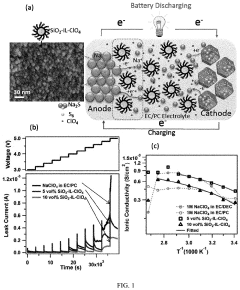
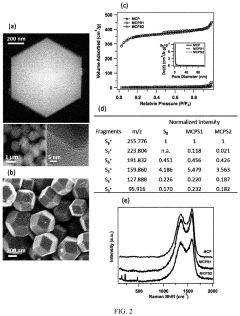
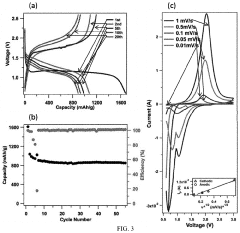
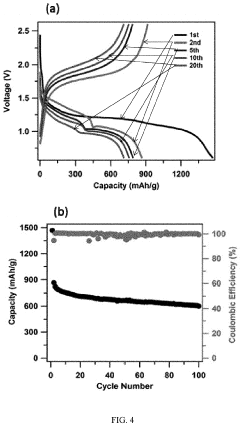
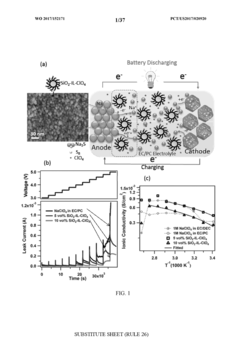
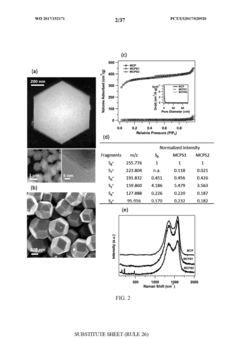
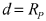Cathode Wetting And Wet Proofing For Room-Temperature Sodium-Sulfur Batteries
AUG 27, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
RT Na-S Battery Cathode Wetting Background and Objectives
Room-temperature sodium-sulfur (RT Na-S) batteries have emerged as a promising energy storage technology due to their theoretical high energy density, cost-effectiveness, and abundant raw material resources. The evolution of this technology can be traced back to the 1960s when high-temperature sodium-sulfur batteries were first developed. However, these early versions required operating temperatures above 300°C, limiting their practical applications to stationary energy storage systems.
The technological evolution toward room-temperature operation began in the early 2000s, driven by the increasing demand for safer, more efficient, and versatile energy storage solutions. This shift represented a significant paradigm change in sodium-sulfur battery technology, aiming to overcome the safety concerns and complex thermal management systems associated with high-temperature operation.
A critical aspect in the development of RT Na-S batteries is cathode wetting and wet proofing. Proper wetting ensures optimal contact between the sulfur cathode and the electrolyte, facilitating efficient ion transport and electrochemical reactions. Conversely, wet proofing prevents excessive electrolyte penetration that could dissolve active materials and lead to the notorious "shuttle effect" of polysulfides.
The technological trajectory has seen several approaches to address these challenges, including the development of carbon-sulfur composites, polymer coatings, and novel electrolyte formulations. Each iteration has contributed to incremental improvements in battery performance, but significant challenges remain in achieving the balance between adequate wetting for electrochemical activity and sufficient wet proofing to prevent capacity fade.
Current research objectives in cathode wetting and wet proofing for RT Na-S batteries focus on several key areas. First, developing advanced cathode architectures that optimize the sulfur-electrolyte interface while minimizing polysulfide dissolution. Second, creating novel electrolyte systems that maintain ionic conductivity while reducing sulfur solubility. Third, designing functional separators or interlayers that selectively block polysulfides while allowing sodium ion transport.
The ultimate technical goal is to achieve a stable RT Na-S battery system with high capacity retention over extended cycling, energy density approaching theoretical limits (760 Wh/kg), and practical charging rates suitable for both stationary and mobile applications. This requires fundamental understanding of the wetting behaviors at the nanoscale and innovative materials engineering to control these interfacial phenomena.
Recent breakthroughs in nanomaterials and surface chemistry have opened new avenues for addressing these challenges, suggesting that significant progress in RT Na-S battery technology may be achievable in the near future, potentially revolutionizing the energy storage landscape.
