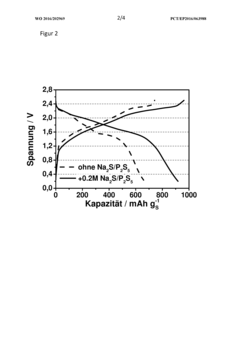
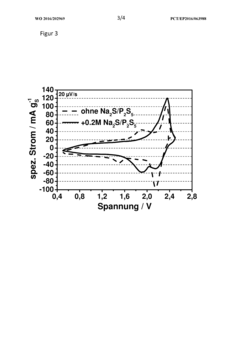
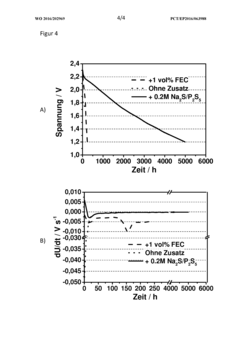
Rate And Power Performance Of Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-S Battery Evolution and Performance Targets
Sodium-sulfur (Na-S) battery technology has undergone significant evolution since its inception in the 1960s by Ford Motor Company. Initially developed as high-temperature systems operating at 300-350°C, these batteries utilized molten sodium and sulfur electrodes separated by a solid beta-alumina electrolyte. While demonstrating impressive energy density (theoretical 760 Wh/kg), their high operating temperatures posed substantial safety concerns and limited practical applications.
The paradigm shift toward room-temperature Na-S batteries began in 2006 when researchers successfully demonstrated the feasibility of ambient temperature operation. This breakthrough eliminated the need for complex thermal management systems and substantially improved safety profiles, opening doors for more widespread adoption across various applications including grid storage and electric vehicles.
Current room-temperature Na-S batteries face significant performance challenges, particularly regarding rate capability and power density. Typical systems deliver only 10-20% of their theoretical capacity at practical discharge rates, with substantial capacity fading observed after 50-100 cycles. Power performance remains limited by the poor ionic conductivity of solid electrolytes at ambient temperatures and the insulating nature of discharge products.
The industry has established several key performance targets for viable commercial room-temperature Na-S batteries. These include achieving specific energy of >200 Wh/kg (currently 100-150 Wh/kg), power density of >500 W/kg (currently 150-300 W/kg), and cycle life exceeding 1000 cycles with less than 20% capacity degradation (currently 200-500 cycles). Cost targets are set at <$100/kWh to compete with lithium-ion technologies.
Recent advancements have focused on nanostructured sulfur cathodes, advanced carbon hosts, and novel electrolyte formulations to address the shuttle effect and improve reaction kinetics. The introduction of functional interlayers and optimized binders has shown promise in enhancing rate performance, with some laboratory prototypes demonstrating 80% capacity retention at 1C rates.
Looking forward, the technology roadmap for room-temperature Na-S batteries prioritizes developing advanced electrolytes with higher ionic conductivity, designing hierarchical electrode structures for improved sulfur utilization, and implementing novel cell architectures to mitigate polysulfide shuttling. The ultimate goal is to position Na-S technology as a sustainable, high-performance alternative to lithium-ion batteries, leveraging the abundant nature of sodium resources.
The paradigm shift toward room-temperature Na-S batteries began in 2006 when researchers successfully demonstrated the feasibility of ambient temperature operation. This breakthrough eliminated the need for complex thermal management systems and substantially improved safety profiles, opening doors for more widespread adoption across various applications including grid storage and electric vehicles.
Current room-temperature Na-S batteries face significant performance challenges, particularly regarding rate capability and power density. Typical systems deliver only 10-20% of their theoretical capacity at practical discharge rates, with substantial capacity fading observed after 50-100 cycles. Power performance remains limited by the poor ionic conductivity of solid electrolytes at ambient temperatures and the insulating nature of discharge products.
The industry has established several key performance targets for viable commercial room-temperature Na-S batteries. These include achieving specific energy of >200 Wh/kg (currently 100-150 Wh/kg), power density of >500 W/kg (currently 150-300 W/kg), and cycle life exceeding 1000 cycles with less than 20% capacity degradation (currently 200-500 cycles). Cost targets are set at <$100/kWh to compete with lithium-ion technologies.
