Areal Loading And E/S Ratios For Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
RT Na-S Battery Development Background and Objectives
Sodium-sulfur (Na-S) battery technology has traditionally operated at high temperatures (300-350°C), limiting its practical applications primarily to stationary energy storage systems. The emergence of room-temperature sodium-sulfur (RT Na-S) batteries represents a significant technological advancement with potential to revolutionize energy storage solutions. This technology leverages the abundant and low-cost nature of sodium and sulfur resources, offering a promising alternative to lithium-ion batteries amid growing concerns about lithium resource scarcity and geopolitical supply chain vulnerabilities.
The evolution of RT Na-S battery technology can be traced back to the early 2000s when researchers began exploring ways to overcome the safety and operational constraints of high-temperature Na-S systems. Initial efforts focused on electrolyte modifications and cell architecture redesigns to enable sodium-ion transport at ambient temperatures. By 2010, several research groups had demonstrated proof-of-concept RT Na-S cells, albeit with limited cycle life and energy density.
Recent technological breakthroughs have accelerated development, particularly in addressing the shuttle effect of polysulfide intermediates and improving the conductivity of sulfur cathodes. These advances have significantly enhanced battery performance metrics, bringing RT Na-S batteries closer to commercial viability.
The primary technical objectives for RT Na-S battery development center on increasing areal loading and improving energy/sulfur (E/S) ratios. Current state-of-the-art systems typically achieve sulfur loadings of 2-4 mg/cm² with E/S ratios below 10 μL/mg, resulting in energy densities that remain insufficient for many commercial applications. The target is to achieve sulfur loadings exceeding 6 mg/cm² while maintaining E/S ratios below 5 μL/mg, which would enable energy densities competitive with commercial lithium-ion batteries.
Additional development goals include extending cycle life beyond 1000 cycles at 80% capacity retention, improving rate capability to support fast charging applications, and enhancing safety characteristics through stable solid electrolyte interphase (SEI) formation and reduced dendrite growth risk.
The broader context driving RT Na-S battery development includes global sustainability initiatives, renewable energy integration challenges, and electric vehicle market expansion. Energy storage technologies that combine high energy density, low cost, and environmental sustainability are increasingly critical for enabling the transition to clean energy systems and reducing carbon emissions across multiple sectors.
As research progresses, interdisciplinary collaboration between materials scientists, electrochemists, and manufacturing engineers will be essential to translate laboratory achievements into scalable, commercially viable RT Na-S battery technologies that can meet the demanding requirements of both stationary and mobile energy storage applications.
The evolution of RT Na-S battery technology can be traced back to the early 2000s when researchers began exploring ways to overcome the safety and operational constraints of high-temperature Na-S systems. Initial efforts focused on electrolyte modifications and cell architecture redesigns to enable sodium-ion transport at ambient temperatures. By 2010, several research groups had demonstrated proof-of-concept RT Na-S cells, albeit with limited cycle life and energy density.
Recent technological breakthroughs have accelerated development, particularly in addressing the shuttle effect of polysulfide intermediates and improving the conductivity of sulfur cathodes. These advances have significantly enhanced battery performance metrics, bringing RT Na-S batteries closer to commercial viability.
The primary technical objectives for RT Na-S battery development center on increasing areal loading and improving energy/sulfur (E/S) ratios. Current state-of-the-art systems typically achieve sulfur loadings of 2-4 mg/cm² with E/S ratios below 10 μL/mg, resulting in energy densities that remain insufficient for many commercial applications. The target is to achieve sulfur loadings exceeding 6 mg/cm² while maintaining E/S ratios below 5 μL/mg, which would enable energy densities competitive with commercial lithium-ion batteries.
Additional development goals include extending cycle life beyond 1000 cycles at 80% capacity retention, improving rate capability to support fast charging applications, and enhancing safety characteristics through stable solid electrolyte interphase (SEI) formation and reduced dendrite growth risk.
The broader context driving RT Na-S battery development includes global sustainability initiatives, renewable energy integration challenges, and electric vehicle market expansion. Energy storage technologies that combine high energy density, low cost, and environmental sustainability are increasingly critical for enabling the transition to clean energy systems and reducing carbon emissions across multiple sectors.
