Grid Storage Value Stacking With Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-S Battery Technology Background and Objectives
Sodium-sulfur (Na-S) battery technology has evolved significantly since its inception in the 1960s by Ford Motor Company. Initially developed as high-temperature systems operating at 300-350°C, these batteries faced substantial challenges in safety, thermal management, and practical deployment despite their promising energy density and use of abundant, low-cost materials. The technological evolution has now reached a critical juncture with the emergence of room-temperature sodium-sulfur batteries, representing a paradigm shift in grid storage applications.
The fundamental chemistry of Na-S batteries leverages the reaction between sodium and sulfur to store and release electrical energy. Traditional high-temperature versions utilize molten sodium and sulfur separated by a solid beta-alumina ceramic electrolyte. Room-temperature variants employ various strategies including polymer electrolytes, liquid electrolytes with additives, and advanced electrode designs to enable sodium-sulfur electrochemistry at ambient conditions without compromising performance metrics.
Current technological objectives for room-temperature Na-S batteries focus on addressing several key challenges. Primary among these is mitigating the shuttle effect, where soluble polysulfide intermediates migrate between electrodes, causing capacity fade and efficiency losses. Researchers aim to develop advanced separator technologies and electrode architectures that minimize this phenomenon while maintaining high ionic conductivity.
Another critical objective involves improving the cycling stability and calendar life of these systems. Room-temperature Na-S batteries currently demonstrate limited cycle life compared to lithium-ion alternatives, necessitating innovations in electrode formulations, electrolyte compositions, and cell design to achieve the 10+ year operational lifetimes required for grid applications.
Energy density enhancement represents a third major objective, with current room-temperature Na-S systems typically delivering 100-150 Wh/kg. Research efforts target pushing these values toward 200-250 Wh/kg through sulfur utilization optimization, sodium anode protection strategies, and advanced cell engineering approaches.
The value stacking potential of these batteries for grid applications drives specific performance objectives related to response time, round-trip efficiency, and operational flexibility. Developers aim to achieve sub-second response capabilities for frequency regulation services while maintaining round-trip efficiencies above 80% for energy arbitrage applications.
Safety and environmental considerations further shape technology objectives, with emphasis on non-flammable electrolyte formulations, thermal runaway prevention, and end-of-life recyclability. These factors are particularly important for grid-scale deployments where safety incidents could impact public perception and regulatory approval.
The fundamental chemistry of Na-S batteries leverages the reaction between sodium and sulfur to store and release electrical energy. Traditional high-temperature versions utilize molten sodium and sulfur separated by a solid beta-alumina ceramic electrolyte. Room-temperature variants employ various strategies including polymer electrolytes, liquid electrolytes with additives, and advanced electrode designs to enable sodium-sulfur electrochemistry at ambient conditions without compromising performance metrics.
Current technological objectives for room-temperature Na-S batteries focus on addressing several key challenges. Primary among these is mitigating the shuttle effect, where soluble polysulfide intermediates migrate between electrodes, causing capacity fade and efficiency losses. Researchers aim to develop advanced separator technologies and electrode architectures that minimize this phenomenon while maintaining high ionic conductivity.
Another critical objective involves improving the cycling stability and calendar life of these systems. Room-temperature Na-S batteries currently demonstrate limited cycle life compared to lithium-ion alternatives, necessitating innovations in electrode formulations, electrolyte compositions, and cell design to achieve the 10+ year operational lifetimes required for grid applications.
Energy density enhancement represents a third major objective, with current room-temperature Na-S systems typically delivering 100-150 Wh/kg. Research efforts target pushing these values toward 200-250 Wh/kg through sulfur utilization optimization, sodium anode protection strategies, and advanced cell engineering approaches.
The value stacking potential of these batteries for grid applications drives specific performance objectives related to response time, round-trip efficiency, and operational flexibility. Developers aim to achieve sub-second response capabilities for frequency regulation services while maintaining round-trip efficiencies above 80% for energy arbitrage applications.