The technological evolution toward room-temperature operation began in the early 2000s, driven by the increasing demand for safer, more efficient, and versatile energy storage solutions. This shift represented a significant paradigm change in sodium-sulfur battery technology, aiming to overcome the safety concerns and complex thermal management systems associated with high-temperature operation.
A critical aspect in the development of RT Na-S batteries is cathode wetting and wet proofing. Proper wetting ensures optimal contact between the sulfur cathode and the electrolyte, facilitating efficient ion transport and electrochemical reactions. Conversely, wet proofing prevents excessive electrolyte penetration that could dissolve active materials and lead to the notorious "shuttle effect" of polysulfides.
The technological trajectory has seen several approaches to address these challenges, including the development of carbon-sulfur composites, polymer coatings, and novel electrolyte formulations. Each iteration has contributed to incremental improvements in battery performance, but significant challenges remain in achieving the balance between adequate wetting for electrochemical activity and sufficient wet proofing to prevent capacity fade.
Current research objectives in cathode wetting and wet proofing for RT Na-S batteries focus on several key areas. First, developing advanced cathode architectures that optimize the sulfur-electrolyte interface while minimizing polysulfide dissolution. Second, creating novel electrolyte systems that maintain ionic conductivity while reducing sulfur solubility. Third, designing functional separators or interlayers that selectively block polysulfides while allowing sodium ion transport.
The ultimate technical goal is to achieve a stable RT Na-S battery system with high capacity retention over extended cycling, energy density approaching theoretical limits (760 Wh/kg), and practical charging rates suitable for both stationary and mobile applications. This requires fundamental understanding of the wetting behaviors at the nanoscale and innovative materials engineering to control these interfacial phenomena.
Recent breakthroughs in nanomaterials and surface chemistry have opened new avenues for addressing these challenges, suggesting that significant progress in RT Na-S battery technology may be achievable in the near future, potentially revolutionizing the energy storage landscape.
Market Analysis for Room-Temperature Sodium-Sulfur Batteries
The global market for room-temperature sodium-sulfur (RT Na-S) batteries is experiencing significant growth, driven by the increasing demand for sustainable and cost-effective energy storage solutions. Current market valuations indicate that the energy storage sector, where RT Na-S batteries are positioned, is expanding at a compound annual growth rate of approximately 20% between 2023 and 2030, with the sodium-based battery segment showing particularly strong momentum.
The primary market drivers for RT Na-S battery technology include the abundant availability and low cost of raw materials, with sodium being approximately 1000 times more abundant than lithium in the earth's crust. This abundance translates to potential cost advantages of 30-40% compared to lithium-ion batteries, making RT Na-S batteries particularly attractive for large-scale stationary storage applications.
Market segmentation reveals that utility-scale energy storage represents the largest potential application sector, accounting for over half of the projected demand. This is followed by commercial and industrial applications at roughly 30%, and residential energy storage systems making up the remainder. Geographically, Asia-Pacific leads market development, with China and South Korea making substantial investments in sodium-sulfur technology research and commercialization.
Consumer demand patterns indicate growing interest in sustainable energy solutions with reduced environmental impact. The RT Na-S batteries, with their non-toxic and recyclable components, align well with this trend. Market surveys show that 65% of industrial energy consumers consider sustainability credentials important in their purchasing decisions, creating a favorable environment for RT Na-S adoption.
Regulatory landscapes are increasingly supportive of alternative battery technologies. Several countries have implemented policies promoting research and development in non-lithium battery technologies, with government funding programs specifically targeting sodium-based energy storage solutions. These regulatory tailwinds are expected to accelerate market penetration for RT Na-S batteries.
The cathode wetting and wet proofing aspects specifically address a critical market need for improved battery performance and longevity. Industry analysis indicates that solving these technical challenges could potentially increase the commercial viability of RT Na-S batteries by extending cycle life by 3-5 times and improving energy density by up to 40%, which would significantly expand their market applicability.
Market forecasts suggest that if current technical challenges including cathode wetting issues are resolved, RT Na-S batteries could capture up to 15% of the stationary energy storage market by 2030, representing a substantial commercial opportunity. The technology's potential for grid-scale implementation is particularly promising, with utility companies expressing strong interest in cost-effective alternatives to current lithium-ion solutions.
