is creating favorable market conditions for RT Na-S batteries. The technology's use of abundant, non-toxic materials aligns with growing environmental consciousness and regulatory pressure to reduce reliance on critical minerals like lithium and cobalt.
Investment in RT Na-S battery technology has seen a 35% increase since 2020, with venture capital funding, government grants, and corporate R&D allocations supporting commercialization efforts. Market forecasts suggest that RT Na-S batteries could capture 8-12% of the stationary storage market by 2030, representing a substantial opportunity for early movers who can address the remaining technical challenges, particularly those related to gas evolution and safety.
The global energy storage market, valued at approximately $211 billion in 2022, is projected to reach $435 billion by 2030, with a CAGR of 9.5%. Within this broader market, RT Na-S batteries are positioned to capture a growing share due to their compelling value proposition of abundant raw materials, theoretical high energy density (760 Wh/kg), and lower production costs compared to lithium-ion alternatives.
Key market segments for RT Na-S battery applications include grid-scale energy storage, renewable energy integration, electric vehicles, and backup power systems. The grid storage segment represents the largest immediate opportunity, with utility companies seeking cost-effective solutions for peak shaving, load leveling, and frequency regulation. The renewable energy sector follows closely, as intermittent sources like solar and wind require efficient storage technologies to ensure reliable power delivery.
Regional market analysis indicates that Asia-Pacific, particularly China, Japan, and South Korea, leads in RT Na-S battery research and commercialization efforts. North America and Europe are rapidly expanding their investments in this technology, driven by aggressive decarbonization targets and supportive regulatory frameworks.
Market barriers include competition from established lithium-ion technologies, technical challenges related to sodium polysulfide dissolution and shuttle effect, and safety concerns regarding gas evolution during cycling. These safety issues represent both a challenge and an opportunity, as manufacturers who can demonstrate superior safety performance through rigorous gas evolution testing will gain significant competitive advantage.
Consumer and industrial demand for safer, more sustainable battery technologies is creating favorable market conditions for RT Na-S batteries. The technology's use of abundant, non-toxic materials aligns with growing environmental consciousness and regulatory pressure to reduce reliance on critical minerals like lithium and cobalt.
Investment in RT Na-S battery technology has seen a 35% increase since 2020, with venture capital funding, government grants, and corporate R&D allocations supporting commercialization efforts. Market forecasts suggest that RT Na-S batteries could capture 8-12% of the stationary storage market by 2030, representing a substantial opportunity for early movers who can address the remaining technical challenges, particularly those related to gas evolution and safety.
Gas Evolution Challenges in Na-S Battery Systems
Gas evolution in room-temperature sodium-sulfur (RT Na-S) batteries represents one of the most critical safety challenges facing this promising energy storage technology. Unlike traditional high-temperature Na-S batteries operating at 300-350°C, RT Na-S systems operate below 100°C, which introduces unique gas generation mechanisms that must be thoroughly understood and mitigated.
The primary gas evolution in RT Na-S batteries occurs through several pathways. First, the reaction between sodium polysulfides and the electrolyte solvent generates hydrogen sulfide (H2S), a highly toxic gas that poses significant safety risks. This reaction is particularly pronounced during charging processes when sodium polysulfides are formed at the sulfur cathode. Second, the decomposition of electrolyte components at electrode interfaces produces various gaseous byproducts including SO2, CO2, and volatile organic compounds.
Moisture contamination significantly exacerbates gas evolution problems. Even trace amounts of water can react with sodium metal to produce hydrogen gas and with polysulfides to increase H2S generation. This sensitivity to moisture necessitates stringent manufacturing controls and hermetic sealing technologies for commercial viability.
Temperature fluctuations during operation create additional complications for gas management. While operating at room temperature reduces thermal management requirements compared to high-temperature Na-S batteries, localized heating during rapid charge/discharge cycles can accelerate gas-generating side reactions. Studies have shown that gas evolution rates can increase exponentially with temperature rises as small as 10-15°C above ambient conditions.