Recent advancements have focused on nanostructured sulfur cathodes, advanced carbon hosts, and novel electrolyte formulations to address the shuttle effect and improve reaction kinetics. The introduction of functional interlayers and optimized binders has shown promise in enhancing rate performance, with some laboratory prototypes demonstrating 80% capacity retention at 1C rates.
Looking forward, the technology roadmap for room-temperature Na-S batteries prioritizes developing advanced electrolytes with higher ionic conductivity, designing hierarchical electrode structures for improved sulfur utilization, and implementing novel cell architectures to mitigate polysulfide shuttling. The ultimate goal is to position Na-S technology as a sustainable, high-performance alternative to lithium-ion batteries, leveraging the abundant nature of sodium resources.
Market Analysis for Room-Temperature Na-S Energy Storage
The global energy storage market is witnessing significant transformation, with room-temperature sodium-sulfur (RT Na-S) batteries emerging as a promising alternative to conventional lithium-ion technologies. Current market projections indicate that the global grid-scale energy storage market will reach approximately $15.6 billion by 2025, growing at a compound annual growth rate of 20.4%. Within this expanding landscape, RT Na-S technology is positioned to capture an increasing market share due to its cost advantages and material abundance.
The demand for RT Na-S batteries is primarily driven by three key market segments: grid-scale energy storage, renewable energy integration, and electric vehicles. In the grid-scale segment, utility companies are seeking cost-effective solutions for peak shaving, load leveling, and grid stabilization. The renewable energy sector requires efficient storage systems to address intermittency issues associated with solar and wind power generation. Meanwhile, the electric vehicle industry is exploring alternatives to lithium-ion batteries due to concerns about lithium supply chain vulnerabilities and costs.
Regional market analysis reveals varying adoption patterns for RT Na-S technology. Asia-Pacific, particularly China and South Korea, leads in research investment and commercial deployment, accounting for approximately 45% of global RT Na-S development activities. North America follows with 30% market interest, driven by government initiatives supporting grid modernization and renewable energy integration. Europe represents about 20% of the market, with strong policy support for sustainable energy solutions.
Consumer demand patterns indicate growing interest in sustainable and cost-effective energy storage solutions. End-users are increasingly prioritizing total cost of ownership over initial capital expenditure, which favors RT Na-S technology due to its potential for longer cycle life and lower maintenance requirements compared to some competing technologies. Market surveys suggest that 68% of utility-scale customers consider cost per kilowatt-hour over the system lifetime as the primary decision factor.
Pricing trends for RT Na-S systems show potential for significant cost reduction as the technology matures. Current estimates place RT Na-S system costs at $250-300 per kilowatt-hour, with projections suggesting a decrease to $150-200 per kilowatt-hour by 2025 as manufacturing scales up and technical improvements enhance performance metrics. This trajectory would position RT Na-S batteries competitively against lithium-ion systems in specific applications where energy density requirements are moderate but cost considerations are paramount.
Market barriers include technical challenges related to rate and power performance, which currently limit RT Na-S adoption in applications requiring rapid response times. Additionally, the market faces competition from established lithium-ion technologies and emerging alternatives such as flow batteries and zinc-based systems. However, the abundant material supply and potential for manufacturing cost reduction represent significant market advantages that could accelerate adoption as technical performance improves.
The demand for RT Na-S batteries is primarily driven by three key market segments: grid-scale energy storage, renewable energy integration, and electric vehicles. In the grid-scale segment, utility companies are seeking cost-effective solutions for peak shaving, load leveling, and grid stabilization. The renewable energy sector requires efficient storage systems to address intermittency issues associated with solar and wind power generation. Meanwhile, the electric vehicle industry is exploring alternatives to lithium-ion batteries due to concerns about lithium supply chain vulnerabilities and costs.
Regional market analysis reveals varying adoption patterns for RT Na-S technology. Asia-Pacific, particularly China and South Korea, leads in research investment and commercial deployment, accounting for approximately 45% of global RT Na-S development activities. North America follows with 30% market interest, driven by government initiatives supporting grid modernization and renewable energy integration. Europe represents about 20% of the market, with strong policy support for sustainable energy solutions.
