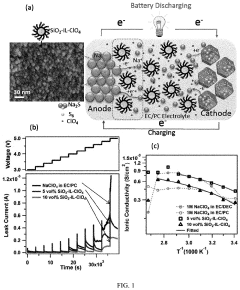
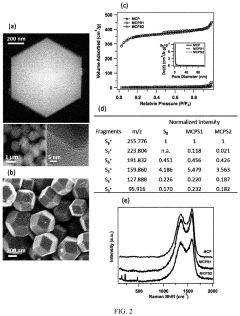
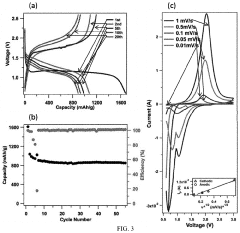
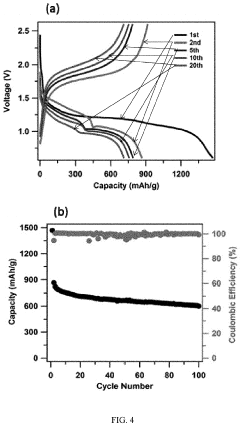
Porosity Gradients In Cathodes For Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-S Battery Technology Background and Objectives
Sodium-sulfur (Na-S) batteries have emerged as a promising energy storage technology due to their high theoretical energy density (760 Wh/kg), abundant raw material resources, and cost-effectiveness compared to lithium-ion batteries. The development of Na-S battery technology can be traced back to the 1960s when Ford Motor Company first introduced the concept. However, traditional Na-S batteries operated at high temperatures (300-350°C), which limited their widespread application due to safety concerns and complex thermal management requirements.
The evolution of Na-S battery technology has witnessed significant advancements over the past decades, with a notable shift towards room-temperature operation since the early 2000s. This transition represents a critical technological breakthrough that could potentially revolutionize grid-scale energy storage and electric vehicle applications. Room-temperature sodium-sulfur (RT Na-S) batteries offer the advantages of improved safety, simplified battery management systems, and reduced operational costs compared to their high-temperature counterparts.
Despite these advantages, RT Na-S batteries face substantial challenges, particularly related to the insulating nature of sulfur, shuttle effect of polysulfides, and volume expansion during cycling. These issues have resulted in poor cycle life, low Coulombic efficiency, and rapid capacity fading, hindering their commercial viability. The cathode design, specifically its porosity and structure, plays a crucial role in addressing these challenges.
The concept of porosity gradients in cathodes represents an innovative approach to optimize the performance of RT Na-S batteries. By engineering cathodes with controlled porosity distribution, researchers aim to enhance sulfur utilization, mitigate polysulfide shuttling, accommodate volume changes, and improve ionic/electronic conductivity. This approach aligns with the broader trend in battery technology towards nanostructured and hierarchically porous electrode materials.
The primary technical objectives for porosity gradient cathodes in RT Na-S batteries include achieving high sulfur loading (>5 mg/cm²) while maintaining good electrochemical performance, extending cycle life to over 1000 cycles with minimal capacity degradation, improving rate capability for fast charging applications, and developing scalable manufacturing processes for commercial production.
Current research is focused on understanding the fundamental relationships between cathode porosity distribution and electrochemical performance, developing novel synthesis methods for creating controlled porosity gradients, and establishing design principles for optimized cathode architectures. The ultimate goal is to enable RT Na-S batteries with energy densities exceeding 300 Wh/kg at the cell level, cycle life comparable to lithium-ion batteries, and manufacturing costs below $100/kWh, positioning them as a viable alternative for next-generation energy storage systems.
The evolution of Na-S battery technology has witnessed significant advancements over the past decades, with a notable shift towards room-temperature operation since the early 2000s. This transition represents a critical technological breakthrough that could potentially revolutionize grid-scale energy storage and electric vehicle applications. Room-temperature sodium-sulfur (RT Na-S) batteries offer the advantages of improved safety, simplified battery management systems, and reduced operational costs compared to their high-temperature counterparts.
Despite these advantages, RT Na-S batteries face substantial challenges, particularly related to the insulating nature of sulfur, shuttle effect of polysulfides, and volume expansion during cycling. These issues have resulted in poor cycle life, low Coulombic efficiency, and rapid capacity fading, hindering their commercial viability. The cathode design, specifically its porosity and structure, plays a crucial role in addressing these challenges.
