Standard Protocols And KPIs For Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
RT-Na-S Battery Development Background and Objectives
Room-temperature sodium-sulfur (RT-Na-S) batteries have emerged as a promising alternative to lithium-ion batteries due to their potential for high energy density, low cost, and environmental sustainability. The development of these batteries traces back to the 1960s when high-temperature sodium-sulfur batteries were first introduced. However, these early versions required operating temperatures above 300°C, limiting their practical applications to large-scale stationary energy storage systems.
The evolution towards room-temperature operation began in the early 2000s, driven by the increasing demand for affordable and sustainable energy storage solutions. This transition represented a significant technological breakthrough, as it expanded the potential applications of Na-S batteries to portable electronics, electric vehicles, and residential energy storage systems.
Despite the promising theoretical energy density of approximately 760 Wh/kg, RT-Na-S batteries have faced persistent challenges that have hindered their commercialization. These include poor cycle life, rapid capacity fading, and safety concerns related to sodium metal anodes and sulfur cathodes.
The current technological landscape shows accelerating research interest in RT-Na-S batteries, with annual publications increasing by over 300% in the past five years. This surge reflects the growing recognition of sodium-based technologies as critical to addressing the limitations of lithium-ion batteries, particularly regarding resource availability and cost.
The primary technical objectives for RT-Na-S battery development include achieving stable cycling performance exceeding 1000 cycles, improving Coulombic efficiency to above 99%, and developing effective strategies to mitigate the shuttle effect of polysulfides. Additionally, enhancing the rate capability to support fast charging applications represents another crucial goal.
Market projections indicate that successful commercialization of RT-Na-S batteries could reduce energy storage costs by up to 70% compared to current lithium-ion technologies, potentially revolutionizing grid-scale storage economics. This cost advantage stems from the abundant nature of sodium resources, which are approximately 1000 times more plentiful in the Earth's crust than lithium.
The establishment of standardized protocols and key performance indicators (KPIs) for RT-Na-S batteries has become increasingly urgent as research activities diversify. These standards would facilitate meaningful comparisons between different research approaches, accelerate technology transfer from laboratory to industry, and provide clear benchmarks for measuring progress toward commercialization goals.
The evolution towards room-temperature operation began in the early 2000s, driven by the increasing demand for affordable and sustainable energy storage solutions. This transition represented a significant technological breakthrough, as it expanded the potential applications of Na-S batteries to portable electronics, electric vehicles, and residential energy storage systems.
Despite the promising theoretical energy density of approximately 760 Wh/kg, RT-Na-S batteries have faced persistent challenges that have hindered their commercialization. These include poor cycle life, rapid capacity fading, and safety concerns related to sodium metal anodes and sulfur cathodes.
The current technological landscape shows accelerating research interest in RT-Na-S batteries, with annual publications increasing by over 300% in the past five years. This surge reflects the growing recognition of sodium-based technologies as critical to addressing the limitations of lithium-ion batteries, particularly regarding resource availability and cost.
The primary technical objectives for RT-Na-S battery development include achieving stable cycling performance exceeding 1000 cycles, improving Coulombic efficiency to above 99%, and developing effective strategies to mitigate the shuttle effect of polysulfides. Additionally, enhancing the rate capability to support fast charging applications represents another crucial goal.
Market projections indicate that successful commercialization of RT-Na-S batteries could reduce energy storage costs by up to 70% compared to current lithium-ion technologies, potentially revolutionizing grid-scale storage economics. This cost advantage stems from the abundant nature of sodium resources, which are approximately 1000 times more plentiful in the Earth's crust than lithium.
The establishment of standardized protocols and key performance indicators (KPIs) for RT-Na-S batteries has become increasingly urgent as research activities diversify. These standards would facilitate meaningful comparisons between different research approaches, accelerate technology transfer from laboratory to industry, and provide clear benchmarks for measuring progress toward commercialization goals.
Market Analysis for Room-Temperature Sodium-Sulfur Energy Storage
The global energy storage market is witnessing significant growth, with projections indicating a compound annual growth rate (CAGR) of 20-25% through 2030. Within this expanding landscape, room-temperature sodium-sulfur (RT-Na-S) batteries are emerging as a promising technology that addresses several critical market needs. The primary market drivers for RT-Na-S batteries include the increasing demand for grid-scale energy storage solutions, the push for renewable energy integration, and concerns about the sustainability and cost of lithium-ion technology.