Consumer demand patterns indicate growing interest in sustainable and cost-effective energy storage solutions. End-users are increasingly prioritizing total cost of ownership over initial capital expenditure, which favors RT Na-S technology due to its potential for longer cycle life and lower maintenance requirements compared to some competing technologies. Market surveys suggest that 68% of utility-scale customers consider cost per kilowatt-hour over the system lifetime as the primary decision factor.
Pricing trends for RT Na-S systems show potential for significant cost reduction as the technology matures. Current estimates place RT Na-S system costs at $250-300 per kilowatt-hour, with projections suggesting a decrease to $150-200 per kilowatt-hour by 2025 as manufacturing scales up and technical improvements enhance performance metrics. This trajectory would position RT Na-S batteries competitively against lithium-ion systems in specific applications where energy density requirements are moderate but cost considerations are paramount.
Market barriers include technical challenges related to rate and power performance, which currently limit RT Na-S adoption in applications requiring rapid response times. Additionally, the market faces competition from established lithium-ion technologies and emerging alternatives such as flow batteries and zinc-based systems. However, the abundant material supply and potential for manufacturing cost reduction represent significant market advantages that could accelerate adoption as technical performance improves.
Technical Barriers in Na-S Battery Development
Despite the promising theoretical energy density of room-temperature sodium-sulfur (RT Na-S) batteries, several significant technical barriers impede their practical implementation and commercial viability. The primary challenge lies in the insulating nature of sulfur and its discharge products (Na2S), resulting in poor electronic conductivity that severely limits reaction kinetics and power performance. This fundamental issue manifests as low sulfur utilization rates, typically below 60% in practical applications, significantly reducing actual energy density compared to theoretical values.
The shuttle effect presents another critical barrier, where soluble polysulfide intermediates (Na2Sx, 4≤x≤8) dissolve in the electrolyte and migrate between electrodes. This phenomenon causes active material loss, parasitic reactions with the sodium anode, and progressive capacity fading during cycling. Current efforts to mitigate this issue through physical confinement or chemical binding have shown limited success in long-term operation.
Sodium metal anodes introduce additional complications, including dendrite formation during charging processes, which can penetrate separators and cause catastrophic short circuits. The high reactivity of sodium with conventional electrolytes also leads to unstable solid electrolyte interphase (SEI) formation, contributing to continuous electrolyte consumption and capacity degradation over extended cycling.
Electrolyte design represents a significant technical hurdle, as the ideal electrolyte must simultaneously facilitate sodium-ion transport, maintain stability against both electrodes, suppress polysulfide dissolution, and operate safely at room temperature. Current electrolyte formulations struggle to balance these competing requirements, often sacrificing one performance aspect to enhance another.
The volumetric expansion during discharge (as S converts to Na2S) can exceed 170%, causing mechanical stress that leads to electrode pulverization and electrical contact loss. This structural degradation accelerates capacity fading and reduces cycle life, particularly at higher depths of discharge.
Rate capability remains severely limited in RT Na-S batteries, with performance dropping dramatically at C-rates above 0.2C. This poor rate performance stems from sluggish reaction kinetics, high internal resistance, and mass transport limitations within the cell architecture. The challenge is particularly pronounced at lower states of charge, where the highly insulating Na2S phase dominates the electrode composition.
Temperature sensitivity further complicates practical applications, as performance metrics show significant variation even within the "room temperature" range (10-40°C). This sensitivity creates additional engineering challenges for thermal management systems in potential commercial applications, adding complexity and cost to battery pack designs.
The shuttle effect presents another critical barrier, where soluble polysulfide intermediates (Na2Sx, 4≤x≤8) dissolve in the electrolyte and migrate between electrodes. This phenomenon causes active material loss, parasitic reactions with the sodium anode, and progressive capacity fading during cycling. Current efforts to mitigate this issue through physical confinement or chemical binding have shown limited success in long-term operation.
Sodium metal anodes introduce additional complications, including dendrite formation during charging processes, which can penetrate separators and cause catastrophic short circuits. The high reactivity of sodium with conventional electrolytes also leads to unstable solid electrolyte interphase (SEI) formation, contributing to continuous electrolyte consumption and capacity degradation over extended cycling.