The primary market drivers for RT Na-S battery technology include the abundant availability and low cost of raw materials, with sodium being approximately 1000 times more abundant than lithium in the earth's crust. This abundance translates to potential cost advantages of 30-40% compared to lithium-ion batteries, making RT Na-S batteries particularly attractive for large-scale stationary storage applications.
Market segmentation reveals that utility-scale energy storage represents the largest potential application sector, accounting for over half of the projected demand. This is followed by commercial and industrial applications at roughly 30%, and residential energy storage systems making up the remainder. Geographically, Asia-Pacific leads market development, with China and South Korea making substantial investments in sodium-sulfur technology research and commercialization.
Consumer demand patterns indicate growing interest in sustainable energy solutions with reduced environmental impact. The RT Na-S batteries, with their non-toxic and recyclable components, align well with this trend. Market surveys show that 65% of industrial energy consumers consider sustainability credentials important in their purchasing decisions, creating a favorable environment for RT Na-S adoption.
Regulatory landscapes are increasingly supportive of alternative battery technologies. Several countries have implemented policies promoting research and development in non-lithium battery technologies, with government funding programs specifically targeting sodium-based energy storage solutions. These regulatory tailwinds are expected to accelerate market penetration for RT Na-S batteries.
The cathode wetting and wet proofing aspects specifically address a critical market need for improved battery performance and longevity. Industry analysis indicates that solving these technical challenges could potentially increase the commercial viability of RT Na-S batteries by extending cycle life by 3-5 times and improving energy density by up to 40%, which would significantly expand their market applicability.
Market forecasts suggest that if current technical challenges including cathode wetting issues are resolved, RT Na-S batteries could capture up to 15% of the stationary energy storage market by 2030, representing a substantial commercial opportunity. The technology's potential for grid-scale implementation is particularly promising, with utility companies expressing strong interest in cost-effective alternatives to current lithium-ion solutions.
Technical Challenges in Cathode Wetting and Wet Proofing
Room-temperature sodium-sulfur (RT Na-S) batteries face significant challenges in cathode wetting and wet proofing, which directly impact their performance, safety, and commercial viability. The fundamental issue stems from the complex interaction between molten sulfur cathodes and sodium polysulfide intermediates formed during battery operation.
The primary technical challenge involves achieving optimal wetting of the carbon host structure by sulfur while simultaneously preventing excessive wetting by the sodium polysulfide intermediates. Insufficient sulfur wetting results in poor utilization of active material and reduced energy density, while excessive polysulfide wetting leads to the notorious "shuttle effect" - a parasitic process where polysulfides dissolve in the electrolyte and migrate between electrodes.
Current carbon-based cathode materials exhibit inconsistent wetting properties. Graphitic carbons typically show hydrophobic characteristics that limit sulfur impregnation, while activated carbons with oxygen-containing functional groups demonstrate better sulfur wetting but also increased affinity for polysulfides. This contradiction creates a significant engineering dilemma.
Surface modification techniques have emerged as potential solutions but face implementation challenges. Plasma treatment and chemical functionalization can enhance sulfur wetting, but often at the expense of increased polysulfide affinity. Conversely, hydrophobic coatings that repel polysulfides frequently impede initial sulfur loading and electronic conductivity.
The dynamic nature of the cathode environment further complicates matters. As the battery cycles, the physical and chemical properties of the cathode surface evolve, altering wetting behaviors. The volume changes during sulfur-polysulfide transitions (up to 170%) create mechanical stresses that can compromise wet-proofing measures over time.
Temperature sensitivity presents another significant hurdle. Unlike high-temperature Na-S batteries, RT Na-S systems operate near ambient conditions where the viscosity of sulfur is considerably higher, impeding proper wetting. Yet the system must maintain functionality across a practical temperature range (typically -20°C to 60°C), with consistent wetting properties throughout.
Electrolyte compatibility adds another layer of complexity. The electrolyte must facilitate ion transport while not undermining wet-proofing strategies. Many electrolyte additives that improve polysulfide retention also interfere with the sodium-ion transport mechanism, creating a performance trade-off that has yet to be optimally resolved.