As research progresses, interdisciplinary collaboration between materials scientists, electrochemists, and manufacturing engineers will be essential to translate laboratory achievements into scalable, commercially viable RT Na-S battery technologies that can meet the demanding requirements of both stationary and mobile energy storage applications.
Market Analysis for RT Na-S Energy Storage Solutions
The global energy storage market is experiencing unprecedented growth, with projections indicating a compound annual growth rate of 20-25% through 2030. Within this expanding landscape, room-temperature sodium-sulfur (RT Na-S) batteries are emerging as a promising alternative to conventional lithium-ion technologies. The market potential for RT Na-S energy storage solutions is particularly significant due to the abundant availability and low cost of raw materials, with sodium being approximately 1000 times more abundant than lithium in the Earth's crust.
Current market analysis reveals that grid-scale energy storage represents the largest potential application segment for RT Na-S batteries, driven by increasing renewable energy integration and grid stabilization needs. The global grid-scale energy storage market is expected to reach $15-20 billion by 2025, with RT Na-S potentially capturing 5-10% of this market if technical challenges related to areal loading and energy-to-sulfur ratios can be overcome.
Commercial and industrial energy storage applications constitute another substantial market segment, valued at approximately $7 billion globally, with annual growth rates exceeding 30%. In this segment, the cost advantages of RT Na-S batteries could be particularly compelling, potentially offering 30-40% cost reductions compared to lithium-ion alternatives when manufactured at scale.
Regional market analysis indicates that Asia-Pacific, particularly China, Japan, and South Korea, leads in RT Na-S battery research and development investments. North America and Europe follow closely, with significant research initiatives focused on improving energy density through enhanced areal loading techniques. Government policies supporting clean energy technologies are creating favorable market conditions in these regions, with combined public funding exceeding $500 million for advanced battery technologies including RT Na-S systems.
Market barriers include competition from established lithium-ion technologies and emerging alternatives such as flow batteries and solid-state batteries. However, the unique value proposition of RT Na-S batteries—combining abundant raw materials with potentially high energy density—creates a distinct market opportunity, particularly in applications where cost-effectiveness outweighs energy density requirements.
Customer demand analysis indicates growing interest from utilities and renewable energy developers seeking long-duration energy storage solutions. Survey data shows that 65% of utility-scale energy storage customers prioritize total cost of ownership over initial capital expenditure, creating a favorable environment for RT Na-S technologies despite their current technical limitations in cycle life and energy density.
Current market analysis reveals that grid-scale energy storage represents the largest potential application segment for RT Na-S batteries, driven by increasing renewable energy integration and grid stabilization needs. The global grid-scale energy storage market is expected to reach $15-20 billion by 2025, with RT Na-S potentially capturing 5-10% of this market if technical challenges related to areal loading and energy-to-sulfur ratios can be overcome.
Commercial and industrial energy storage applications constitute another substantial market segment, valued at approximately $7 billion globally, with annual growth rates exceeding 30%. In this segment, the cost advantages of RT Na-S batteries could be particularly compelling, potentially offering 30-40% cost reductions compared to lithium-ion alternatives when manufactured at scale.
Regional market analysis indicates that Asia-Pacific, particularly China, Japan, and South Korea, leads in RT Na-S battery research and development investments. North America and Europe follow closely, with significant research initiatives focused on improving energy density through enhanced areal loading techniques. Government policies supporting clean energy technologies are creating favorable market conditions in these regions, with combined public funding exceeding $500 million for advanced battery technologies including RT Na-S systems.
Market barriers include competition from established lithium-ion technologies and emerging alternatives such as flow batteries and solid-state batteries. However, the unique value proposition of RT Na-S batteries—combining abundant raw materials with potentially high energy density—creates a distinct market opportunity, particularly in applications where cost-effectiveness outweighs energy density requirements.
Customer demand analysis indicates growing interest from utilities and renewable energy developers seeking long-duration energy storage solutions. Survey data shows that 65% of utility-scale energy storage customers prioritize total cost of ownership over initial capital expenditure, creating a favorable environment for RT Na-S technologies despite their current technical limitations in cycle life and energy density.