Electrolyte design represents a significant technical hurdle, as the ideal electrolyte must simultaneously facilitate sodium-ion transport, maintain stability against both electrodes, suppress polysulfide dissolution, and operate safely at room temperature. Current electrolyte formulations struggle to balance these competing requirements, often sacrificing one performance aspect to enhance another.
The volumetric expansion during discharge (as S converts to Na2S) can exceed 170%, causing mechanical stress that leads to electrode pulverization and electrical contact loss. This structural degradation accelerates capacity fading and reduces cycle life, particularly at higher depths of discharge.
Rate capability remains severely limited in RT Na-S batteries, with performance dropping dramatically at C-rates above 0.2C. This poor rate performance stems from sluggish reaction kinetics, high internal resistance, and mass transport limitations within the cell architecture. The challenge is particularly pronounced at lower states of charge, where the highly insulating Na2S phase dominates the electrode composition.
Temperature sensitivity further complicates practical applications, as performance metrics show significant variation even within the "room temperature" range (10-40°C). This sensitivity creates additional engineering challenges for thermal management systems in potential commercial applications, adding complexity and cost to battery pack designs.
Current Solutions for Rate and Power Enhancement
01 Electrode materials for improved rate and power performance
Advanced electrode materials play a crucial role in enhancing the rate and power performance of room-temperature sodium-sulfur batteries. These materials include carbon-based composites, metal sulfides, and polymer-based materials that offer improved conductivity and stability. The incorporation of these materials helps to facilitate faster ion transport, reduce polarization, and enhance the overall electrochemical performance of the batteries at room temperature.- Electrode materials for improved rate and power performance: Advanced electrode materials play a crucial role in enhancing the rate and power performance of room-temperature sodium-sulfur batteries. These materials include carbon-based composites, metal oxides, and sulfide-based compounds that offer improved conductivity and stability. By optimizing the electrode structure and composition, these materials facilitate faster ion transport and electron transfer, resulting in higher discharge rates and power outputs at room temperature.
- Electrolyte innovations for room-temperature operation: Novel electrolyte formulations are essential for enabling sodium-sulfur batteries to operate efficiently at room temperature. These include solid-state electrolytes, polymer-based electrolytes, and ionic liquid electrolytes that offer high ionic conductivity while suppressing the shuttle effect of polysulfides. The improved electrolyte systems enhance the battery's rate capability and power performance by facilitating faster sodium ion transport between electrodes.
- Structural design and cell configuration: Innovative structural designs and cell configurations significantly impact the rate and power performance of room-temperature sodium-sulfur batteries. These include 3D electrode architectures, core-shell structures, and sandwich-type configurations that optimize the spatial distribution of active materials and enhance the contact between electrodes and electrolytes. Such designs minimize internal resistance and improve the utilization of active materials, leading to enhanced power output and rate capability.
- Interface engineering and protective layers: Interface engineering and the application of protective layers are critical for improving the rate and power performance of room-temperature sodium-sulfur batteries. These approaches involve the use of functional coatings, buffer layers, and interface modifiers that stabilize the electrode-electrolyte interface, prevent side reactions, and facilitate ion transport. By reducing interfacial resistance and enhancing the stability of the electrochemical system, these strategies enable higher power outputs and better rate capabilities.
- Additives and dopants for performance enhancement: Various additives and dopants are employed to enhance the rate and power performance of room-temperature sodium-sulfur batteries. These include metal nanoparticles, conductive polymers, and heteroatom dopants that improve the electronic conductivity, catalytic activity, and structural stability of the battery components. By incorporating these additives in appropriate concentrations and distributions, the electrochemical kinetics and overall battery performance can be significantly improved at room temperature.