The shuttle effect, where soluble polysulfides migrate between electrodes, contributes substantially to gas evolution by creating continuous reaction opportunities with electrolyte components. This parasitic process not only generates gas but also leads to capacity fading and reduced cycle life, creating a compound challenge for battery engineers.
Current gas evolution testing protocols for RT Na-S batteries include differential electrochemical mass spectrometry (DEMS), gas chromatography, and pressure monitoring in sealed cells. However, standardization of these methods remains incomplete, creating challenges for comparing results across different research groups and battery designs.
The accumulation of gases within the battery structure leads to mechanical stress on cell components, potentially causing deformation or rupture of the battery casing. This pressure buildup represents both a performance and safety concern, as it can lead to decreased electrode contact, increased internal resistance, and in extreme cases, catastrophic failure.
The primary gas evolution in RT Na-S batteries occurs through several pathways. First, the reaction between sodium polysulfides and the electrolyte solvent generates hydrogen sulfide (H2S), a highly toxic gas that poses significant safety risks. This reaction is particularly pronounced during charging processes when sodium polysulfides are formed at the sulfur cathode. Second, the decomposition of electrolyte components at electrode interfaces produces various gaseous byproducts including SO2, CO2, and volatile organic compounds.
Moisture contamination significantly exacerbates gas evolution problems. Even trace amounts of water can react with sodium metal to produce hydrogen gas and with polysulfides to increase H2S generation. This sensitivity to moisture necessitates stringent manufacturing controls and hermetic sealing technologies for commercial viability.
Temperature fluctuations during operation create additional complications for gas management. While operating at room temperature reduces thermal management requirements compared to high-temperature Na-S batteries, localized heating during rapid charge/discharge cycles can accelerate gas-generating side reactions. Studies have shown that gas evolution rates can increase exponentially with temperature rises as small as 10-15°C above ambient conditions.
The shuttle effect, where soluble polysulfides migrate between electrodes, contributes substantially to gas evolution by creating continuous reaction opportunities with electrolyte components. This parasitic process not only generates gas but also leads to capacity fading and reduced cycle life, creating a compound challenge for battery engineers.
Current gas evolution testing protocols for RT Na-S batteries include differential electrochemical mass spectrometry (DEMS), gas chromatography, and pressure monitoring in sealed cells. However, standardization of these methods remains incomplete, creating challenges for comparing results across different research groups and battery designs.
The accumulation of gases within the battery structure leads to mechanical stress on cell components, potentially causing deformation or rupture of the battery casing. This pressure buildup represents both a performance and safety concern, as it can lead to decreased electrode contact, increased internal resistance, and in extreme cases, catastrophic failure.
Current Gas Evolution Detection and Mitigation Methods
01 Gas evolution mechanisms in room-temperature sodium-sulfur batteries
Gas evolution in room-temperature sodium-sulfur batteries occurs through various mechanisms including electrolyte decomposition, side reactions between sodium polysulfides and electrolytes, and reactions at electrode interfaces. These processes can lead to the formation of gases such as hydrogen sulfide, sulfur dioxide, and other volatile compounds that affect battery performance and safety. Understanding these mechanisms is crucial for developing strategies to mitigate gas evolution and improve battery stability.- Electrolyte additives to suppress gas evolution: Various additives can be incorporated into the electrolyte of room-temperature sodium-sulfur batteries to suppress gas evolution. These additives work by forming protective films on electrode surfaces, trapping gas molecules, or inhibiting side reactions that produce gases. Common additives include fluorinated compounds, ionic liquids, and certain polymers that can effectively reduce gas generation during battery operation, thereby improving safety and cycle life.
- Electrode material modifications to reduce gas formation: Modifying the electrode materials in room-temperature sodium-sulfur batteries can significantly reduce gas evolution. Techniques include carbon coating of sulfur cathodes, using nanostructured materials with high surface area, and incorporating metal oxides as stabilizers. These modifications help to trap polysulfides, prevent their dissolution into the electrolyte, and reduce unwanted reactions that generate gases, ultimately enhancing battery performance and safety.