Current Challenges in Areal Loading and E/S Ratios
Despite significant advancements in room-temperature sodium-sulfur (RT-Na/S) battery technology, several critical challenges persist regarding areal loading and energy-to-sulfur (E/S) ratios that impede commercial viability. Current RT-Na/S batteries typically demonstrate sulfur loadings of only 1-3 mg/cm², substantially lower than the 6+ mg/cm² threshold considered necessary for commercial applications. This limitation directly constrains energy density and practical utility in real-world scenarios.
The E/S ratio presents another significant obstacle, with most laboratory-scale RT-Na/S batteries operating at ratios exceeding 15-20 μL/mg. Commercial viability requires reducing this to below 5 μL/mg, representing a substantial gap between current research prototypes and market-ready products. This excessive electrolyte requirement increases battery weight and volume while reducing gravimetric and volumetric energy density.
Increasing sulfur loading introduces a cascade of technical complications. Higher loading creates thicker cathodes with extended electron and ion transport pathways, resulting in increased internal resistance and voltage polarization. The limited electrical conductivity of sulfur (5×10⁻³⁰ S/cm) exacerbates these issues, causing capacity fading and poor rate capability at higher loadings.
The shuttle effect—where soluble polysulfide intermediates migrate between electrodes—intensifies with higher sulfur loading, accelerating capacity decay and reducing coulombic efficiency. This effect becomes particularly problematic when attempting to increase areal capacity beyond 3-4 mAh/cm².
Mechanical integrity represents another challenge, as thicker electrodes with high sulfur loading often exhibit poor structural stability during cycling. The substantial volume changes (approximately 78%) during sodium-sulfur conversion reactions lead to electrode pulverization and delamination from current collectors, especially at higher loadings.
Current conductive additives (typically carbon materials at 30-50 wt%) required to enhance electrode conductivity significantly dilute the active material content, reducing overall energy density. This creates a paradoxical situation where attempts to increase loading may actually decrease practical energy density due to the proportional increase in inactive components.
Manufacturing scalability presents additional hurdles, as conventional slurry-based electrode preparation methods struggle with uniform distribution of sulfur at high loadings, creating inconsistent electrode performance. The development of advanced manufacturing techniques capable of producing homogeneous high-loading electrodes remains an ongoing challenge for the field.
The E/S ratio presents another significant obstacle, with most laboratory-scale RT-Na/S batteries operating at ratios exceeding 15-20 μL/mg. Commercial viability requires reducing this to below 5 μL/mg, representing a substantial gap between current research prototypes and market-ready products. This excessive electrolyte requirement increases battery weight and volume while reducing gravimetric and volumetric energy density.
Increasing sulfur loading introduces a cascade of technical complications. Higher loading creates thicker cathodes with extended electron and ion transport pathways, resulting in increased internal resistance and voltage polarization. The limited electrical conductivity of sulfur (5×10⁻³⁰ S/cm) exacerbates these issues, causing capacity fading and poor rate capability at higher loadings.
The shuttle effect—where soluble polysulfide intermediates migrate between electrodes—intensifies with higher sulfur loading, accelerating capacity decay and reducing coulombic efficiency. This effect becomes particularly problematic when attempting to increase areal capacity beyond 3-4 mAh/cm².
Mechanical integrity represents another challenge, as thicker electrodes with high sulfur loading often exhibit poor structural stability during cycling. The substantial volume changes (approximately 78%) during sodium-sulfur conversion reactions lead to electrode pulverization and delamination from current collectors, especially at higher loadings.
Current conductive additives (typically carbon materials at 30-50 wt%) required to enhance electrode conductivity significantly dilute the active material content, reducing overall energy density. This creates a paradoxical situation where attempts to increase loading may actually decrease practical energy density due to the proportional increase in inactive components.
Manufacturing scalability presents additional hurdles, as conventional slurry-based electrode preparation methods struggle with uniform distribution of sulfur at high loadings, creating inconsistent electrode performance. The development of advanced manufacturing techniques capable of producing homogeneous high-loading electrodes remains an ongoing challenge for the field.