02 Electrolyte optimization for room-temperature operation
Specialized electrolyte formulations are essential for enabling sodium-sulfur batteries to operate efficiently at room temperature. These formulations typically include ionic liquids, polymer electrolytes, or solid-state electrolytes with additives that enhance sodium-ion conductivity while suppressing the shuttle effect of polysulfides. Optimized electrolytes contribute significantly to improved rate capability and power performance by facilitating faster ion transport and maintaining stability during cycling.Expand Specific Solutions03 Structural design and cell configuration
The structural design and configuration of room-temperature sodium-sulfur batteries significantly impact their rate and power performance. Innovative cell designs include 3D architectures, sandwich structures, and core-shell configurations that optimize the spatial distribution of active materials and facilitate efficient ion and electron transport. These designs help to accommodate volume changes during cycling and maintain structural integrity, resulting in enhanced power output and rate capability.Expand Specific Solutions04 Interface engineering and protective layers
Interface engineering and the application of protective layers are critical for improving the rate and power performance of room-temperature sodium-sulfur batteries. These approaches involve the use of functional coatings, buffer layers, or interlayers that stabilize the electrode-electrolyte interface, suppress side reactions, and facilitate ion transport. By mitigating interfacial resistance and enhancing the stability of the electrochemical interface, these strategies contribute to superior rate capability and power output.Expand Specific Solutions05 Advanced characterization and performance optimization techniques
Advanced characterization and optimization techniques are employed to enhance the rate and power performance of room-temperature sodium-sulfur batteries. These include in-situ/operando spectroscopy, electrochemical impedance spectroscopy, and computational modeling to understand reaction mechanisms and identify performance bottlenecks. Based on these insights, optimization strategies such as doping, defect engineering, and hierarchical structuring are implemented to achieve superior rate capability and power performance under various operating conditions.Expand Specific Solutions
Leading Companies and Research Institutions in Na-S Battery Field
The room-temperature sodium-sulfur (RT-Na-S) battery market is in an early growth phase, characterized by increasing research activity but limited commercial deployment. Current market size remains modest compared to lithium-ion technologies, though projections indicate significant expansion potential due to abundant raw materials and lower costs. From a technical maturity perspective, RT-Na-S batteries face challenges in rate capability and power performance. Leading players include NGK Insulators with established high-temperature Na-S technology now transitioning to room-temperature solutions, while academic institutions like Zhejiang University and Central South University contribute fundamental research. Commercial entities including LG Energy Solution, CATL, and SK Innovation are investing in development programs, with specialized companies like Honeycomb Battery and Lyten focusing on novel materials to overcome performance limitations.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a comprehensive room-temperature sodium-sulfur battery technology focusing on grid-scale energy storage applications. Their approach utilizes a carbon-sulfur composite cathode with a specialized pore structure design that maximizes active material utilization while minimizing polysulfide dissolution. LG's proprietary electrolyte formulation incorporates flame-retardant additives and optimized sodium salt concentrations that enable stable room-temperature operation with enhanced safety characteristics. Their anode design employs a sodium-tin alloy that provides improved cycling stability compared to pure sodium metal while maintaining high capacity. LG Energy Solution's manufacturing process leverages their extensive battery production expertise, utilizing roll-to-roll electrode fabrication techniques adapted for sodium-sulfur chemistry. Their cells demonstrate energy densities of 250-300 Wh/kg with power capabilities suitable for grid applications, achieving over 1500 cycles with 80% capacity retention at moderate rates. LG has also developed advanced battery management systems specifically calibrated for the unique characteristics of room-temperature sodium-sulfur chemistry, enabling precise state-of-charge estimation and thermal management.
Strengths: Established manufacturing expertise and global supply chain; comprehensive system-level approach including battery management; sodium-tin alloy anode provides enhanced safety and stability. Weaknesses: Lower energy density compared to some competing technologies; primarily focused on stationary applications rather than mobile use cases; rate capability limitations restrict high-power applications.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered commercial sodium-sulfur (NaS) battery technology since the 1980s, developing high-temperature (300°C) systems. For room-temperature sodium-sulfur batteries, NGK has developed innovative solid electrolyte materials that enable operation at ambient conditions while maintaining high energy density. Their approach incorporates a specialized beta-alumina solid electrolyte (BASE) modified for lower temperature conductivity, combined with a proprietary sulfur cathode structure that mitigates the shuttle effect. NGK's room-temperature NaS technology employs a carbon-sulfur composite cathode with optimized pore structure to enhance sulfur utilization and reaction kinetics. The company has demonstrated cells achieving energy densities of 350-400 Wh/kg with significantly improved cycle life compared to earlier room-temperature designs, maintaining approximately 80% capacity retention after 500 cycles at moderate discharge rates.