The concept of porosity gradients in cathodes represents an innovative approach to optimize the performance of RT Na-S batteries. By engineering cathodes with controlled porosity distribution, researchers aim to enhance sulfur utilization, mitigate polysulfide shuttling, accommodate volume changes, and improve ionic/electronic conductivity. This approach aligns with the broader trend in battery technology towards nanostructured and hierarchically porous electrode materials.
The primary technical objectives for porosity gradient cathodes in RT Na-S batteries include achieving high sulfur loading (>5 mg/cm²) while maintaining good electrochemical performance, extending cycle life to over 1000 cycles with minimal capacity degradation, improving rate capability for fast charging applications, and developing scalable manufacturing processes for commercial production.
Current research is focused on understanding the fundamental relationships between cathode porosity distribution and electrochemical performance, developing novel synthesis methods for creating controlled porosity gradients, and establishing design principles for optimized cathode architectures. The ultimate goal is to enable RT Na-S batteries with energy densities exceeding 300 Wh/kg at the cell level, cycle life comparable to lithium-ion batteries, and manufacturing costs below $100/kWh, positioning them as a viable alternative for next-generation energy storage systems.
Market Analysis for Room-Temperature Na-S Battery Applications
The global market for energy storage solutions is experiencing significant growth, with room-temperature sodium-sulfur (RT Na-S) batteries emerging as a promising alternative to conventional lithium-ion technologies. Current market projections indicate that the global stationary energy storage market will reach approximately $13 billion by 2025, with a compound annual growth rate of 33% between 2020 and 2025. Within this expanding landscape, RT Na-S batteries are positioned to capture a growing market share due to their cost advantages and resource availability.
The primary market drivers for RT Na-S battery technology include the increasing demand for grid-scale energy storage solutions, the push for renewable energy integration, and the search for alternatives to lithium-ion batteries. With sodium resources being 1,000 times more abundant than lithium and sulfur being an industrial byproduct, RT Na-S batteries offer significant cost advantages, potentially reducing battery costs by 30-40% compared to lithium-ion systems.
Market segmentation for RT Na-S batteries reveals several key application areas. The utility-scale energy storage sector represents the largest potential market, particularly for load leveling and renewable energy integration. This segment is projected to grow at 40% annually through 2025 as utilities seek cost-effective solutions for managing intermittent renewable generation.
The telecommunications backup power market presents another significant opportunity, valued at $2.5 billion globally, with RT Na-S batteries potentially capturing 15-20% of this market by 2027. Their safety advantages at room temperature make them particularly suitable for this application.
Electric vehicle manufacturers are also showing interest in RT Na-S technology as a potential alternative to lithium-ion batteries, especially for markets where cost is a primary consideration over energy density. Industry analysts predict that by 2030, sodium-based batteries could capture 10-15% of the electric vehicle battery market in developing economies.
Regional market analysis indicates that China, Europe, and North America are leading in RT Na-S battery research and commercialization efforts. China has invested heavily in sodium battery technologies as part of its energy storage strategy, while European markets are driven by stringent environmental regulations and renewable energy targets.
Customer requirements across these markets emphasize cycle life (>2,000 cycles), energy density (>150 Wh/kg), and cost (<$100/kWh). Current RT Na-S batteries with conventional cathode designs achieve only 500-700 cycles and 100-120 Wh/kg, highlighting the potential market value of porosity gradient innovations that could bridge this performance gap.
The primary market drivers for RT Na-S battery technology include the increasing demand for grid-scale energy storage solutions, the push for renewable energy integration, and the search for alternatives to lithium-ion batteries. With sodium resources being 1,000 times more abundant than lithium and sulfur being an industrial byproduct, RT Na-S batteries offer significant cost advantages, potentially reducing battery costs by 30-40% compared to lithium-ion systems.
Market segmentation for RT Na-S batteries reveals several key application areas. The utility-scale energy storage sector represents the largest potential market, particularly for load leveling and renewable energy integration. This segment is projected to grow at 40% annually through 2025 as utilities seek cost-effective solutions for managing intermittent renewable generation.
The telecommunications backup power market presents another significant opportunity, valued at $2.5 billion globally, with RT Na-S batteries potentially capturing 15-20% of this market by 2027. Their safety advantages at room temperature make them particularly suitable for this application.