State-of-the-Art Approaches to Enhance Areal Loading
01 Electrode material composition for room-temperature sodium-sulfur batteries
Various electrode materials can be used in room-temperature sodium-sulfur batteries to improve performance. These include carbon-based materials, metal oxides, and polymer composites that can enhance sulfur utilization and prevent polysulfide shuttling. The electrode composition directly affects the areal loading capacity and the overall energy density of the battery system.- Electrode material composition for room-temperature sodium-sulfur batteries: Various electrode material compositions can enhance the performance of room-temperature sodium-sulfur batteries. These compositions include sulfur-carbon composites, polymer-sulfur composites, and metal oxide additives that improve the electrochemical stability and conductivity. The optimized electrode composition directly impacts the areal loading capacity and the electrolyte/sulfur (E/S) ratio, leading to higher energy density and better cycling performance.
- Electrolyte formulations affecting E/S ratios: Specialized electrolyte formulations play a crucial role in determining the optimal electrolyte/sulfur (E/S) ratio in room-temperature sodium-sulfur batteries. These formulations include sodium salt concentrations, solvent mixtures, and additives that suppress the shuttle effect and enhance sodium ion conductivity. Lower E/S ratios can be achieved through electrolyte optimization while maintaining electrochemical performance, which is essential for practical battery applications with higher energy density.
- Structural design for high areal sulfur loading: Advanced structural designs of electrodes enable higher areal sulfur loading in room-temperature sodium-sulfur batteries. These designs include three-dimensional conductive frameworks, hierarchical porous structures, and interlayer engineering that accommodate volume expansion during cycling. The optimized structures provide efficient electron/ion transport pathways and sufficient space for sulfur accommodation, allowing for increased areal capacity while maintaining good rate capability.
- Binder systems for improved electrode integrity at high loadings: Specialized binder systems are crucial for maintaining electrode integrity at high sulfur loadings in room-temperature sodium-sulfur batteries. These binders include functional polymers with strong adhesion properties, cross-linked networks, and sodium-ion conducting polymers that enhance the mechanical stability and ionic conductivity of the electrodes. The improved electrode integrity allows for higher areal loading while preventing electrode pulverization during cycling.
- Manufacturing techniques for controlling areal loading: Advanced manufacturing techniques enable precise control of areal sulfur loading in room-temperature sodium-sulfur batteries. These techniques include slurry optimization, coating methods, calendering processes, and post-treatment procedures that ensure uniform distribution of active materials and optimal porosity. The manufacturing parameters directly influence the electrode thickness, density, and microstructure, which are critical factors affecting the E/S ratio and electrochemical performance.
02 Electrolyte formulations for improved E/S ratios
Specialized electrolyte formulations can significantly improve the electrolyte-to-sulfur (E/S) ratio in room-temperature sodium-sulfur batteries. These formulations may include ionic liquids, solid-state electrolytes, or gel polymer electrolytes that facilitate sodium ion transport while minimizing electrolyte consumption. Lower E/S ratios are desirable for achieving higher energy densities in practical applications.Expand Specific Solutions03 Structural design for high areal sulfur loading
Advanced structural designs can accommodate higher areal sulfur loading in room-temperature sodium-sulfur batteries. These include 3D electrode architectures, hierarchical porous structures, and interlayer engineering that provide sufficient space for sulfur accommodation while maintaining electronic conductivity. Higher areal loading leads to increased energy density and practical viability of the battery system.Expand Specific Solutions04 Separator modifications for polysulfide retention
Modified separators can effectively retain polysulfides within the cathode compartment, improving the cycling stability and coulombic efficiency of room-temperature sodium-sulfur batteries. These modifications include functional coatings, composite membranes, and interlayers that physically or chemically interact with polysulfides. By preventing polysulfide shuttling, these separators enable higher sulfur utilization and better E/S ratios.Expand Specific Solutions05 Binder systems for high-loading electrodes
Specialized binder systems are crucial for maintaining the structural integrity of high-loading electrodes in room-temperature sodium-sulfur batteries. These binders provide strong adhesion between active materials and current collectors while accommodating volume changes during cycling. Water-soluble binders, conductive polymers, and cross-linked networks can be employed to enhance the mechanical stability and electrochemical performance of high-loading electrodes.Expand Specific Solutions