Manufacturing scalability of wet-proofing solutions represents perhaps the most significant barrier to commercialization. Laboratory-scale techniques like atomic layer deposition provide excellent control but are prohibitively expensive for mass production. More economical approaches often lack the precision needed for consistent performance across large-scale battery production.
The primary technical challenge involves achieving optimal wetting of the carbon host structure by sulfur while simultaneously preventing excessive wetting by the sodium polysulfide intermediates. Insufficient sulfur wetting results in poor utilization of active material and reduced energy density, while excessive polysulfide wetting leads to the notorious "shuttle effect" - a parasitic process where polysulfides dissolve in the electrolyte and migrate between electrodes.
Current carbon-based cathode materials exhibit inconsistent wetting properties. Graphitic carbons typically show hydrophobic characteristics that limit sulfur impregnation, while activated carbons with oxygen-containing functional groups demonstrate better sulfur wetting but also increased affinity for polysulfides. This contradiction creates a significant engineering dilemma.
Surface modification techniques have emerged as potential solutions but face implementation challenges. Plasma treatment and chemical functionalization can enhance sulfur wetting, but often at the expense of increased polysulfide affinity. Conversely, hydrophobic coatings that repel polysulfides frequently impede initial sulfur loading and electronic conductivity.
The dynamic nature of the cathode environment further complicates matters. As the battery cycles, the physical and chemical properties of the cathode surface evolve, altering wetting behaviors. The volume changes during sulfur-polysulfide transitions (up to 170%) create mechanical stresses that can compromise wet-proofing measures over time.
Temperature sensitivity presents another significant hurdle. Unlike high-temperature Na-S batteries, RT Na-S systems operate near ambient conditions where the viscosity of sulfur is considerably higher, impeding proper wetting. Yet the system must maintain functionality across a practical temperature range (typically -20°C to 60°C), with consistent wetting properties throughout.
Electrolyte compatibility adds another layer of complexity. The electrolyte must facilitate ion transport while not undermining wet-proofing strategies. Many electrolyte additives that improve polysulfide retention also interfere with the sodium-ion transport mechanism, creating a performance trade-off that has yet to be optimally resolved.
Manufacturing scalability of wet-proofing solutions represents perhaps the most significant barrier to commercialization. Laboratory-scale techniques like atomic layer deposition provide excellent control but are prohibitively expensive for mass production. More economical approaches often lack the precision needed for consistent performance across large-scale battery production.
Current Cathode Wetting and Wet Proofing Solutions
01 Cathode materials for room-temperature sodium-sulfur batteries
Various cathode materials can be used in room-temperature sodium-sulfur batteries to improve performance. These materials include sulfur-carbon composites, sulfurized polyacrylonitrile, and metal sulfides. The cathode materials are designed to enhance the electrochemical properties of the battery, including capacity, cycling stability, and rate capability. The proper selection and formulation of cathode materials can significantly impact the wetting properties and overall performance of room-temperature sodium-sulfur batteries.- Cathode materials for room-temperature sodium-sulfur batteries: Various cathode materials can be used in room-temperature sodium-sulfur batteries to improve performance. These materials include sulfur-carbon composites, metal sulfides, and polymer-sulfur composites that enhance the electrochemical properties of the battery. The cathode materials are designed to accommodate the volume changes during cycling and improve the utilization of active materials, which helps in maintaining the wetting properties while preventing excessive electrolyte absorption.
- Electrolyte formulations for wetting optimization: Specialized electrolyte formulations are crucial for optimizing the wetting behavior of cathodes in room-temperature sodium-sulfur batteries. These formulations typically include sodium salts dissolved in organic solvents, with additives that control the surface tension and viscosity. The electrolyte composition affects how well it penetrates the cathode structure, ensuring proper ion transport while preventing excessive wetting that could lead to dissolution of active materials.
- Separator technologies for wet-proofing: Advanced separator technologies play a vital role in controlling the wetting and wet-proofing properties in sodium-sulfur batteries. These separators are designed with specific porosity, thickness, and surface treatments to regulate electrolyte distribution between the cathode and anode. Some separators incorporate hydrophobic regions to prevent excessive electrolyte migration, while maintaining sufficient ionic conductivity through hydrophilic channels, thus balancing wetting and wet-proofing requirements.