The energy storage market can be segmented into utility-scale, commercial and industrial, and residential applications. RT-Na-S batteries show particular promise in the utility-scale segment, where their potential for long-duration storage aligns with grid stabilization requirements. The technology's ability to operate efficiently at ambient temperatures represents a significant market advantage over traditional high-temperature sodium-sulfur batteries, which require energy-intensive heating systems.
From a geographical perspective, regions with aggressive renewable energy targets and substantial investments in grid modernization present the most immediate market opportunities. These include the European Union, which aims to achieve carbon neutrality by 2050; China, which continues to expand its renewable energy capacity; and the United States, particularly following recent legislative support for clean energy technologies.
Cost considerations remain paramount in the energy storage market. Current lithium-ion battery costs have decreased substantially over the past decade, setting a competitive benchmark for alternative technologies. RT-Na-S batteries offer potential cost advantages due to the abundance and low cost of sodium and sulfur raw materials. Market analysis suggests that if manufacturing scales up, RT-Na-S batteries could achieve costs below $100/kWh, making them economically viable for widespread deployment.
Market acceptance will depend on demonstrated performance metrics. Energy density, cycle life, and safety characteristics will determine which applications RT-Na-S batteries can effectively serve. Current market requirements for grid storage applications typically demand energy densities of 100-150 Wh/kg, cycle life exceeding 2,000 cycles, and calendar life of 10+ years.
Competition in the energy storage market remains intense, with lithium-ion technology currently dominating. However, supply chain vulnerabilities and raw material constraints for lithium-ion batteries create market opportunities for alternative technologies like RT-Na-S. Other competing technologies include flow batteries, compressed air energy storage, and thermal storage solutions, each with distinct advantages in specific applications.
The regulatory landscape significantly impacts market development. Policies supporting renewable energy integration, carbon reduction targets, and energy security initiatives all create favorable conditions for advanced energy storage technologies like RT-Na-S batteries. Additionally, increasing emphasis on sustainable and ethical supply chains may provide RT-Na-S batteries with a market advantage over technologies dependent on critical minerals from geopolitically sensitive regions.
The energy storage market can be segmented into utility-scale, commercial and industrial, and residential applications. RT-Na-S batteries show particular promise in the utility-scale segment, where their potential for long-duration storage aligns with grid stabilization requirements. The technology's ability to operate efficiently at ambient temperatures represents a significant market advantage over traditional high-temperature sodium-sulfur batteries, which require energy-intensive heating systems.
From a geographical perspective, regions with aggressive renewable energy targets and substantial investments in grid modernization present the most immediate market opportunities. These include the European Union, which aims to achieve carbon neutrality by 2050; China, which continues to expand its renewable energy capacity; and the United States, particularly following recent legislative support for clean energy technologies.
Cost considerations remain paramount in the energy storage market. Current lithium-ion battery costs have decreased substantially over the past decade, setting a competitive benchmark for alternative technologies. RT-Na-S batteries offer potential cost advantages due to the abundance and low cost of sodium and sulfur raw materials. Market analysis suggests that if manufacturing scales up, RT-Na-S batteries could achieve costs below $100/kWh, making them economically viable for widespread deployment.
Market acceptance will depend on demonstrated performance metrics. Energy density, cycle life, and safety characteristics will determine which applications RT-Na-S batteries can effectively serve. Current market requirements for grid storage applications typically demand energy densities of 100-150 Wh/kg, cycle life exceeding 2,000 cycles, and calendar life of 10+ years.
Competition in the energy storage market remains intense, with lithium-ion technology currently dominating. However, supply chain vulnerabilities and raw material constraints for lithium-ion batteries create market opportunities for alternative technologies like RT-Na-S. Other competing technologies include flow batteries, compressed air energy storage, and thermal storage solutions, each with distinct advantages in specific applications.
The regulatory landscape significantly impacts market development. Policies supporting renewable energy integration, carbon reduction targets, and energy security initiatives all create favorable conditions for advanced energy storage technologies like RT-Na-S batteries. Additionally, increasing emphasis on sustainable and ethical supply chains may provide RT-Na-S batteries with a market advantage over technologies dependent on critical minerals from geopolitically sensitive regions.
Technical Challenges in RT-Na-S Battery Development
Room-temperature sodium-sulfur (RT-Na-S) batteries face significant technical challenges that have hindered their widespread commercialization despite their promising theoretical energy density of 1274 Wh/kg. The primary obstacle lies in the shuttle effect of polysulfides, where soluble sodium polysulfide intermediates dissolve in the electrolyte during cycling, causing active material loss, capacity fading, and reduced coulombic efficiency.