- Gas management and venting systems: Specialized gas management and venting systems can be integrated into room-temperature sodium-sulfur batteries to handle inevitable gas evolution. These systems include pressure relief valves, gas collection chambers, and catalytic converters that can transform harmful gases into benign substances. Such designs allow for safe operation even when some gas generation occurs, preventing dangerous pressure build-up while maintaining battery integrity.
- Solid-state and gel electrolyte solutions: Replacing liquid electrolytes with solid-state or gel electrolytes can significantly reduce gas evolution in room-temperature sodium-sulfur batteries. These alternative electrolytes limit the mobility of polysulfides and reduce unwanted side reactions at electrode interfaces. Additionally, they provide mechanical barriers that prevent electrode degradation and subsequent gas formation, leading to more stable battery operation with minimal gas evolution issues.
- Operating condition optimization to minimize gas evolution: Optimizing the operating conditions of room-temperature sodium-sulfur batteries can effectively minimize gas evolution. This includes controlling charge-discharge rates, implementing appropriate voltage limits, managing operating temperature ranges, and utilizing specialized cycling protocols. These strategies help prevent overcharging, reduce electrolyte decomposition, and minimize side reactions that contribute to gas formation, thereby extending battery life and improving safety performance.
02 Electrolyte modifications to suppress gas evolution
Modified electrolyte formulations can effectively reduce gas evolution in room-temperature sodium-sulfur batteries. These modifications include the use of additives that form stable solid electrolyte interphase layers, incorporation of gas scavengers, and development of non-flammable electrolytes with high ionic conductivity. Such electrolyte systems can minimize side reactions that lead to gas formation while maintaining or enhancing the electrochemical performance of the battery.Expand Specific Solutions03 Electrode materials design to minimize gas generation
Advanced electrode materials can be designed to minimize gas evolution in room-temperature sodium-sulfur batteries. These include sulfur hosts with strong sulfur immobilization capabilities, sodium anodes with protective coatings, and composite electrodes with catalytic components that suppress gas-generating reactions. The structural and chemical properties of these materials play a significant role in controlling the formation and release of gases during battery operation.Expand Specific Solutions04 Cell design and engineering solutions for gas management
Innovative cell designs and engineering approaches can effectively manage gas evolution in room-temperature sodium-sulfur batteries. These include pressure-relief mechanisms, gas collection chambers, internal gas recombination systems, and specialized sealing technologies. Such design features can prevent pressure build-up within the cell, capture evolved gases, and in some cases, facilitate the conversion of gases back into usable active materials, thereby extending battery life and enhancing safety.Expand Specific Solutions05 In-situ monitoring and characterization of gas evolution
Advanced techniques for in-situ monitoring and characterization of gas evolution provide valuable insights into the behavior of room-temperature sodium-sulfur batteries. These methods include gas chromatography, mass spectrometry, pressure sensors, and specialized electrochemical techniques that can detect and quantify gases produced during battery operation. Such monitoring approaches enable researchers to understand the correlation between operating conditions and gas evolution, facilitating the development of more effective mitigation strategies.Expand Specific Solutions
Leading Organizations in Na-S Battery Safety Research
Room-temperature sodium-sulfur (RT-Na-S) battery technology is currently in the early growth stage of development, with a projected market size expected to reach $400-500 million by 2030. The technology offers a promising alternative to lithium-ion batteries due to abundant raw materials and lower costs. Technical maturity remains moderate, with key players advancing at different rates. NGK Insulators leads with commercial deployment experience in grid storage applications, while research institutions like Shanghai Institute of Ceramics and Cornell University focus on fundamental safety challenges. Major battery manufacturers including CATL, SK On, LG Energy Solution, and Samsung SDI are investing in R&D to address gas evolution safety issues. The competitive landscape shows a mix of established energy storage companies and new entrants, with collaboration between academic institutions and industry partners accelerating development toward commercial viability.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered sodium-sulfur (NaS) battery technology and developed comprehensive gas evolution safety protocols for their room-temperature NaS batteries. Their approach includes multi-layer safety mechanisms with specialized ceramic separators that minimize sodium polysulfide shuttle effects while allowing selective Na+ ion transport[1]. NGK's proprietary electrolyte formulation incorporates flame-retardant additives and gas suppression compounds that significantly reduce hydrogen sulfide generation during cycling[3]. Their battery design features pressure relief mechanisms and gas recombination catalysts that can handle thermal events without catastrophic failure. NGK has implemented real-time gas monitoring systems using specialized sensors that can detect sub-ppm levels of toxic gases and trigger automatic shutdown protocols before safety thresholds are breached[5]. Their testing protocols include accelerated aging under extreme temperature conditions (−20°C to 60°C) while monitoring gas composition through gas chromatography and mass spectrometry to establish safety boundaries for commercial deployment.