Electric vehicle manufacturers are also showing interest in RT Na-S technology as a potential alternative to lithium-ion batteries, especially for markets where cost is a primary consideration over energy density. Industry analysts predict that by 2030, sodium-based batteries could capture 10-15% of the electric vehicle battery market in developing economies.
Regional market analysis indicates that China, Europe, and North America are leading in RT Na-S battery research and commercialization efforts. China has invested heavily in sodium battery technologies as part of its energy storage strategy, while European markets are driven by stringent environmental regulations and renewable energy targets.
Customer requirements across these markets emphasize cycle life (>2,000 cycles), energy density (>150 Wh/kg), and cost (<$100/kWh). Current RT Na-S batteries with conventional cathode designs achieve only 500-700 cycles and 100-120 Wh/kg, highlighting the potential market value of porosity gradient innovations that could bridge this performance gap.
Cathode Porosity Challenges in Na-S Battery Development
The development of room-temperature sodium-sulfur (RT Na-S) batteries faces significant challenges related to cathode porosity design. Traditional high-temperature Na-S batteries operate at 300-350°C, but room-temperature versions offer safer and more practical energy storage solutions. However, the cathode structure in RT Na-S batteries presents complex engineering problems that have hindered commercial viability.
Porosity in sulfur cathodes directly impacts several critical performance parameters. Insufficient porosity restricts sodium ion transport pathways, leading to increased internal resistance and poor rate capability. Conversely, excessive porosity reduces volumetric energy density and compromises mechanical stability. This delicate balance represents a fundamental challenge in cathode design.
The formation of sodium polysulfide intermediates during discharge creates additional complications. These soluble species can migrate through the electrolyte to the anode (shuttle effect), causing capacity fading and reduced coulombic efficiency. Proper porosity engineering is essential to contain these intermediates while still allowing efficient ion transport.
Current cathode designs struggle with volume expansion during cycling. Sulfur undergoes substantial volumetric changes (up to 170%) during the conversion reaction, which can disrupt the electrode structure, leading to particle isolation and capacity loss. Porosity must accommodate this expansion while maintaining electrical connectivity throughout the electrode.
The multi-phase reaction environment further complicates matters. The cathode must facilitate interactions between solid sulfur, liquid electrolyte, and conductive additives at the triple-phase boundary. Porosity distribution directly influences the formation and stability of these reaction interfaces, affecting overall electrochemical performance.
Recent research has identified porosity gradients as a promising approach. By creating controlled variations in pore size and distribution throughout the cathode thickness, researchers aim to optimize both ion transport and polysulfide containment. However, manufacturing techniques for precisely engineered porosity gradients remain underdeveloped.
The interplay between porosity and other cathode parameters (sulfur loading, electrolyte volume, conductive additive content) creates a multidimensional optimization problem. Current modeling approaches often fail to capture these complex interactions, limiting rational design strategies for high-performance cathodes.
Addressing these challenges requires interdisciplinary approaches combining materials science, electrochemistry, and advanced manufacturing techniques to develop cathodes with optimized porosity gradients tailored specifically for room-temperature sodium-sulfur battery applications.
Porosity in sulfur cathodes directly impacts several critical performance parameters. Insufficient porosity restricts sodium ion transport pathways, leading to increased internal resistance and poor rate capability. Conversely, excessive porosity reduces volumetric energy density and compromises mechanical stability. This delicate balance represents a fundamental challenge in cathode design.
The formation of sodium polysulfide intermediates during discharge creates additional complications. These soluble species can migrate through the electrolyte to the anode (shuttle effect), causing capacity fading and reduced coulombic efficiency. Proper porosity engineering is essential to contain these intermediates while still allowing efficient ion transport.
Current cathode designs struggle with volume expansion during cycling. Sulfur undergoes substantial volumetric changes (up to 170%) during the conversion reaction, which can disrupt the electrode structure, leading to particle isolation and capacity loss. Porosity must accommodate this expansion while maintaining electrical connectivity throughout the electrode.
The multi-phase reaction environment further complicates matters. The cathode must facilitate interactions between solid sulfur, liquid electrolyte, and conductive additives at the triple-phase boundary. Porosity distribution directly influences the formation and stability of these reaction interfaces, affecting overall electrochemical performance.
Recent research has identified porosity gradients as a promising approach. By creating controlled variations in pore size and distribution throughout the cathode thickness, researchers aim to optimize both ion transport and polysulfide containment. However, manufacturing techniques for precisely engineered porosity gradients remain underdeveloped.