Another critical challenge is the insulating nature of sulfur and its discharge product Na2S, resulting in poor electronic conductivity. This limitation necessitates the incorporation of conductive additives, which reduces the overall energy density of the battery system. The volume expansion during the sulfur to Na2S conversion (approximately 170%) further complicates electrode design and stability.
Sodium metal anodes present their own set of challenges, including dendrite formation during cycling, which can lead to internal short circuits and safety hazards. The high reactivity of sodium metal with conventional electrolytes creates unstable solid electrolyte interphase (SEI) layers, contributing to continuous electrolyte consumption and capacity degradation over extended cycling.
Electrolyte optimization remains particularly challenging for RT-Na-S batteries. The electrolyte must simultaneously suppress the shuttle effect, facilitate sodium-ion transport, maintain stability against both sodium metal and sulfur species, and operate efficiently at room temperature. Current electrolyte formulations struggle to meet all these requirements simultaneously.
The development of suitable separators that can effectively block polysulfide migration while maintaining high ionic conductivity presents another technical hurdle. Conventional polyolefin separators are insufficient for preventing the shuttle effect in RT-Na-S batteries.
Interface engineering between electrodes and electrolytes requires significant improvement to enhance reaction kinetics and cycling stability. Poor interfacial contact and high interfacial resistance limit the rate capability and practical energy density of these battery systems.
Scale-up and manufacturing processes for RT-Na-S batteries introduce additional challenges related to material consistency, electrode fabrication, and cell assembly under controlled environments due to the air-sensitivity of sodium metal. The lack of standardized testing protocols and key performance indicators (KPIs) further complicates the assessment and comparison of different RT-Na-S battery technologies across research groups and manufacturers.
Addressing these multifaceted challenges requires interdisciplinary approaches combining materials science, electrochemistry, and engineering innovations to develop practical RT-Na-S batteries that can fulfill their theoretical potential as cost-effective, high-energy-density energy storage solutions.
Another critical challenge is the insulating nature of sulfur and its discharge product Na2S, resulting in poor electronic conductivity. This limitation necessitates the incorporation of conductive additives, which reduces the overall energy density of the battery system. The volume expansion during the sulfur to Na2S conversion (approximately 170%) further complicates electrode design and stability.
Sodium metal anodes present their own set of challenges, including dendrite formation during cycling, which can lead to internal short circuits and safety hazards. The high reactivity of sodium metal with conventional electrolytes creates unstable solid electrolyte interphase (SEI) layers, contributing to continuous electrolyte consumption and capacity degradation over extended cycling.
Electrolyte optimization remains particularly challenging for RT-Na-S batteries. The electrolyte must simultaneously suppress the shuttle effect, facilitate sodium-ion transport, maintain stability against both sodium metal and sulfur species, and operate efficiently at room temperature. Current electrolyte formulations struggle to meet all these requirements simultaneously.
The development of suitable separators that can effectively block polysulfide migration while maintaining high ionic conductivity presents another technical hurdle. Conventional polyolefin separators are insufficient for preventing the shuttle effect in RT-Na-S batteries.
Interface engineering between electrodes and electrolytes requires significant improvement to enhance reaction kinetics and cycling stability. Poor interfacial contact and high interfacial resistance limit the rate capability and practical energy density of these battery systems.
Scale-up and manufacturing processes for RT-Na-S batteries introduce additional challenges related to material consistency, electrode fabrication, and cell assembly under controlled environments due to the air-sensitivity of sodium metal. The lack of standardized testing protocols and key performance indicators (KPIs) further complicates the assessment and comparison of different RT-Na-S battery technologies across research groups and manufacturers.
Addressing these multifaceted challenges requires interdisciplinary approaches combining materials science, electrochemistry, and engineering innovations to develop practical RT-Na-S batteries that can fulfill their theoretical potential as cost-effective, high-energy-density energy storage solutions.