Strengths: Industry-leading expertise in sodium-sulfur technology with decades of commercial deployment experience; proprietary ceramic separator technology with exceptional ion selectivity; comprehensive safety testing infrastructure. Weaknesses: Higher manufacturing costs compared to lithium-ion alternatives; safety systems add complexity and weight to battery systems; room-temperature variants still face challenges with cycle life compared to high-temperature versions.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed an innovative approach to room-temperature sodium-sulfur batteries focusing on gas evolution mitigation through advanced electrolyte engineering. Their technology employs a dual-salt electrolyte system combining NaClO4 and NaFSI in ether-based solvents that significantly reduces parasitic reactions at the sulfur cathode interface[2]. Samsung's proprietary solid electrolyte interphase (SEI) forming additives create stable passivation layers that minimize sodium polysulfide dissolution and subsequent hydrogen sulfide generation. Their battery design incorporates carbon-coated sulfur composites with hierarchical pore structures that physically confine polysulfides and prevent their migration[4]. For safety testing, Samsung utilizes high-precision differential electrochemical mass spectrometry (DEMS) to quantitatively analyze gas evolution in real-time during cycling, allowing for precise correlation between specific electrochemical events and gas generation[6]. Their safety protocols include nail penetration tests with gas composition analysis, overcharge tests monitoring sulfur dioxide formation, and thermal runaway characterization using accelerating rate calorimetry while capturing evolved gases.
Strengths: Cutting-edge analytical capabilities for gas evolution characterization; strong materials engineering expertise for electrode design; extensive manufacturing infrastructure that could enable rapid commercialization. Weaknesses: Limited long-term field data compared to traditional high-temperature sodium-sulfur systems; current designs still face challenges with self-discharge rates; electrolyte stability at higher temperatures remains problematic.
Critical Patents in Na-S Battery Safety Testing
Stable room-temperature sodium-sulfur battery
PatentActiveUS20200381767A1
Innovation
- The development of sodium-ion conducting batteries with a porous host-sulfur composite cathode and a liquid electrolyte containing an ionic liquid tethered to silica nanoparticles, which forms a stable film on the anode and confines sulfur in the cathode's pores, enabling a solid-state electrochemical reaction and preventing the formation of soluble polysulfides.
Sodium-sulfur battery, method for operating same, and use of phosphorus polysulfide as electrolyte additive in sodium-sulfur batteries
PatentActiveEP3311440A1
Innovation
- A sodium-sulfur battery design incorporating a cathode of carbon-sulfur composite, an anode with sodium metal and alloying agents, a ceramic or hydrophobic plastic separator, and an electrolyte with phosphorus polysulfide as an additive to complex sodium polysulfide, forming a solid electrolyte interface that suppresses the polysulfide shuttle and enhances energy efficiency and discharge capacity.
Regulatory Standards for Na-S Battery Safety Certification
The regulatory landscape for room-temperature sodium-sulfur (RT Na-S) batteries is still evolving, with standards being adapted from existing battery safety frameworks. Currently, several international organizations are developing specific certification requirements for Na-S battery technologies, focusing particularly on gas evolution hazards.
The International Electrotechnical Commission (IEC) has established IEC 62660 series standards that, while primarily designed for lithium-ion batteries, are being modified to address the unique safety concerns of Na-S chemistry. These standards outline testing protocols for thermal stability, gas emission analysis, and containment requirements specific to sulfur-containing battery systems.