Safety and environmental considerations further shape technology objectives, with emphasis on non-flammable electrolyte formulations, thermal runaway prevention, and end-of-life recyclability. These factors are particularly important for grid-scale deployments where safety incidents could impact public perception and regulatory approval.
Grid Storage Market Demand Analysis
The global grid storage market is experiencing unprecedented growth, driven by the increasing integration of renewable energy sources and the need for grid stability. As of 2023, the market size has reached approximately $8.5 billion and is projected to grow at a CAGR of 24% through 2030, potentially reaching $45 billion by the end of the decade. This remarkable expansion reflects the critical role that energy storage systems play in modern power infrastructure.
Room-temperature sodium-sulfur (RT-Na-S) batteries represent a particularly promising technology within this expanding market. Unlike traditional lithium-ion solutions, RT-Na-S batteries offer significant cost advantages due to the abundant nature of their core materials. Sodium is approximately 1,000 times more abundant in the Earth's crust than lithium, potentially reducing raw material costs by 30-40% compared to lithium-based alternatives.
The demand for grid-scale storage solutions is being driven by several converging factors. First, the increasing penetration of variable renewable energy sources such as wind and solar necessitates effective storage solutions to manage intermittency issues. Countries with ambitious renewable energy targets, including Germany (65% by 2030), China (35% by 2030), and the United States (various state-level commitments), are creating substantial market pull for advanced storage technologies.
Regulatory frameworks are increasingly favorable for storage deployment. For instance, FERC Order 841 in the United States has opened wholesale electricity markets to energy storage participation, while the European Union's Clean Energy Package explicitly recognizes storage as a distinct asset class in electricity markets. These regulatory developments have created new revenue opportunities for storage assets.
Value stacking—the ability to provide multiple grid services from a single storage asset—has emerged as a critical market requirement. RT-Na-S batteries excel in this regard due to their operational flexibility. Market analysis indicates that storage systems capable of providing multiple services (frequency regulation, peak shaving, renewable integration, etc.) can increase their revenue potential by 40-60% compared to single-service applications.
The commercial and industrial (C&I) segment represents a particularly promising market for RT-Na-S technology. With electricity costs for industrial consumers rising by an average of 15% globally over the past five years, behind-the-meter storage solutions that can reduce demand charges and provide backup power are seeing increased adoption. This segment is expected to grow at 32% annually through 2028, outpacing the overall storage market.
Geographically, the Asia-Pacific region currently leads in grid storage deployment, accounting for 45% of global installations, followed by North America (30%) and Europe (20%). However, emerging markets in Africa and South America are showing accelerated growth rates as they seek to improve grid reliability and integrate renewable resources.
Room-temperature sodium-sulfur (RT-Na-S) batteries represent a particularly promising technology within this expanding market. Unlike traditional lithium-ion solutions, RT-Na-S batteries offer significant cost advantages due to the abundant nature of their core materials. Sodium is approximately 1,000 times more abundant in the Earth's crust than lithium, potentially reducing raw material costs by 30-40% compared to lithium-based alternatives.
The demand for grid-scale storage solutions is being driven by several converging factors. First, the increasing penetration of variable renewable energy sources such as wind and solar necessitates effective storage solutions to manage intermittency issues. Countries with ambitious renewable energy targets, including Germany (65% by 2030), China (35% by 2030), and the United States (various state-level commitments), are creating substantial market pull for advanced storage technologies.
Regulatory frameworks are increasingly favorable for storage deployment. For instance, FERC Order 841 in the United States has opened wholesale electricity markets to energy storage participation, while the European Union's Clean Energy Package explicitly recognizes storage as a distinct asset class in electricity markets. These regulatory developments have created new revenue opportunities for storage assets.
Value stacking—the ability to provide multiple grid services from a single storage asset—has emerged as a critical market requirement. RT-Na-S batteries excel in this regard due to their operational flexibility. Market analysis indicates that storage systems capable of providing multiple services (frequency regulation, peak shaving, renewable integration, etc.) can increase their revenue potential by 40-60% compared to single-service applications.