Strengths: Extensive experience with sodium-sulfur chemistry; established manufacturing infrastructure; proven reliability in grid applications. Weaknesses: Room-temperature versions still face challenges with lower power density compared to their high-temperature counterparts; sodium dendrite formation remains a concern for long-term stability.
Key Patents and Breakthroughs in Na-S Battery Technology
Sodium-sulfur battery, method for operating same, and use of phosphorus polysulfide as electrolyte additive in sodium-sulfur batteries
PatentWO2016202969A1
Innovation
- A sodium-sulfur battery design using a carbon-sulfur composite cathode, an organic solvent-based electrolyte with a conductive salt and phosphorus polysulfide as an additive to suppress the polysulfide shuttle, allowing operation at room temperature with enhanced energy efficiency and discharge capacity.
Material Supply Chain Analysis for Na-S Battery Production
The sodium-sulfur battery supply chain presents unique challenges and opportunities compared to traditional lithium-ion battery production. Raw material availability forms the foundation of this supply chain, with sodium resources being abundantly available worldwide through seawater and mineral deposits. Unlike lithium, sodium does not face significant geopolitical supply constraints, offering greater supply security. Global sodium reserves are estimated to be virtually unlimited, with production costs significantly lower than lithium.
Sulfur, the cathode material, is primarily sourced as a byproduct from petroleum refining and natural gas processing. The global sulfur market produces approximately 70 million tons annually, with prices ranging from $100-200 per ton, making it substantially more economical than lithium-based cathode materials. This abundance creates a cost advantage for Na-S battery production.
The manufacturing process requires specialized equipment for handling molten sodium and sulfur in high-temperature versions, though room-temperature variants have less stringent requirements. Key manufacturing components include sodium purification systems, sulfur processing equipment, and specialized separator production facilities. Current production capacity remains limited, with only a few manufacturers globally capable of large-scale Na-S battery production.
Supply chain bottlenecks primarily center around the beta-alumina solid electrolyte production for high-temperature versions, while room-temperature variants face challenges with separator materials that can effectively prevent polysulfide shuttling while maintaining adequate ionic conductivity. Carbon host materials for sulfur cathodes represent another potential constraint, requiring specialized manufacturing processes to create optimal porous structures.
Regional distribution of manufacturing capabilities shows concentration in Japan, China, and limited facilities in North America and Europe. NGK Insulators (Japan) maintains the dominant market position for high-temperature systems, while room-temperature Na-S battery production remains largely in research and pilot production phases.
Cost analysis reveals that room-temperature Na-S batteries offer potential production costs of $70-120 per kWh at scale, compared to $150-200 for lithium-ion batteries. However, achieving these economics requires overcoming technical challenges related to cycle life and power performance, which currently limit widespread commercialization despite the favorable raw material economics.
Sulfur, the cathode material, is primarily sourced as a byproduct from petroleum refining and natural gas processing. The global sulfur market produces approximately 70 million tons annually, with prices ranging from $100-200 per ton, making it substantially more economical than lithium-based cathode materials. This abundance creates a cost advantage for Na-S battery production.
The manufacturing process requires specialized equipment for handling molten sodium and sulfur in high-temperature versions, though room-temperature variants have less stringent requirements. Key manufacturing components include sodium purification systems, sulfur processing equipment, and specialized separator production facilities. Current production capacity remains limited, with only a few manufacturers globally capable of large-scale Na-S battery production.
Supply chain bottlenecks primarily center around the beta-alumina solid electrolyte production for high-temperature versions, while room-temperature variants face challenges with separator materials that can effectively prevent polysulfide shuttling while maintaining adequate ionic conductivity. Carbon host materials for sulfur cathodes represent another potential constraint, requiring specialized manufacturing processes to create optimal porous structures.
Regional distribution of manufacturing capabilities shows concentration in Japan, China, and limited facilities in North America and Europe. NGK Insulators (Japan) maintains the dominant market position for high-temperature systems, while room-temperature Na-S battery production remains largely in research and pilot production phases.