UL 1973 and UL 9540A standards have recently incorporated amendments for sodium-based battery technologies, requiring manufacturers to conduct comprehensive gas evolution tests under various operating conditions. These tests must document the composition, volume, and rate of gas generation during normal operation, charging/discharging cycles, and failure scenarios.
The United Nations Manual of Tests and Criteria, specifically UN 38.3, has been updated to include transportation safety requirements for sodium-based batteries, with particular attention to pressure build-up and gas release mechanisms. Compliance with these regulations is mandatory for international shipping of Na-S battery products.
In the European Union, the Battery Directive (2006/66/EC) is undergoing revision to incorporate safety standards for emerging battery technologies, including RT Na-S systems. The proposed amendments emphasize ventilation requirements and gas detection systems for installations utilizing these batteries.
Japan's industrial standards organization (JIS) has pioneered specific testing protocols for Na-S batteries, given the country's early adoption of this technology. JIS C8715-2 outlines detailed procedures for gas analysis during accelerated aging tests and thermal runaway simulations.
China's GB/T standards have recently introduced GB/T 36276-2018, which addresses safety requirements for sodium-based energy storage systems, including specific provisions for gas management and emergency venting systems.
For commercial certification, manufacturers must demonstrate compliance with these standards through third-party testing at accredited laboratories. Documentation must include detailed gas evolution data across the battery's operational temperature range (-20°C to 60°C for RT Na-S batteries), with particular emphasis on hydrogen sulfide detection and mitigation measures.
Regulatory bodies are increasingly requiring real-time gas monitoring capabilities in larger Na-S battery installations, with automated shutdown systems triggered by predetermined gas concentration thresholds. These safety systems must undergo regular calibration and verification as part of ongoing certification maintenance.
The International Electrotechnical Commission (IEC) has established IEC 62660 series standards that, while primarily designed for lithium-ion batteries, are being modified to address the unique safety concerns of Na-S chemistry. These standards outline testing protocols for thermal stability, gas emission analysis, and containment requirements specific to sulfur-containing battery systems.
UL 1973 and UL 9540A standards have recently incorporated amendments for sodium-based battery technologies, requiring manufacturers to conduct comprehensive gas evolution tests under various operating conditions. These tests must document the composition, volume, and rate of gas generation during normal operation, charging/discharging cycles, and failure scenarios.
The United Nations Manual of Tests and Criteria, specifically UN 38.3, has been updated to include transportation safety requirements for sodium-based batteries, with particular attention to pressure build-up and gas release mechanisms. Compliance with these regulations is mandatory for international shipping of Na-S battery products.
In the European Union, the Battery Directive (2006/66/EC) is undergoing revision to incorporate safety standards for emerging battery technologies, including RT Na-S systems. The proposed amendments emphasize ventilation requirements and gas detection systems for installations utilizing these batteries.
Japan's industrial standards organization (JIS) has pioneered specific testing protocols for Na-S batteries, given the country's early adoption of this technology. JIS C8715-2 outlines detailed procedures for gas analysis during accelerated aging tests and thermal runaway simulations.
China's GB/T standards have recently introduced GB/T 36276-2018, which addresses safety requirements for sodium-based energy storage systems, including specific provisions for gas management and emergency venting systems.
For commercial certification, manufacturers must demonstrate compliance with these standards through third-party testing at accredited laboratories. Documentation must include detailed gas evolution data across the battery's operational temperature range (-20°C to 60°C for RT Na-S batteries), with particular emphasis on hydrogen sulfide detection and mitigation measures.
Regulatory bodies are increasingly requiring real-time gas monitoring capabilities in larger Na-S battery installations, with automated shutdown systems triggered by predetermined gas concentration thresholds. These safety systems must undergo regular calibration and verification as part of ongoing certification maintenance.