The commercial and industrial (C&I) segment represents a particularly promising market for RT-Na-S technology. With electricity costs for industrial consumers rising by an average of 15% globally over the past five years, behind-the-meter storage solutions that can reduce demand charges and provide backup power are seeing increased adoption. This segment is expected to grow at 32% annually through 2028, outpacing the overall storage market.
Geographically, the Asia-Pacific region currently leads in grid storage deployment, accounting for 45% of global installations, followed by North America (30%) and Europe (20%). However, emerging markets in Africa and South America are showing accelerated growth rates as they seek to improve grid reliability and integrate renewable resources.
Room-Temperature Na-S Battery Development Status
Room-temperature sodium-sulfur (RT Na-S) batteries have emerged as a promising alternative to conventional high-temperature Na-S systems, which typically operate at 300-350°C. The development of RT Na-S batteries has progressed significantly over the past decade, driven by the need for cost-effective grid storage solutions that can operate safely at ambient temperatures.
Early research on RT Na-S batteries began in the 2000s, with pioneering work focusing on addressing the fundamental challenges of using sodium and sulfur electrodes at room temperature. The initial prototypes suffered from severe capacity fading, low Coulombic efficiency, and short cycle life due to the shuttle effect of soluble polysulfides and the formation of insulating discharge products.
A breakthrough came in 2012-2013 when researchers successfully demonstrated improved RT Na-S batteries using carbon-sulfur composites as cathodes and optimized electrolyte formulations. These advancements helped mitigate the shuttle effect and enhance the electrochemical performance, achieving initial discharge capacities of 400-600 mAh/g.
Between 2015 and 2018, significant progress was made in electrode materials engineering. Researchers developed hierarchical carbon structures, metal oxide additives, and polymer binders specifically designed to trap polysulfides and facilitate electron/ion transport. These innovations extended cycle life from tens of cycles to hundreds of cycles.
Recent developments (2019-2023) have focused on electrolyte optimization, with the introduction of solid-state and gel polymer electrolytes showing promise in suppressing the shuttle effect. Advanced separator designs and functional interlayers have also contributed to enhanced battery performance and stability.
Current state-of-the-art RT Na-S batteries demonstrate energy densities of 200-300 Wh/kg, power densities of 100-200 W/kg, and cycle lives of 500-1000 cycles at moderate depths of discharge. However, these metrics still fall short of commercial requirements for grid storage applications, which typically demand 2000+ cycles and stable operation over 10+ years.
Several research institutions and companies are actively working on scaling up RT Na-S technology from coin cells to pouch and prismatic formats suitable for grid applications. Pilot demonstrations have begun in controlled environments, though commercial deployment remains limited to specialized applications where the technology's current limitations are acceptable.
The cost trajectory for RT Na-S batteries is promising, with current estimates suggesting potential system costs of $70-100/kWh at scale, significantly lower than lithium-ion alternatives for stationary storage applications. This economic advantage, combined with the abundant nature of sodium and sulfur resources, positions RT Na-S batteries as a compelling option for future grid storage value stacking applications.
Early research on RT Na-S batteries began in the 2000s, with pioneering work focusing on addressing the fundamental challenges of using sodium and sulfur electrodes at room temperature. The initial prototypes suffered from severe capacity fading, low Coulombic efficiency, and short cycle life due to the shuttle effect of soluble polysulfides and the formation of insulating discharge products.
A breakthrough came in 2012-2013 when researchers successfully demonstrated improved RT Na-S batteries using carbon-sulfur composites as cathodes and optimized electrolyte formulations. These advancements helped mitigate the shuttle effect and enhance the electrochemical performance, achieving initial discharge capacities of 400-600 mAh/g.