Current Standardization Protocols for RT-Na-S Batteries
01 Electrolyte compositions for room-temperature sodium-sulfur batteries
Various electrolyte compositions have been developed to enable sodium-sulfur batteries to operate at room temperature. These include solid polymer electrolytes, gel polymer electrolytes, and ionic liquid-based electrolytes that provide sufficient ionic conductivity while preventing polysulfide shuttling. The electrolyte compositions often incorporate additives to enhance stability and performance, allowing the batteries to function effectively without the high temperatures traditionally required for sodium-sulfur systems.- Electrolyte compositions for room-temperature sodium-sulfur batteries: Various electrolyte compositions have been developed to enable sodium-sulfur batteries to operate at room temperature. These include polymer electrolytes, solid-state electrolytes, and liquid electrolytes with specific additives that enhance ionic conductivity while preventing polysulfide shuttling. The electrolyte composition is critical for achieving stable cycling performance and high energy density in room-temperature sodium-sulfur batteries.
- Electrode materials and structures for enhanced performance: Advanced electrode materials and structures are essential for room-temperature sodium-sulfur batteries. These include sulfur hosts with high surface area, sodium anodes with protective layers, and carbon-based materials for improved conductivity. The electrode design affects key performance indicators such as capacity retention, cycling stability, and rate capability, which are standard metrics for evaluating battery performance.
- Testing protocols and performance evaluation standards: Standardized testing protocols have been established for evaluating room-temperature sodium-sulfur batteries. These include cycling tests at various current densities, temperature performance tests, and long-term stability assessments. Key performance indicators (KPIs) typically measured include specific capacity, Coulombic efficiency, energy density, power density, and cycle life. These standardized protocols enable consistent comparison between different battery designs.
- Safety mechanisms and thermal management: Safety is a critical aspect of room-temperature sodium-sulfur batteries, requiring specific protocols for testing and evaluation. Various safety mechanisms have been developed, including pressure relief valves, thermal management systems, and separator designs that prevent short circuits. Standard safety tests include thermal stability tests, overcharge/overdischarge tests, and mechanical abuse tests to ensure the batteries meet safety requirements for commercial applications.
- Cell design and manufacturing processes: The cell design and manufacturing processes significantly impact the performance of room-temperature sodium-sulfur batteries. Standardized cell designs include pouch cells, cylindrical cells, and prismatic cells, each with specific advantages. Manufacturing protocols cover electrode preparation, cell assembly, electrolyte filling, and sealing processes. Quality control measures during manufacturing ensure consistency in performance metrics and are essential for scaling up production for commercial applications.
02 Electrode materials and structures for enhanced performance
Advanced electrode materials and structures are critical for room-temperature sodium-sulfur batteries. These include sulfur cathodes with carbon hosts to improve conductivity and contain polysulfides, sodium anodes with protective layers to prevent dendrite formation, and novel composite electrodes that enhance reaction kinetics. The electrode design focuses on maximizing active material utilization while maintaining structural stability during cycling, which directly impacts key performance indicators such as capacity and cycle life.Expand Specific Solutions03 Testing protocols and performance evaluation standards
Standardized testing protocols have been established to evaluate room-temperature sodium-sulfur batteries consistently. These protocols specify testing conditions including charge-discharge rates, temperature ranges, and cycle numbers. Key performance indicators (KPIs) commonly measured include energy density, power density, coulombic efficiency, cycle life, and self-discharge rate. These standardized methods enable objective comparison between different battery designs and technologies, facilitating research advancement and commercialization efforts.Expand Specific Solutions04 Safety mechanisms and thermal management systems
Safety is a critical concern for room-temperature sodium-sulfur batteries due to the reactivity of sodium and sulfur. Various safety mechanisms have been developed, including pressure relief designs, thermal fuses, and battery management systems that monitor temperature and voltage. Thermal management systems help prevent thermal runaway and maintain optimal operating conditions. These safety features are essential for commercial viability and are evaluated through specific testing protocols such as nail penetration tests, overcharge tests, and thermal stability assessments.Expand Specific Solutions05 Cell design and manufacturing processes