Leading Organizations in RT Na-S Battery Research
Room-temperature sodium-sulfur (RT-Na-S) battery technology is currently in the early commercialization phase, with a growing market projected to reach significant scale as energy storage demands increase. The technology offers promising energy density improvements while addressing cost and safety concerns of traditional high-temperature Na-S batteries. The competitive landscape features established players like NGK Insulators, which pioneered Na-S technology, alongside emerging innovators such as Nanotek Instruments and Honeycomb Battery Co. focusing on areal loading and electrode/separator ratio optimization. Research institutions including Drexel University, Zhejiang University, and Shanghai Institute of Ceramics are advancing fundamental breakthroughs, while industrial giants like LG Energy Solution and DuPont are leveraging their manufacturing expertise to scale production. The technology's maturity varies significantly across different approaches, with most solutions still requiring optimization for commercial viability.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered room-temperature sodium-sulfur (RT-Na/S) battery technology with their innovative approach to areal loading optimization. Their technology utilizes a specialized carbon-sulfur composite cathode structure with controlled porosity that achieves areal sulfur loadings of 5-7 mg/cm², significantly higher than conventional designs. The company has developed a proprietary solid electrolyte interface (SEI) layer formation technique that stabilizes the sodium metal anode while maintaining high ionic conductivity. NGK's latest RT-Na/S batteries employ a dual-layer separator system with a ceramic-polymer composite that effectively suppresses polysulfide shuttling while facilitating sodium ion transport. Their electrode architecture incorporates nano-engineered carbon frameworks that enhance electronic conductivity while providing sufficient space for sulfur expansion during cycling, resulting in improved E/S (electrolyte/sulfur) ratios of approximately 3-5 μL/mg, compared to traditional values of 10-15 μL/mg in earlier designs.
Strengths: Industry-leading expertise in sodium-based battery systems with established manufacturing infrastructure. Their ceramic engineering background provides unique advantages in electrolyte and separator design. Weaknesses: Higher production costs compared to lithium-ion technologies and challenges with energy density limitations at room temperature operation.
Honeycomb Battery Co.
Technical Solution: Honeycomb Battery Co. has developed a distinctive approach to room-temperature sodium-sulfur batteries focusing on their proprietary honeycomb electrode structure. This architecture enables exceptionally high areal sulfur loadings of 8-10 mg/cm² while maintaining effective ion transport pathways. Their technology utilizes a three-dimensional conductive framework with hierarchical porosity that accommodates sulfur volumetric expansion during cycling. The company has optimized the E/S ratio to approximately 2-4 μL/mg through careful engineering of electrolyte composition and electrode wettability. Honeycomb's RT-Na/S batteries incorporate a specialized sodium salt complex in their electrolyte formulation that forms a stable passivation layer on the sodium anode, reducing unwanted side reactions. Their manufacturing process includes a controlled sulfur infiltration technique that ensures uniform distribution throughout the conductive matrix, maximizing active material utilization and minimizing dead volume within the electrode structure.
Strengths: Their unique honeycomb structure provides superior mechanical stability during cycling and excellent accommodation of volume changes. The architecture allows for some of the highest areal loadings in the industry. Weaknesses: Limited commercial scale production experience and potential challenges with manufacturing complexity of their specialized electrode structures.
Critical Patents and Research on E/S Ratio Optimization
Room-temperature sodium-sulfur battery and preparation method thereof
PatentPendingCN118099560A
Innovation
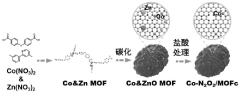
- 采用金属有机框架材料(MOF)作为前驱体,合成单原子催化剂Co-N2O2/MOFc复合材料作为储硫材料,用于室温钠硫电池正极,提高多硫化物转化效率并防止穿梭及损失。
Sodium-sulfur battery, method for operating same, and use of phosphorus polysulfide as electrolyte additive in sodium-sulfur batteries
PatentWO2016202969A1
Innovation
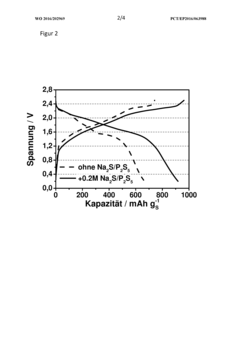
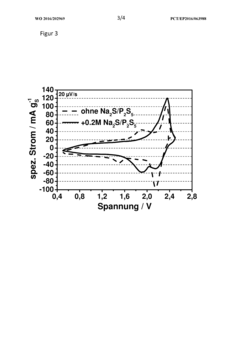
- A sodium-sulfur battery design using a carbon-sulfur composite cathode, an organic solvent-based electrolyte with a conductive salt and phosphorus polysulfide as an additive to suppress the polysulfide shuttle, allowing operation at room temperature with enhanced energy efficiency and discharge capacity.