Between 2015 and 2018, significant progress was made in electrode materials engineering. Researchers developed hierarchical carbon structures, metal oxide additives, and polymer binders specifically designed to trap polysulfides and facilitate electron/ion transport. These innovations extended cycle life from tens of cycles to hundreds of cycles.
Recent developments (2019-2023) have focused on electrolyte optimization, with the introduction of solid-state and gel polymer electrolytes showing promise in suppressing the shuttle effect. Advanced separator designs and functional interlayers have also contributed to enhanced battery performance and stability.
Current state-of-the-art RT Na-S batteries demonstrate energy densities of 200-300 Wh/kg, power densities of 100-200 W/kg, and cycle lives of 500-1000 cycles at moderate depths of discharge. However, these metrics still fall short of commercial requirements for grid storage applications, which typically demand 2000+ cycles and stable operation over 10+ years.
Several research institutions and companies are actively working on scaling up RT Na-S technology from coin cells to pouch and prismatic formats suitable for grid applications. Pilot demonstrations have begun in controlled environments, though commercial deployment remains limited to specialized applications where the technology's current limitations are acceptable.
The cost trajectory for RT Na-S batteries is promising, with current estimates suggesting potential system costs of $70-100/kWh at scale, significantly lower than lithium-ion alternatives for stationary storage applications. This economic advantage, combined with the abundant nature of sodium and sulfur resources, positions RT Na-S batteries as a compelling option for future grid storage value stacking applications.
Current Value Stacking Approaches for Grid Storage
01 Electrode materials for room-temperature sodium-sulfur batteries
Various electrode materials can be used in room-temperature sodium-sulfur batteries to enhance performance. These include carbon-based materials, metal oxides, and composite electrodes that improve sulfur utilization and prevent polysulfide shuttling. Advanced electrode designs incorporate conductive frameworks that facilitate ion transport while containing active materials, resulting in higher capacity and cycle stability at room temperature.- Electrode materials for room-temperature sodium-sulfur batteries: Various electrode materials can be used in room-temperature sodium-sulfur batteries to enhance performance. These include carbon-based materials, metal oxides, and composite structures that improve the conductivity and stability of the electrodes. The electrode materials are designed to address issues such as the shuttle effect and to increase the energy density of the batteries, making them more suitable for practical applications.
- Electrolyte solutions for improved ionic conductivity: Specialized electrolyte solutions are developed to enhance the ionic conductivity in room-temperature sodium-sulfur batteries. These electrolytes typically contain sodium salts dissolved in organic solvents or polymer matrices. Some formulations include additives that suppress the shuttle effect and improve the stability of the solid-electrolyte interphase. Advanced electrolyte designs enable faster charging rates and longer cycle life at ambient temperatures.
- Sulfur cathode modifications for enhanced performance: Modifications to the sulfur cathode are essential for improving the performance of room-temperature sodium-sulfur batteries. These modifications include encapsulation of sulfur in porous materials, use of sulfur-carbon composites, and incorporation of conductive additives. Such approaches help to contain polysulfides, increase the utilization of active material, and enhance the overall energy density and cycle stability of the batteries.
- Battery system design and thermal management: The design of room-temperature sodium-sulfur battery systems includes considerations for thermal management, safety features, and integration with energy storage applications. These designs incorporate cooling systems, protective circuitry, and structural elements that ensure stable operation under various conditions. Advanced battery management systems monitor and control the charging and discharging processes to optimize performance and prevent degradation.
- Value stacking applications and grid integration: Room-temperature sodium-sulfur batteries offer multiple value stacking opportunities through various applications. They can be used for grid stabilization, renewable energy integration, peak shaving, and backup power. The ability to operate at ambient temperatures without heating systems provides cost advantages over traditional high-temperature sodium-sulfur batteries. These batteries are particularly valuable for stationary energy storage applications where cost-effectiveness and safety are priorities.