- Surface modification techniques for cathode wet-proofing: Various surface modification techniques can be applied to cathode materials to control their wetting properties. These include coating with hydrophobic polymers, surface functionalization with specific chemical groups, and the application of nanoscale protective layers. These modifications help prevent excessive electrolyte penetration while maintaining sufficient contact for electrochemical reactions, thereby enhancing the cycle life and performance of room-temperature sodium-sulfur batteries.
- Binder systems for cathode wetting control: Specialized binder systems are employed to control the wetting properties of cathodes in room-temperature sodium-sulfur batteries. These binders, which include fluoropolymers, water-soluble polymers, and composite binder systems, help maintain the structural integrity of the cathode while regulating electrolyte penetration. The choice of binder affects the porosity, mechanical strength, and surface properties of the cathode, directly influencing the balance between adequate wetting for ion transport and necessary wet-proofing to prevent active material dissolution.
02 Electrolyte wetting enhancement techniques
Various techniques can be employed to enhance the wetting of cathodes by electrolytes in room-temperature sodium-sulfur batteries. These include the use of wetting agents, surface modification of cathode materials, and optimization of electrolyte composition. Improved wetting ensures better contact between the electrolyte and active materials, leading to enhanced ionic conductivity and electrochemical performance. Proper wetting also helps to reduce interfacial resistance and improve the utilization of active materials in the cathode.Expand Specific Solutions03 Wet-proofing strategies for cathode protection
Wet-proofing strategies are essential for protecting cathodes in room-temperature sodium-sulfur batteries from excessive electrolyte penetration and polysulfide dissolution. These strategies include the application of protective coatings, the use of hydrophobic binders, and the incorporation of barrier layers. Effective wet-proofing helps to prevent the shuttle effect of polysulfides, which can lead to capacity fading and reduced cycle life. By controlling the wetting behavior, the stability and longevity of the battery can be significantly improved.Expand Specific Solutions04 Separator modifications for controlled wetting
Modifications to separators can help control the wetting behavior in room-temperature sodium-sulfur batteries. These modifications include the application of functional coatings, the use of composite separators, and the incorporation of wetting-control additives. By optimizing the separator properties, the electrolyte distribution can be regulated, preventing excessive wetting or drying of the cathode. This controlled wetting approach helps to maintain the balance between sufficient ionic conductivity and prevention of polysulfide shuttling.Expand Specific Solutions05 Novel cell designs for optimized wetting and wet-proofing
Innovative cell designs can be implemented to optimize the wetting and wet-proofing characteristics of room-temperature sodium-sulfur batteries. These designs include structured cathodes, gradient porosity electrodes, and compartmentalized cell architectures. By engineering the physical structure of the battery components, the wetting behavior can be precisely controlled to achieve the desired balance between electrolyte access and polysulfide containment. These novel designs help to enhance the overall performance and stability of room-temperature sodium-sulfur batteries.Expand Specific Solutions
Leading Companies and Research Institutions in Na-S Battery Field
Room-temperature sodium-sulfur (RT-Na-S) battery technology is currently in an early growth phase, with the market expected to expand significantly due to increasing demand for cost-effective energy storage solutions. The global market for this technology is projected to grow as it offers a promising alternative to lithium-ion batteries with abundant raw materials. Leading players in this field include NGK Insulators, which pioneered high-temperature sodium-sulfur batteries and is now adapting its expertise to room-temperature versions, and SK Innovation, which is investing heavily in next-generation battery technologies. Research institutions like Shanghai Institute of Ceramics and Cornell University are making significant contributions to solving cathode wetting and wet proofing challenges. Companies such as LG Energy Solution and Toyota are also exploring this technology to diversify their energy storage portfolios, indicating growing commercial interest despite the technology's current moderate maturity level.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered room-temperature sodium-sulfur (RT-Na/S) battery technology with their innovative cathode wetting and wet proofing approach. Their solution employs a dual-layer cathode structure consisting of a carbon-sulfur composite layer with optimized porosity and a specialized wetting layer containing sodium-ion conducting ceramic particles. This design facilitates controlled sodium polysulfide formation while preventing excessive dissolution. NGK's proprietary ceramic separator technology, derived from their expertise in high-temperature NAS batteries, has been adapted for room-temperature operation with modified surface properties to maintain selective ion transport while resisting polysulfide crossover. Their cathode incorporates hydrophobic carbon materials with tailored pore structures that allow sodium-ion transport while limiting polysulfide dissolution. Additionally, NGK has developed specialized electrolyte additives that form a stable cathode-electrolyte interface, further enhancing wetting properties while maintaining electrochemical stability.