Innovative cell designs and manufacturing processes have been developed specifically for room-temperature sodium-sulfur batteries. These include novel cell configurations such as pouch, cylindrical, and prismatic formats adapted for the unique chemistry requirements. Manufacturing processes focus on electrode preparation techniques, electrolyte filling methods, and sealing procedures that ensure consistent quality and performance. Advanced quality control measures during production help achieve the reliability needed for commercial applications, with specific KPIs tracking manufacturing consistency and defect rates.Expand Specific Solutions
Leading Organizations in RT-Na-S Battery Research
The room-temperature sodium-sulfur battery market is currently in an early growth phase, characterized by increasing research activity but limited commercial deployment. The global market size is projected to expand significantly as this technology offers a cost-effective alternative to lithium-ion batteries for grid-scale energy storage. Technical maturity varies across key players: NGK Insulators leads with established high-temperature sodium-sulfur technology but faces challenges in room-temperature adaptations; academic institutions like Drexel University, Cornell, and University of Maryland are advancing fundamental research; while companies including Toyota Motor Corp., SK Innovation, and Honeycomb Battery are developing proprietary solutions to overcome stability and cycle life limitations. Chinese entities such as Shanghai Electric and Hefei Guoxuan are rapidly accelerating R&D efforts, positioning themselves as emerging competitors in this promising energy storage segment.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered room-temperature sodium-sulfur (RT-Na/S) battery technology with their proprietary ceramic electrolyte system. Their approach focuses on developing NASICON (Na Super Ionic CONductor) solid electrolytes that enable stable operation at ambient temperatures. NGK's protocol involves a multi-layer cell design with a specialized interface management system that prevents polysulfide shuttling - a common failure mechanism in Na/S batteries. Their standardized testing protocol includes 1000+ cycle endurance tests at various discharge rates (0.1C-2C), temperature stability verification (0-40°C), and safety validation under mechanical stress conditions. NGK has established specific KPIs including energy density targets of 300-350 Wh/kg, cycle life exceeding 1000 cycles with less than 20% capacity fade, and self-discharge rates below 3% per month. Their manufacturing protocol incorporates precise control of sodium anode interfaces using proprietary coating technologies to prevent dendrite formation.
Strengths: Industry-leading expertise in ceramic electrolytes with decades of experience in high-temperature Na/S systems that transfers to room-temperature applications. Established manufacturing infrastructure and quality control systems. Weaknesses: Their solid electrolyte approach may face challenges with interfacial resistance at room temperature, potentially limiting power density compared to some liquid electrolyte systems.
SK Innovation Co., Ltd.
Technical Solution: SK Innovation has developed a comprehensive approach to room-temperature sodium-sulfur batteries focusing on nanostructured carbon-sulfur composite cathodes. Their protocol centers on a dual-confinement strategy where sulfur is first embedded in mesoporous carbon matrices (pore size 2-5nm) and then encapsulated in a sodium-ion conductive polymer layer. This hierarchical structure effectively suppresses polysulfide dissolution while maintaining high ionic conductivity. SK Innovation's standardized testing protocol includes accelerated aging tests at elevated temperatures (40-60°C), rate capability assessments from 0.1C to 5C, and long-term cycling under various depth-of-discharge conditions. Their established KPIs include initial capacity targets of 1200-1500 mAh/g of sulfur, capacity retention above 80% after 500 cycles, and Coulombic efficiency exceeding 99.5%. SK Innovation has also developed specialized electrolyte formulations containing sodium salt complexes and additives that form stable solid electrolyte interphase layers, critical for preventing continuous electrolyte decomposition during cycling.
Strengths: Strong expertise in carbon-sulfur composite materials and electrolyte engineering from their lithium-sulfur battery research that translates well to sodium-sulfur systems. Established battery manufacturing capabilities and supply chain integration. Weaknesses: Their approach relies heavily on expensive carbon host materials and specialized polymer coatings that may increase production costs and limit mass-market adoption.
Key Performance Indicators and Testing Methodologies
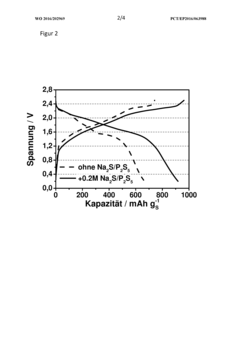
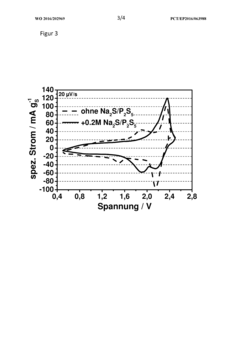
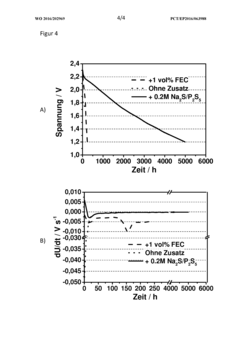
Sodium-sulfur battery, method for operating same, and use of phosphorus polysulfide as electrolyte additive in sodium-sulfur batteries
PatentWO2016202969A1
Innovation
- A sodium-sulfur battery design using a carbon-sulfur composite cathode, an organic solvent-based electrolyte with a conductive salt and phosphorus polysulfide as an additive to suppress the polysulfide shuttle, allowing operation at room temperature with enhanced energy efficiency and discharge capacity.