Cost analysis reveals that room-temperature Na-S batteries offer potential production costs of $70-120 per kWh at scale, compared to $150-200 for lithium-ion batteries. However, achieving these economics requires overcoming technical challenges related to cycle life and power performance, which currently limit widespread commercialization despite the favorable raw material economics.
Safety and Environmental Considerations for Na-S Battery Systems
Safety considerations for room-temperature sodium-sulfur (RT Na-S) batteries are paramount due to the reactive nature of sodium metal and sulfur compounds. Unlike high-temperature Na-S batteries operating at 300-350°C, RT Na-S systems present different but equally significant safety challenges. The primary concern involves sodium's high reactivity with moisture and oxygen, potentially causing fires or explosions if cell integrity is compromised. Additionally, during charging-discharging cycles, sodium dendrite formation can lead to internal short circuits, particularly at higher power rates.
Environmental considerations extend to both manufacturing processes and end-of-life management. The production of RT Na-S batteries involves less energy consumption compared to high-temperature versions, reducing the carbon footprint. However, the extraction of raw materials, particularly sulfur, must be evaluated for environmental impact. Sulfur, while abundant as a byproduct of petroleum refining, requires proper handling to prevent environmental contamination.
Containment strategies for RT Na-S batteries include advanced cell designs with robust separators and electrolytes that inhibit dendrite growth while maintaining power performance. Solid-state electrolytes show promise in enhancing safety by eliminating flammable liquid components, though often at the cost of reduced rate capability. Polymer-based or ceramic electrolytes provide physical barriers against sodium penetration while allowing sufficient ion transport.
Thermal management systems are essential even for room-temperature operation, as localized heating during rapid charging or high-power applications can trigger thermal runaway. Advanced battery management systems (BMS) with real-time monitoring capabilities can detect abnormal temperature fluctuations or voltage irregularities before safety incidents occur.
Regulatory frameworks for Na-S battery systems continue to evolve, with standards focusing on transportation safety, installation requirements, and recycling protocols. The UN Transportation of Dangerous Goods regulations classify sodium batteries according to hazard levels, imposing specific packaging and handling requirements. As RT Na-S technology advances toward commercialization, these regulations will likely become more specific to address the unique characteristics of room-temperature systems.
Recycling infrastructure for Na-S batteries remains underdeveloped compared to lithium-ion technologies. The recovery of sodium and sulfur compounds presents both challenges and opportunities, as these materials are relatively abundant but require specialized processes to separate safely. Developing efficient recycling pathways will be crucial for the long-term sustainability of RT Na-S battery technology, particularly as deployment scales increase.
Environmental considerations extend to both manufacturing processes and end-of-life management. The production of RT Na-S batteries involves less energy consumption compared to high-temperature versions, reducing the carbon footprint. However, the extraction of raw materials, particularly sulfur, must be evaluated for environmental impact. Sulfur, while abundant as a byproduct of petroleum refining, requires proper handling to prevent environmental contamination.
Containment strategies for RT Na-S batteries include advanced cell designs with robust separators and electrolytes that inhibit dendrite growth while maintaining power performance. Solid-state electrolytes show promise in enhancing safety by eliminating flammable liquid components, though often at the cost of reduced rate capability. Polymer-based or ceramic electrolytes provide physical barriers against sodium penetration while allowing sufficient ion transport.
Thermal management systems are essential even for room-temperature operation, as localized heating during rapid charging or high-power applications can trigger thermal runaway. Advanced battery management systems (BMS) with real-time monitoring capabilities can detect abnormal temperature fluctuations or voltage irregularities before safety incidents occur.
Regulatory frameworks for Na-S battery systems continue to evolve, with standards focusing on transportation safety, installation requirements, and recycling protocols. The UN Transportation of Dangerous Goods regulations classify sodium batteries according to hazard levels, imposing specific packaging and handling requirements. As RT Na-S technology advances toward commercialization, these regulations will likely become more specific to address the unique characteristics of room-temperature systems.
Recycling infrastructure for Na-S batteries remains underdeveloped compared to lithium-ion technologies. The recovery of sodium and sulfur compounds presents both challenges and opportunities, as these materials are relatively abundant but require specialized processes to separate safely. Developing efficient recycling pathways will be crucial for the long-term sustainability of RT Na-S battery technology, particularly as deployment scales increase.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!