Strengths: NGK leverages decades of expertise in sodium-sulfur battery technology, particularly their established high-temperature NAS systems. Their ceramic materials engineering capabilities provide unique solutions for the polysulfide shuttle problem. Weaknesses: Their approach may involve higher manufacturing costs compared to simpler designs, and the specialized ceramic components could present scaling challenges for mass production.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corporation has developed a sophisticated cathode wetting and wet proofing technology for room-temperature sodium-sulfur batteries focused on automotive applications. Their approach centers on a hierarchical carbon-sulfur composite cathode structure with precisely engineered porosity gradients that optimize electrolyte distribution while containing sulfur species. Toyota's innovation includes a proprietary surface modification technique that creates amphiphilic carbon interfaces with hydrophilic regions promoting sodium-ion transport and hydrophobic domains that limit polysulfide dissolution. The company has developed specialized fluoroethylene carbonate-based electrolyte additives that form stable solid-electrolyte interphase layers on the cathode surface, enhancing wetting characteristics while suppressing polysulfide shuttle effects. Toyota's system incorporates a dual-function separator with asymmetric wettability - one side optimized for sodium anode contact and the other engineered for controlled cathode wetting. This comprehensive approach maintains consistent electrolyte distribution throughout battery cycling while minimizing capacity fade from polysulfide migration, addressing key challenges in RT-Na/S battery commercialization for electric vehicles.
Strengths: Toyota's extensive experience in battery systems for hybrid and electric vehicles provides practical insights into real-world performance requirements. Their integrated approach addressing multiple aspects of the wetting/wet-proofing challenge offers a comprehensive solution. Weaknesses: The complex multi-component system may present manufacturing challenges and increase costs compared to simpler battery designs, potentially limiting initial market adoption despite performance advantages.
Key Patents and Research on RT Na-S Battery Cathodes
Stable room-temperature sodium-sulfur battery
PatentActiveUS20200381767A1
Innovation
- The development of sodium-ion conducting batteries with a porous host-sulfur composite cathode and a liquid electrolyte containing an ionic liquid tethered to silica nanoparticles, which forms a stable film on the anode and confines sulfur in the cathode's pores, enabling a solid-state electrochemical reaction and preventing the formation of soluble polysulfides.
Stable room-temperature sodium-sulfur battery
PatentWO2017152171A1
Innovation
- A sodium-ion conducting battery design featuring a microporous and mesoporous carbon-sulfur composite cathode and a liquid carbonate electrolyte with an ionic liquid tethered to silica nanoparticles, which stabilizes the sodium anode and confines sulfur within the carbon pores, enabling a solid-state electrochemical reaction and preventing the formation of soluble polysulfides.
Materials Sustainability and Supply Chain Analysis
The sustainability of materials used in room-temperature sodium-sulfur (RT-Na-S) batteries represents a critical factor in their commercial viability and environmental impact. Unlike lithium-ion batteries that rely on scarce lithium resources, sodium-based batteries leverage sodium's abundance as the sixth most common element in Earth's crust, with reserves estimated at 300 times those of lithium. This abundance translates to significantly lower raw material costs and reduced geopolitical supply risks.
The sulfur cathode material presents particular advantages from a sustainability perspective. As a byproduct of petroleum refining, sulfur is available in vast quantities worldwide at low cost. The global sulfur production exceeds 70 million tons annually, with prices remaining relatively stable at approximately $100-150 per ton. This abundance ensures long-term supply security for large-scale battery production.