The interplay between porosity and other cathode parameters (sulfur loading, electrolyte volume, conductive additive content) creates a multidimensional optimization problem. Current modeling approaches often fail to capture these complex interactions, limiting rational design strategies for high-performance cathodes.
Addressing these challenges requires interdisciplinary approaches combining materials science, electrochemistry, and advanced manufacturing techniques to develop cathodes with optimized porosity gradients tailored specifically for room-temperature sodium-sulfur battery applications.
Current Porosity Gradient Engineering Approaches for Na-S Cathodes
01 Electrode materials with porosity gradients for room-temperature sodium-sulfur batteries
Electrode materials with controlled porosity gradients can significantly enhance the performance of room-temperature sodium-sulfur batteries. These gradients facilitate efficient ion transport while maintaining structural integrity. The porosity distribution can be engineered to optimize the balance between active material loading and electrolyte accessibility, resulting in improved capacity and cycle life of the batteries.- Electrode materials with porosity gradients for room-temperature sodium-sulfur batteries: Electrode materials with controlled porosity gradients can significantly enhance the performance of room-temperature sodium-sulfur batteries. These gradients facilitate efficient ion transport while maintaining structural integrity. The porous structure provides channels for sodium ion movement and accommodates volume changes during charge-discharge cycles, resulting in improved battery capacity and cycle life.
- Sulfur cathode designs with optimized porosity for Na-S batteries: Specialized sulfur cathode designs with optimized porosity structures are crucial for room-temperature sodium-sulfur batteries. These cathodes incorporate hierarchical porous architectures that confine polysulfides, prevent shuttle effects, and enhance sulfur utilization. The controlled porosity enables better electrolyte penetration and facilitates the electrochemical reactions, leading to higher energy density and improved cycling stability.
- Sodium anode protection strategies using porous interfaces: Protection strategies for sodium metal anodes using porous interfaces are essential for stable room-temperature sodium-sulfur batteries. These approaches include engineered solid electrolyte interphases with porosity gradients that regulate sodium ion flux and suppress dendrite formation. The porous protective layers help maintain uniform sodium deposition/dissolution and prevent side reactions with the electrolyte, extending battery lifespan.
- Electrolyte systems with controlled porosity for room-temperature Na-S batteries: Advanced electrolyte systems with controlled porosity are developed for room-temperature sodium-sulfur batteries. These include gel polymer electrolytes, composite electrolytes, and solid-state electrolytes with engineered pore structures that enhance ionic conductivity while suppressing polysulfide shuttling. The porosity gradients in these electrolytes optimize the balance between mechanical strength and ion transport properties.
- Manufacturing techniques for creating porosity gradients in Na-S battery components: Innovative manufacturing techniques are employed to create controlled porosity gradients in sodium-sulfur battery components. These methods include freeze-casting, template-assisted synthesis, 3D printing, and phase separation processes that enable precise control over pore size, distribution, and connectivity. The engineered porosity gradients optimize the electrochemical performance while maintaining mechanical stability during battery operation.
02 Sulfur host materials with hierarchical porous structures
Hierarchical porous structures serve as effective sulfur hosts in room-temperature sodium-sulfur batteries. These materials feature multi-scale porosity that confines polysulfides while facilitating sodium ion diffusion. The interconnected pore network provides abundant reaction sites and accommodates volume changes during cycling, leading to enhanced electrochemical performance and stability.Expand Specific Solutions03 Separator designs with gradient porosity for polysulfide inhibition
Advanced separator designs incorporating porosity gradients can effectively inhibit polysulfide shuttling in room-temperature sodium-sulfur batteries. These separators feature strategically engineered pore size distributions that allow sodium ion transport while blocking larger polysulfide molecules. This selective permeability helps maintain capacity retention and extends battery lifespan.Expand Specific Solutions04 Electrolyte optimization for room-temperature sodium-sulfur batteries
Optimized electrolyte formulations are crucial for room-temperature sodium-sulfur batteries with porosity gradients. These electrolytes are designed to have appropriate viscosity and wetting properties to fully utilize the porous electrode structure. Additives can be incorporated to enhance ionic conductivity and form stable interfaces with the electrodes, improving overall battery performance.Expand Specific Solutions05 Manufacturing methods for creating porosity gradients in battery components