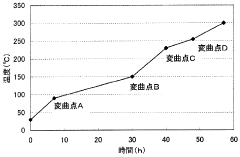
Temperature increasing method for sodium-sulfur battery
PatentWO2010082528A1
Innovation
- Implementing a multi-step temperature gradient method with three or more heating rates, including inflection points at 90°C ± 5°C and 150°C ± 5°C, with specific temperature gradients ranging from 2°C/h to 7°C/h in different intervals to ensure efficient heating without compromising battery quality.
Safety and Environmental Considerations for Na-S Battery Systems
Safety considerations for room-temperature sodium-sulfur (RT Na-S) batteries are paramount due to the reactive nature of both sodium and sulfur components. Unlike traditional high-temperature Na-S batteries operating at 300-350°C, RT Na-S systems present different safety challenges that require standardized protocols. The primary concern involves sodium's high reactivity with moisture and oxygen, potentially leading to fire hazards if cell integrity is compromised. Additionally, polysulfide shuttle effects can create internal short circuits, thermal runaway risks, and capacity degradation over time.
Effective safety protocols must include robust encapsulation techniques to prevent sodium exposure to air and moisture. Standardized testing should evaluate cell behavior under various abuse conditions including overcharging, over-discharging, mechanical impact, and thermal stress. Current industry standards like IEC 62660 and UL 1642 require adaptation to address the specific characteristics of RT Na-S chemistry.
Environmental considerations for Na-S battery systems present both advantages and challenges. The abundant nature of sodium and sulfur resources offers significant sustainability benefits compared to lithium-ion technologies that rely on critical raw materials. However, end-of-life management requires careful attention due to the reactive nature of battery components. Recycling protocols must address safe extraction of sodium and sulfur while preventing environmental contamination.
Life cycle assessment (LCA) studies indicate that RT Na-S batteries potentially have lower environmental impact than lithium-ion alternatives, particularly regarding resource depletion and greenhouse gas emissions during manufacturing. However, these advantages depend on establishing efficient recycling infrastructure and standardized decommissioning procedures.
Key performance indicators for environmental assessment should include resource efficiency metrics, toxicity potential, energy return on investment, and recyclability rate. Standardized protocols must establish threshold values for leachable substances and define safe disposal methods for non-recyclable components. The development of closed-loop recycling systems represents a critical area for industry advancement.
Regulatory frameworks governing RT Na-S batteries remain underdeveloped compared to lithium-ion technologies. Future standards should incorporate both safety and environmental considerations throughout the product lifecycle, from material sourcing to end-of-life management. This integrated approach will ensure that RT Na-S batteries fulfill their potential as a sustainable energy storage solution while minimizing risks to human health and environmental integrity.
Effective safety protocols must include robust encapsulation techniques to prevent sodium exposure to air and moisture. Standardized testing should evaluate cell behavior under various abuse conditions including overcharging, over-discharging, mechanical impact, and thermal stress. Current industry standards like IEC 62660 and UL 1642 require adaptation to address the specific characteristics of RT Na-S chemistry.
Environmental considerations for Na-S battery systems present both advantages and challenges. The abundant nature of sodium and sulfur resources offers significant sustainability benefits compared to lithium-ion technologies that rely on critical raw materials. However, end-of-life management requires careful attention due to the reactive nature of battery components. Recycling protocols must address safe extraction of sodium and sulfur while preventing environmental contamination.
Life cycle assessment (LCA) studies indicate that RT Na-S batteries potentially have lower environmental impact than lithium-ion alternatives, particularly regarding resource depletion and greenhouse gas emissions during manufacturing. However, these advantages depend on establishing efficient recycling infrastructure and standardized decommissioning procedures.
Key performance indicators for environmental assessment should include resource efficiency metrics, toxicity potential, energy return on investment, and recyclability rate. Standardized protocols must establish threshold values for leachable substances and define safe disposal methods for non-recyclable components. The development of closed-loop recycling systems represents a critical area for industry advancement.