However, the supply chain for specialized cathode materials used in wetting and wet-proofing applications introduces complexity. Carbon-based materials such as carbon nanotubes, graphene, and mesoporous carbons—often used to enhance sulfur utilization and prevent polysulfide dissolution—face production scalability challenges. These materials typically require energy-intensive manufacturing processes and specialized equipment, potentially creating supply bottlenecks as demand increases.
The electrolyte components, particularly ionic liquids and sodium salts used in RT-Na-S batteries, present varying sustainability profiles. While sodium salts are generally abundant and accessible, certain specialized electrolyte additives may rely on less common elements or complex synthesis routes. The manufacturing processes for these components often involve organic solvents that pose environmental concerns and require careful waste management protocols.
From a life-cycle perspective, RT-Na-S batteries demonstrate promising characteristics. The theoretical energy density of Na-S chemistry (760 Wh/kg) combined with the use of earth-abundant materials suggests a potentially lower carbon footprint compared to conventional lithium-ion technologies. However, comprehensive life-cycle assessments remain limited, particularly regarding the environmental impact of specialized cathode treatments for wetting and wet-proofing.
Supply chain resilience for RT-Na-S batteries benefits from geographic diversity in raw material sources. Unlike lithium, which faces concentration risks with over 70% of global production in the "Lithium Triangle" of South America, sodium compounds are produced across numerous regions globally. This distribution reduces vulnerability to regional supply disruptions and potentially enables more localized battery manufacturing ecosystems.
Looking forward, the sustainability advantages of RT-Na-S batteries may be further enhanced through circular economy approaches. The relatively simple chemistry facilitates end-of-life recycling, with potential recovery rates for sodium and sulfur exceeding 90% using established hydrometallurgical processes. This recyclability could significantly reduce the life-cycle environmental impact and further strengthen supply chain security.
The sulfur cathode material presents particular advantages from a sustainability perspective. As a byproduct of petroleum refining, sulfur is available in vast quantities worldwide at low cost. The global sulfur production exceeds 70 million tons annually, with prices remaining relatively stable at approximately $100-150 per ton. This abundance ensures long-term supply security for large-scale battery production.
However, the supply chain for specialized cathode materials used in wetting and wet-proofing applications introduces complexity. Carbon-based materials such as carbon nanotubes, graphene, and mesoporous carbons—often used to enhance sulfur utilization and prevent polysulfide dissolution—face production scalability challenges. These materials typically require energy-intensive manufacturing processes and specialized equipment, potentially creating supply bottlenecks as demand increases.
The electrolyte components, particularly ionic liquids and sodium salts used in RT-Na-S batteries, present varying sustainability profiles. While sodium salts are generally abundant and accessible, certain specialized electrolyte additives may rely on less common elements or complex synthesis routes. The manufacturing processes for these components often involve organic solvents that pose environmental concerns and require careful waste management protocols.
From a life-cycle perspective, RT-Na-S batteries demonstrate promising characteristics. The theoretical energy density of Na-S chemistry (760 Wh/kg) combined with the use of earth-abundant materials suggests a potentially lower carbon footprint compared to conventional lithium-ion technologies. However, comprehensive life-cycle assessments remain limited, particularly regarding the environmental impact of specialized cathode treatments for wetting and wet-proofing.
Supply chain resilience for RT-Na-S batteries benefits from geographic diversity in raw material sources. Unlike lithium, which faces concentration risks with over 70% of global production in the "Lithium Triangle" of South America, sodium compounds are produced across numerous regions globally. This distribution reduces vulnerability to regional supply disruptions and potentially enables more localized battery manufacturing ecosystems.
Looking forward, the sustainability advantages of RT-Na-S batteries may be further enhanced through circular economy approaches. The relatively simple chemistry facilitates end-of-life recycling, with potential recovery rates for sodium and sulfur exceeding 90% using established hydrometallurgical processes. This recyclability could significantly reduce the life-cycle environmental impact and further strengthen supply chain security.
Safety Standards and Performance Benchmarks
The establishment of comprehensive safety standards for room-temperature sodium-sulfur (RT-Na-S) batteries is critical due to the reactive nature of sodium metal and sulfur compounds. Current safety protocols primarily focus on thermal stability, with requirements that RT-Na-S batteries maintain structural integrity and prevent thermal runaway at temperatures ranging from -20°C to 60°C under normal operating conditions. International standards such as IEC 62660 and UL 1642, originally developed for lithium-ion systems, are being adapted to address the unique safety concerns of sodium-based technologies.