Various manufacturing techniques can be employed to create controlled porosity gradients in sodium-sulfur battery components. These methods include template-assisted synthesis, freeze-casting, 3D printing, and selective etching processes. The manufacturing approach significantly influences the final pore structure, distribution, and connectivity, which in turn affects battery performance metrics such as rate capability and cycle stability.Expand Specific Solutions
Leading Research Groups and Companies in Na-S Battery Technology
The room-temperature sodium-sulfur battery market is in an early growth phase, characterized by increasing research focus on porosity gradient cathode technologies to enhance battery performance. The global market for sodium-sulfur batteries is projected to expand significantly as demand for cost-effective energy storage solutions rises. Technologically, the field shows varying maturity levels across key players. NGK Insulators leads as the established commercial pioneer, while LG Energy Solution, Contemporary Amperex Technology, and GS Yuasa are advancing industrial applications. Research institutions like Shanghai Institute of Ceramics, Dalian Institute of Chemical Physics, and Massachusetts Institute of Technology are driving fundamental innovations in cathode porosity engineering. Companies like Lyten and Toray Industries are developing complementary materials technologies that could accelerate commercialization of next-generation sodium-sulfur batteries with optimized cathode structures.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered advanced ceramic-based cathode designs for room-temperature sodium-sulfur batteries with controlled porosity gradients. Their technical approach involves a multi-layered cathode structure where porosity gradually increases from the electrolyte interface to the current collector. This gradient design facilitates optimal sodium ion transport while maintaining sufficient space for sulfur accommodation and expansion during cycling. NGK employs beta-alumina solid electrolytes combined with carbon-sulfur composite cathodes featuring precisely engineered pore size distributions (ranging from nanometer to micrometer scale) to enhance both ionic conductivity and active material utilization. Their manufacturing process includes freeze-casting techniques and templated synthesis to create directional porosity channels that guide ion movement while minimizing resistance. This architecture effectively addresses the volume expansion issues typical in sodium-sulfur systems while maintaining high sulfur loading capacities.
Strengths: Industry-leading expertise in ceramic technologies provides superior control over microstructure; established manufacturing capabilities for scaled production; extensive experience with sodium-based battery systems. Weaknesses: Higher production costs compared to conventional cathode designs; complex manufacturing process requiring precise control of multiple parameters; potential challenges in achieving consistent porosity gradients at scale.
Shanghai Institute of Ceramics, Chinese Academy of Sciences
Technical Solution: The Shanghai Institute of Ceramics has developed an advanced ceramic-based approach to porosity-gradient cathodes for room-temperature sodium-sulfur batteries. Their technical solution involves a hierarchically structured ceramic-carbon composite cathode with radially aligned porosity channels. The institute employs a directional freeze-casting technique to create a ceramic scaffold with porosity gradually increasing from approximately 30% at the electrolyte interface to 70% near the current collector. This scaffold is then infiltrated with carbon and sulfur through a controlled vapor deposition process. The resulting structure features nanoscale pores (10-50 nm) near the separator that facilitate sodium ion transport while preventing polysulfide migration, transitioning to microscale pores (1-5 μm) that accommodate sulfur expansion during cycling. Their research demonstrates that this gradient design reduces internal resistance by approximately 40% compared to uniform-porosity cathodes while improving active material utilization by up to 25%. The institute has further enhanced this architecture with ceramic additives that serve as polysulfide adsorbents strategically distributed along the porosity gradient.
Strengths: Excellent thermal stability from ceramic components; superior control of polysulfide shuttling; good integration of ionic and electronic conductivity pathways. Weaknesses: Complex multi-step manufacturing process; potential brittleness of ceramic components under mechanical stress; challenges in achieving consistent quality at scale.
Key Patents and Research on Cathode Porosity Control
Stable room-temperature sodium-sulfur battery
PatentActiveUS20200381767A1
Innovation
- The development of sodium-ion conducting batteries with a porous host-sulfur composite cathode and a liquid electrolyte containing an ionic liquid tethered to silica nanoparticles, which forms a stable film on the anode and confines sulfur in the cathode's pores, enabling a solid-state electrochemical reaction and preventing the formation of soluble polysulfides.