Regulatory frameworks governing RT Na-S batteries remain underdeveloped compared to lithium-ion technologies. Future standards should incorporate both safety and environmental considerations throughout the product lifecycle, from material sourcing to end-of-life management. This integrated approach will ensure that RT Na-S batteries fulfill their potential as a sustainable energy storage solution while minimizing risks to human health and environmental integrity.
Comparative Analysis with Other Emerging Battery Technologies
Room-temperature sodium-sulfur (RT Na-S) batteries represent a promising energy storage solution that must be evaluated against other emerging battery technologies to understand their competitive position. When comparing RT Na-S batteries with alternatives such as lithium-sulfur, sodium-ion, potassium-ion, and solid-state batteries, several key performance metrics reveal distinctive advantages and limitations.
Energy density comparisons show that RT Na-S batteries (theoretical: 760 Wh/kg) offer higher energy density than conventional sodium-ion batteries (300-350 Wh/kg) but remain below lithium-sulfur systems (2600 Wh/kg theoretical). However, RT Na-S batteries demonstrate superior practical energy density realization compared to Li-S systems, which suffer from significant theoretical-to-practical performance gaps.
Cost analysis reveals RT Na-S batteries' strongest competitive advantage. Using abundant, low-cost materials (sodium and sulfur), they present manufacturing costs potentially 30-40% lower than lithium-based alternatives. This economic advantage becomes increasingly significant as energy storage deployment scales globally.
Safety performance positions RT Na-S batteries favorably compared to lithium-based technologies. The absence of dendrite formation issues that plague lithium metal anodes and reduced thermal runaway risks represent critical advantages, particularly for grid-scale and residential applications where safety concerns are paramount.
Cycle life remains a challenge for RT Na-S batteries, typically achieving 500-1000 cycles before significant capacity degradation, compared to 2000+ cycles for commercial sodium-ion and lithium-ion technologies. This limitation particularly affects their competitiveness in applications requiring frequent cycling.
Environmental impact assessment strongly favors RT Na-S technology. The abundant, non-toxic nature of sodium and the potential for closed-loop sulfur recycling create a substantially lower ecological footprint compared to lithium and cobalt-dependent technologies, which face resource scarcity and extraction-related environmental concerns.
Manufacturing scalability analysis indicates RT Na-S batteries can leverage existing production infrastructure with moderate modifications, unlike solid-state technologies requiring entirely new manufacturing paradigms. This adaptability potentially enables faster market penetration and cost reduction through economies of scale.
Application-specific performance evaluation reveals RT Na-S batteries are particularly well-positioned for stationary energy storage applications where energy density requirements are less stringent than in transportation, and their cost advantages become decisive competitive factors.
Energy density comparisons show that RT Na-S batteries (theoretical: 760 Wh/kg) offer higher energy density than conventional sodium-ion batteries (300-350 Wh/kg) but remain below lithium-sulfur systems (2600 Wh/kg theoretical). However, RT Na-S batteries demonstrate superior practical energy density realization compared to Li-S systems, which suffer from significant theoretical-to-practical performance gaps.
Cost analysis reveals RT Na-S batteries' strongest competitive advantage. Using abundant, low-cost materials (sodium and sulfur), they present manufacturing costs potentially 30-40% lower than lithium-based alternatives. This economic advantage becomes increasingly significant as energy storage deployment scales globally.
Safety performance positions RT Na-S batteries favorably compared to lithium-based technologies. The absence of dendrite formation issues that plague lithium metal anodes and reduced thermal runaway risks represent critical advantages, particularly for grid-scale and residential applications where safety concerns are paramount.
Cycle life remains a challenge for RT Na-S batteries, typically achieving 500-1000 cycles before significant capacity degradation, compared to 2000+ cycles for commercial sodium-ion and lithium-ion technologies. This limitation particularly affects their competitiveness in applications requiring frequent cycling.
Environmental impact assessment strongly favors RT Na-S technology. The abundant, non-toxic nature of sodium and the potential for closed-loop sulfur recycling create a substantially lower ecological footprint compared to lithium and cobalt-dependent technologies, which face resource scarcity and extraction-related environmental concerns.
Manufacturing scalability analysis indicates RT Na-S batteries can leverage existing production infrastructure with moderate modifications, unlike solid-state technologies requiring entirely new manufacturing paradigms. This adaptability potentially enables faster market penetration and cost reduction through economies of scale.
Application-specific performance evaluation reveals RT Na-S batteries are particularly well-positioned for stationary energy storage applications where energy density requirements are less stringent than in transportation, and their cost advantages become decisive competitive factors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!