Performance benchmarks for commercial viability of RT-Na-S batteries have been established by industry consortiums and research institutions. These include energy density targets of >300 Wh/kg at the cell level, cycle life exceeding 1000 cycles with less than 20% capacity degradation, and coulombic efficiency consistently above 99.5%. Specific to cathode wetting and wet proofing technologies, performance standards require uniform electrolyte distribution with wetting angles below 30° while maintaining electronic isolation between sulfur species and the sodium anode.
Accelerated testing protocols have been developed to evaluate the long-term stability of wetting agents and wet-proofing materials. These tests simulate extended cycling under various temperature and current density conditions, with requirements that cathode materials maintain their hydrophilic/hydrophobic properties as appropriate after 500 equivalent cycles. Material degradation metrics specify that less than 5% change in wetting characteristics is acceptable for commercial applications.
Safety certification for RT-Na-S batteries incorporating novel wetting technologies must address potential failure modes including electrolyte leakage, polysulfide shuttle effects, and dendrite formation. Current standards mandate that batteries undergo nail penetration tests, crush tests, and external short circuit tests without exhibiting violent reactions or fires. The unique challenge of sodium's reactivity with moisture necessitates additional hermetic sealing requirements with leak rates below 10^-6 mbar·L/s.
Regulatory frameworks across major markets are evolving to incorporate sodium-based battery technologies. The European Battery Directive is currently being updated to include specific provisions for sodium batteries, while China's GB/T standards have already introduced preliminary guidelines for RT-Na-S systems. In the United States, the Department of Energy has funded initiatives to develop standardized testing protocols specifically addressing the interface stability issues common in wet-proofed cathode designs.
Industry adoption of these standards remains in early stages, with most manufacturers currently using internal benchmarks supplemented by applicable portions of lithium-ion standards. A coordinated international effort through organizations like the International Electrotechnical Commission is underway to harmonize safety and performance requirements for next-generation sodium battery technologies, with particular attention to the unique challenges presented by cathode wetting and wet-proofing mechanisms.
Performance benchmarks for commercial viability of RT-Na-S batteries have been established by industry consortiums and research institutions. These include energy density targets of >300 Wh/kg at the cell level, cycle life exceeding 1000 cycles with less than 20% capacity degradation, and coulombic efficiency consistently above 99.5%. Specific to cathode wetting and wet proofing technologies, performance standards require uniform electrolyte distribution with wetting angles below 30° while maintaining electronic isolation between sulfur species and the sodium anode.
Accelerated testing protocols have been developed to evaluate the long-term stability of wetting agents and wet-proofing materials. These tests simulate extended cycling under various temperature and current density conditions, with requirements that cathode materials maintain their hydrophilic/hydrophobic properties as appropriate after 500 equivalent cycles. Material degradation metrics specify that less than 5% change in wetting characteristics is acceptable for commercial applications.
Safety certification for RT-Na-S batteries incorporating novel wetting technologies must address potential failure modes including electrolyte leakage, polysulfide shuttle effects, and dendrite formation. Current standards mandate that batteries undergo nail penetration tests, crush tests, and external short circuit tests without exhibiting violent reactions or fires. The unique challenge of sodium's reactivity with moisture necessitates additional hermetic sealing requirements with leak rates below 10^-6 mbar·L/s.
Regulatory frameworks across major markets are evolving to incorporate sodium-based battery technologies. The European Battery Directive is currently being updated to include specific provisions for sodium batteries, while China's GB/T standards have already introduced preliminary guidelines for RT-Na-S systems. In the United States, the Department of Energy has funded initiatives to develop standardized testing protocols specifically addressing the interface stability issues common in wet-proofed cathode designs.
Industry adoption of these standards remains in early stages, with most manufacturers currently using internal benchmarks supplemented by applicable portions of lithium-ion standards. A coordinated international effort through organizations like the International Electrotechnical Commission is underway to harmonize safety and performance requirements for next-generation sodium battery technologies, with particular attention to the unique challenges presented by cathode wetting and wet-proofing mechanisms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!