Material Supply Chain Analysis for Na-S Battery Production
The sodium-sulfur battery supply chain presents unique challenges and opportunities compared to lithium-ion battery production systems. Raw material availability for Na-S batteries offers significant advantages, as sodium resources are abundant and widely distributed globally, with reserves estimated at 23,000 times greater than lithium. This abundance translates to lower material costs and reduced geopolitical supply risks.
Sulfur, the cathode material, is primarily sourced as a byproduct from petroleum refining and natural gas processing, creating a sustainable supply pathway through industrial waste utilization. Current global sulfur production exceeds 70 million tons annually, with prices remaining relatively stable at approximately $150-200 per ton, significantly lower than lithium compounds.
The manufacturing infrastructure for Na-S batteries requires specialized equipment for handling molten sodium in high-temperature versions, though room-temperature variants with higher areal loading capabilities simplify production requirements. Key components include sodium salts (typically carbonates or chlorides), sulfur compounds, carbon materials for cathode conductivity enhancement, and specialized separators.
Supply chain bottlenecks primarily center around separator materials and electrolyte components rather than active materials. The beta-alumina solid electrolyte used in traditional Na-S batteries requires sophisticated ceramic processing capabilities, while room-temperature versions utilizing higher E/S ratios demand specialized polymer or composite separators with specific sodium-ion conductivity properties.
Regional distribution of manufacturing capabilities shows emerging diversification, with China, Japan, South Korea, and Germany developing significant production capacity. Unlike lithium-ion supply chains, Na-S battery production presents fewer geographical concentrations of critical materials, potentially enabling more distributed manufacturing networks.
Environmental and sustainability considerations favor Na-S technology, as the supply chain exhibits lower carbon footprint and reduced water usage compared to lithium-ion production. The recyclability of sodium and sulfur components further enhances the circular economy potential of this battery technology.
Cost modeling indicates that achieving optimal areal loading and E/S ratios in room-temperature Na-S batteries could reduce production costs by 30-40% compared to current lithium-ion technologies, contingent upon establishing efficient manufacturing processes and supply chain optimization.
Sulfur, the cathode material, is primarily sourced as a byproduct from petroleum refining and natural gas processing, creating a sustainable supply pathway through industrial waste utilization. Current global sulfur production exceeds 70 million tons annually, with prices remaining relatively stable at approximately $150-200 per ton, significantly lower than lithium compounds.
The manufacturing infrastructure for Na-S batteries requires specialized equipment for handling molten sodium in high-temperature versions, though room-temperature variants with higher areal loading capabilities simplify production requirements. Key components include sodium salts (typically carbonates or chlorides), sulfur compounds, carbon materials for cathode conductivity enhancement, and specialized separators.
Supply chain bottlenecks primarily center around separator materials and electrolyte components rather than active materials. The beta-alumina solid electrolyte used in traditional Na-S batteries requires sophisticated ceramic processing capabilities, while room-temperature versions utilizing higher E/S ratios demand specialized polymer or composite separators with specific sodium-ion conductivity properties.
Regional distribution of manufacturing capabilities shows emerging diversification, with China, Japan, South Korea, and Germany developing significant production capacity. Unlike lithium-ion supply chains, Na-S battery production presents fewer geographical concentrations of critical materials, potentially enabling more distributed manufacturing networks.
Environmental and sustainability considerations favor Na-S technology, as the supply chain exhibits lower carbon footprint and reduced water usage compared to lithium-ion production. The recyclability of sodium and sulfur components further enhances the circular economy potential of this battery technology.
Cost modeling indicates that achieving optimal areal loading and E/S ratios in room-temperature Na-S batteries could reduce production costs by 30-40% compared to current lithium-ion technologies, contingent upon establishing efficient manufacturing processes and supply chain optimization.