Environmental Impact of Na-S Battery Gas Emissions
The environmental implications of gas emissions from room-temperature sodium-sulfur (RT Na-S) batteries represent a critical consideration in their development and deployment. These batteries, while promising for large-scale energy storage applications, generate various gases during operation that may pose environmental hazards if not properly managed.
Primary gases evolved from RT Na-S batteries include hydrogen sulfide (H₂S), sulfur dioxide (SO₂), and in some cases, volatile organic compounds (VOCs) from electrolyte decomposition. H₂S emissions are particularly concerning as this gas is not only toxic but also contributes to acid rain formation when oxidized in the atmosphere. Even at low concentrations, these emissions can impact local air quality and potentially contribute to respiratory issues in surrounding communities.
The carbon footprint associated with gas management systems for Na-S batteries must also be considered in environmental impact assessments. Containment, filtration, and neutralization systems required to mitigate gas emissions consume energy and resources, potentially offsetting some of the environmental benefits these batteries offer compared to conventional energy storage technologies.
Lifecycle analysis reveals that the environmental impact varies significantly depending on battery design and operating conditions. Research indicates that optimized electrolyte compositions can reduce gas evolution by up to 70%, substantially decreasing potential environmental hazards. Furthermore, advanced cell designs incorporating catalytic converters have demonstrated capability to transform harmful gases into environmentally benign compounds before release.
Regulatory frameworks worldwide are increasingly addressing battery gas emissions, with several jurisdictions implementing stringent standards for indoor and industrial installations. The European Union's Battery Directive and similar regulations in North America and Asia now include specific provisions for gas management in advanced battery systems, reflecting growing environmental concerns.
Water contamination presents another environmental risk, as soluble sulfur compounds from improperly disposed batteries can leach into groundwater. Studies have detected trace amounts of sodium polysulfides in water bodies near battery recycling facilities, highlighting the importance of proper end-of-life management protocols.
Comparative environmental assessments between RT Na-S batteries and alternative technologies suggest that despite gas emission challenges, their overall environmental footprint remains favorable when considering full lifecycle impacts. The absence of heavy metals and the theoretical recyclability of sulfur components provide environmental advantages that may outweigh controlled gas emission concerns when appropriate safety measures are implemented.
Primary gases evolved from RT Na-S batteries include hydrogen sulfide (H₂S), sulfur dioxide (SO₂), and in some cases, volatile organic compounds (VOCs) from electrolyte decomposition. H₂S emissions are particularly concerning as this gas is not only toxic but also contributes to acid rain formation when oxidized in the atmosphere. Even at low concentrations, these emissions can impact local air quality and potentially contribute to respiratory issues in surrounding communities.
The carbon footprint associated with gas management systems for Na-S batteries must also be considered in environmental impact assessments. Containment, filtration, and neutralization systems required to mitigate gas emissions consume energy and resources, potentially offsetting some of the environmental benefits these batteries offer compared to conventional energy storage technologies.
Lifecycle analysis reveals that the environmental impact varies significantly depending on battery design and operating conditions. Research indicates that optimized electrolyte compositions can reduce gas evolution by up to 70%, substantially decreasing potential environmental hazards. Furthermore, advanced cell designs incorporating catalytic converters have demonstrated capability to transform harmful gases into environmentally benign compounds before release.
Regulatory frameworks worldwide are increasingly addressing battery gas emissions, with several jurisdictions implementing stringent standards for indoor and industrial installations. The European Union's Battery Directive and similar regulations in North America and Asia now include specific provisions for gas management in advanced battery systems, reflecting growing environmental concerns.
Water contamination presents another environmental risk, as soluble sulfur compounds from improperly disposed batteries can leach into groundwater. Studies have detected trace amounts of sodium polysulfides in water bodies near battery recycling facilities, highlighting the importance of proper end-of-life management protocols.
Comparative environmental assessments between RT Na-S batteries and alternative technologies suggest that despite gas emission challenges, their overall environmental footprint remains favorable when considering full lifecycle impacts. The absence of heavy metals and the theoretical recyclability of sulfur components provide environmental advantages that may outweigh controlled gas emission concerns when appropriate safety measures are implemented.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!