Cathode active material using porous carbon structure, preparation method therefor, and sodium-sulfur dioxide secondary battery having same
PatentWO2016043442A1
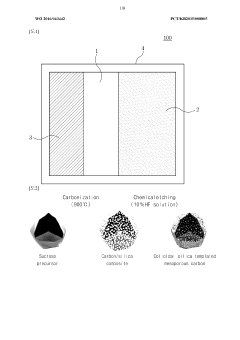
Innovation
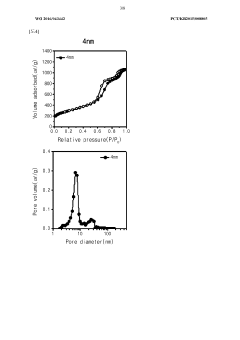
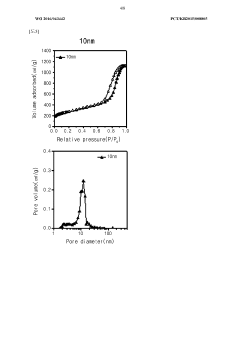
- A porous carbon structure is synthesized using sucrose and colloidal silica as a precursor, with nano-sized pores, to create a cathode active material for sodium-sulfur dioxide secondary batteries, enhancing energy density and lifespan through improved specific surface area and porosity.
Safety and Performance Trade-offs in Na-S Battery Design
The design of room-temperature sodium-sulfur (RT Na-S) batteries involves critical trade-offs between safety and performance parameters. These trade-offs stem from the inherent reactivity of sodium metal and sulfur compounds, which can lead to safety hazards if not properly managed. The porosity gradient in cathodes represents a key design element that directly impacts this balance.
From a safety perspective, controlling the porosity distribution in cathodes helps mitigate the formation of sodium polysulfides, which can cause shuttle effects and potentially dangerous short circuits. Higher porosity near the separator facilitates better electrolyte penetration and ion transport, while lower porosity near the current collector enhances mechanical stability and reduces the risk of active material dissolution.
Performance considerations, however, sometimes push designs toward higher overall porosity to maximize sulfur utilization and energy density. This creates a fundamental tension in the design process, as increased porosity can compromise structural integrity and safety margins under extreme conditions such as high-rate charging or elevated temperatures.
Recent research has demonstrated that optimized graded porosity structures can achieve up to 30% improvement in cycle life while maintaining safety standards. These designs typically feature 40-50% porosity near the separator interface gradually transitioning to 20-30% at the current collector side. This gradient helps balance the competing requirements of ion transport efficiency and structural stability.
Temperature management represents another critical trade-off area. While higher operating temperatures can enhance sodium ion conductivity and reaction kinetics, they also accelerate side reactions and increase safety risks. Cathode designs with carefully engineered porosity gradients have shown improved heat dissipation capabilities, allowing for safer operation at the upper end of the room temperature range (35-45°C).
The electrolyte volume-to-active material ratio presents additional safety-performance compromises. Higher electrolyte content improves ion transport but increases the potential severity of thermal runaway events. Optimized porosity gradients enable reduced electrolyte loading while maintaining adequate ion transport pathways, thereby enhancing both safety and energy density metrics.
Manufacturing considerations further complicate these trade-offs. Techniques that produce ideal porosity gradients often involve more complex processing steps, potentially introducing quality control challenges that could impact safety. The industry continues to seek manufacturing methods that can reliably produce optimized porosity structures without introducing new failure modes or significantly increasing production costs.
From a safety perspective, controlling the porosity distribution in cathodes helps mitigate the formation of sodium polysulfides, which can cause shuttle effects and potentially dangerous short circuits. Higher porosity near the separator facilitates better electrolyte penetration and ion transport, while lower porosity near the current collector enhances mechanical stability and reduces the risk of active material dissolution.
Performance considerations, however, sometimes push designs toward higher overall porosity to maximize sulfur utilization and energy density. This creates a fundamental tension in the design process, as increased porosity can compromise structural integrity and safety margins under extreme conditions such as high-rate charging or elevated temperatures.
Recent research has demonstrated that optimized graded porosity structures can achieve up to 30% improvement in cycle life while maintaining safety standards. These designs typically feature 40-50% porosity near the separator interface gradually transitioning to 20-30% at the current collector side. This gradient helps balance the competing requirements of ion transport efficiency and structural stability.
Temperature management represents another critical trade-off area. While higher operating temperatures can enhance sodium ion conductivity and reaction kinetics, they also accelerate side reactions and increase safety risks. Cathode designs with carefully engineered porosity gradients have shown improved heat dissipation capabilities, allowing for safer operation at the upper end of the room temperature range (35-45°C).