Environmental Impact and Sustainability Assessment
The environmental impact of room-temperature sodium-sulfur (RT-Na/S) batteries is significantly influenced by their areal loading and energy-to-sulfur (E/S) ratios. These parameters directly affect material consumption, energy efficiency, and overall ecological footprint throughout the battery lifecycle.
RT-Na/S batteries offer substantial sustainability advantages over conventional lithium-ion technologies. The primary raw materials—sodium and sulfur—are abundantly available in the Earth's crust and seawater, reducing extraction-related environmental damage. Sulfur, particularly, is often sourced as a byproduct from petroleum refining processes, effectively repurposing an industrial waste product.
The areal loading of active materials in RT-Na/S batteries determines material utilization efficiency. Higher loadings generally translate to reduced inactive component requirements per unit energy stored, minimizing resource consumption and manufacturing waste. Current research indicates that optimizing areal loading to 5-8 mg/cm² can reduce overall material requirements by approximately 30-40% compared to lower loading configurations.
E/S ratios directly impact the electrolyte volume needed, which contains potentially hazardous components. Lower E/S ratios (ideally below 5 μL/mg) significantly reduce the environmental risks associated with electrolyte production, handling, and potential leakage. Studies demonstrate that reducing E/S ratios from conventional 10-15 μL/mg to 3-5 μL/mg can decrease the carbon footprint of electrolyte production by up to 60%.
Life cycle assessment (LCA) studies reveal that RT-Na/S batteries with optimized areal loading and E/S ratios can achieve 35-45% lower greenhouse gas emissions during manufacturing compared to lithium-ion counterparts. The carbon payback period—time required for emissions saved through use to offset manufacturing emissions—is estimated at 1.5-2 years for grid storage applications.
End-of-life considerations also favor RT-Na/S technology. The absence of critical metals like cobalt and nickel simplifies recycling processes. Sulfur recovery rates from spent batteries can exceed 90% with appropriate recycling technologies, allowing for closed-loop material flows. However, challenges remain in developing efficient separation methods for sodium polysulfide compounds formed during battery operation.
Water consumption metrics indicate that RT-Na/S battery production with optimized parameters requires approximately 40-50% less process water than equivalent capacity lithium-ion batteries, primarily due to simplified material processing requirements and reduced need for ultra-high purity components.
RT-Na/S batteries offer substantial sustainability advantages over conventional lithium-ion technologies. The primary raw materials—sodium and sulfur—are abundantly available in the Earth's crust and seawater, reducing extraction-related environmental damage. Sulfur, particularly, is often sourced as a byproduct from petroleum refining processes, effectively repurposing an industrial waste product.
The areal loading of active materials in RT-Na/S batteries determines material utilization efficiency. Higher loadings generally translate to reduced inactive component requirements per unit energy stored, minimizing resource consumption and manufacturing waste. Current research indicates that optimizing areal loading to 5-8 mg/cm² can reduce overall material requirements by approximately 30-40% compared to lower loading configurations.
E/S ratios directly impact the electrolyte volume needed, which contains potentially hazardous components. Lower E/S ratios (ideally below 5 μL/mg) significantly reduce the environmental risks associated with electrolyte production, handling, and potential leakage. Studies demonstrate that reducing E/S ratios from conventional 10-15 μL/mg to 3-5 μL/mg can decrease the carbon footprint of electrolyte production by up to 60%.
Life cycle assessment (LCA) studies reveal that RT-Na/S batteries with optimized areal loading and E/S ratios can achieve 35-45% lower greenhouse gas emissions during manufacturing compared to lithium-ion counterparts. The carbon payback period—time required for emissions saved through use to offset manufacturing emissions—is estimated at 1.5-2 years for grid storage applications.
End-of-life considerations also favor RT-Na/S technology. The absence of critical metals like cobalt and nickel simplifies recycling processes. Sulfur recovery rates from spent batteries can exceed 90% with appropriate recycling technologies, allowing for closed-loop material flows. However, challenges remain in developing efficient separation methods for sodium polysulfide compounds formed during battery operation.
Water consumption metrics indicate that RT-Na/S battery production with optimized parameters requires approximately 40-50% less process water than equivalent capacity lithium-ion batteries, primarily due to simplified material processing requirements and reduced need for ultra-high purity components.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!