The electrolyte volume-to-active material ratio presents additional safety-performance compromises. Higher electrolyte content improves ion transport but increases the potential severity of thermal runaway events. Optimized porosity gradients enable reduced electrolyte loading while maintaining adequate ion transport pathways, thereby enhancing both safety and energy density metrics.
Manufacturing considerations further complicate these trade-offs. Techniques that produce ideal porosity gradients often involve more complex processing steps, potentially introducing quality control challenges that could impact safety. The industry continues to seek manufacturing methods that can reliably produce optimized porosity structures without introducing new failure modes or significantly increasing production costs.
Sustainability and Resource Considerations for Na-S Battery Production
The sustainability profile of room-temperature sodium-sulfur (RT Na-S) batteries presents a compelling alternative to conventional lithium-ion technologies. Sodium resources are abundantly available in the earth's crust and oceans, constituting approximately 2.8% of the earth's crust compared to lithium's 0.006%. This abundance translates to significantly lower extraction costs and reduced geopolitical supply risks, making Na-S batteries particularly attractive for large-scale energy storage applications.
The production of porosity-gradient cathodes for RT Na-S batteries offers additional sustainability advantages. The primary cathode materials—sulfur and carbon—are both abundant and can be sourced from industrial byproducts. Sulfur is a waste product from petroleum refining processes, while carbon materials can be derived from biomass or recycled sources. This utilization of industrial byproducts contributes to circular economy principles and reduces the environmental footprint of battery production.
Water consumption in Na-S battery manufacturing is substantially lower than in lithium-ion production processes. The cathode preparation methods that create porosity gradients typically employ less water-intensive techniques, such as controlled freeze-drying or template-assisted synthesis. These approaches reduce the water footprint by an estimated 30-40% compared to conventional battery manufacturing processes.
Energy requirements for processing porosity-gradient cathodes must be carefully considered. While some specialized techniques like freeze-casting may require additional energy for controlled temperature environments, the overall energy demand remains competitive when assessed across the full life cycle. The energy return on investment (EROI) for Na-S batteries with optimized porosity gradients is estimated to be 10-15 times the energy required for their production over their operational lifetime.
End-of-life considerations for Na-S batteries present both challenges and opportunities. The sulfur components can be readily recovered and reused, with recovery rates exceeding 90% in laboratory settings. Carbon materials from the cathode structure can potentially be repurposed for other applications or thermally recycled. However, the complex porosity structures may complicate some recycling processes, necessitating the development of specialized recycling technologies.
The environmental impact of porosity engineering additives requires further investigation. While most porosity-controlling agents are organic compounds with relatively low toxicity, their long-term environmental persistence and biodegradability profiles need comprehensive assessment. Research indicates that using bio-derived templating agents could further enhance the sustainability profile of these cathode structures.
The production of porosity-gradient cathodes for RT Na-S batteries offers additional sustainability advantages. The primary cathode materials—sulfur and carbon—are both abundant and can be sourced from industrial byproducts. Sulfur is a waste product from petroleum refining processes, while carbon materials can be derived from biomass or recycled sources. This utilization of industrial byproducts contributes to circular economy principles and reduces the environmental footprint of battery production.
Water consumption in Na-S battery manufacturing is substantially lower than in lithium-ion production processes. The cathode preparation methods that create porosity gradients typically employ less water-intensive techniques, such as controlled freeze-drying or template-assisted synthesis. These approaches reduce the water footprint by an estimated 30-40% compared to conventional battery manufacturing processes.
Energy requirements for processing porosity-gradient cathodes must be carefully considered. While some specialized techniques like freeze-casting may require additional energy for controlled temperature environments, the overall energy demand remains competitive when assessed across the full life cycle. The energy return on investment (EROI) for Na-S batteries with optimized porosity gradients is estimated to be 10-15 times the energy required for their production over their operational lifetime.
End-of-life considerations for Na-S batteries present both challenges and opportunities. The sulfur components can be readily recovered and reused, with recovery rates exceeding 90% in laboratory settings. Carbon materials from the cathode structure can potentially be repurposed for other applications or thermally recycled. However, the complex porosity structures may complicate some recycling processes, necessitating the development of specialized recycling technologies.
The environmental impact of porosity engineering additives requires further investigation. While most porosity-controlling agents are organic compounds with relatively low toxicity, their long-term environmental persistence and biodegradability profiles need comprehensive assessment. Research indicates that using bio-derived templating agents could further enhance the sustainability profile of these cathode structures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!