02 Electrolyte innovations for room-temperature operation
Specialized electrolytes enable sodium-sulfur batteries to function effectively at room temperature. These include solid-state electrolytes, gel polymer electrolytes, and liquid electrolytes with additives that suppress dendrite formation and enhance ionic conductivity. The electrolyte formulations also address challenges related to the reactivity between sodium metal and sulfur compounds, improving overall battery safety and performance.Expand Specific Solutions03 Separator technologies for preventing polysulfide shuttling
Advanced separator designs are crucial for room-temperature sodium-sulfur batteries to prevent polysulfide shuttling. These separators incorporate functional coatings, nanostructured materials, or modified polymers that selectively block polysulfides while allowing sodium ion transport. Some designs feature dual-layer or composite structures that combine mechanical strength with ion selectivity, extending battery cycle life and efficiency.Expand Specific Solutions04 Battery system integration and value stacking applications
Room-temperature sodium-sulfur batteries offer significant value stacking opportunities through integration with renewable energy systems, grid storage, and other applications. These batteries can provide multiple services simultaneously, including peak shaving, frequency regulation, and backup power. System designs incorporate thermal management, battery management systems, and modular architectures that optimize performance while enabling scalability for various deployment scenarios.Expand Specific Solutions05 Manufacturing processes and cost reduction strategies
Innovative manufacturing techniques help reduce costs and improve the commercial viability of room-temperature sodium-sulfur batteries. These include scalable production methods for electrode materials, automated assembly processes, and techniques for encapsulating sulfur to prevent loss during cycling. Some approaches utilize abundant, low-cost materials and simplified cell designs that maintain performance while reducing manufacturing complexity and overall system costs.Expand Specific Solutions
Key Industry Players in Grid Storage Solutions
The room-temperature sodium-sulfur battery market for grid storage value stacking is in an early growth phase, characterized by increasing commercial interest due to its cost-effectiveness and sustainability advantages. The global market size is expanding rapidly, projected to reach significant scale as grid storage demands increase worldwide. Technologically, these batteries are advancing from early commercial deployment to broader adoption, with NGK Insulators leading as the pioneer commercial manufacturer with decades of experience. Other key players showing technological maturity include BASF, Tesla, and LG Energy Solution, who are leveraging their battery expertise to develop room-temperature variants. Research institutions like MIT, Cornell University, and Shanghai Institute of Ceramics are driving fundamental innovations, while utility companies such as Tokyo Electric Power and State Grid Shanghai are conducting field implementations to validate performance in real-world grid applications.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered high-temperature sodium-sulfur (NaS) battery technology and is now advancing room-temperature sodium-sulfur solutions for grid storage applications. Their approach involves a proprietary solid electrolyte material that enables stable sodium ion transport at ambient temperatures while preventing dendrite formation. NGK's room-temperature NaS batteries utilize a modified beta-alumina ceramic separator and specially formulated sulfur cathodes with carbon additives to enhance conductivity. The company has developed modular containerized systems that integrate these batteries with advanced thermal management and battery management systems, allowing for multiple grid services including frequency regulation, peak shaving, and renewable integration. Their systems demonstrate energy densities of approximately 150-200 Wh/kg and cycle life exceeding 4,000 cycles at 80% depth of discharge, with response times under 100 milliseconds for grid frequency support applications.
Strengths: Decades of experience with sodium-sulfur technology; established grid-scale deployment infrastructure; proven safety record with large-scale energy storage systems; vertical integration from materials to system level. Weaknesses: Higher initial costs compared to lithium-ion alternatives; lower energy density than competing technologies; limited track record specifically with room-temperature variants.
BASF Corp.
Technical Solution: BASF has developed an innovative approach to room-temperature sodium-sulfur batteries focusing on advanced materials engineering. Their technology utilizes specially designed carbon-sulfur composite cathodes with tailored pore structures that effectively contain polysulfide intermediates, addressing the shuttle effect that typically plagues Na-S batteries. BASF's electrolyte formulations incorporate flame-retardant additives and ionic liquid components that enhance safety while maintaining high ionic conductivity at room temperature. The company has created a proprietary sodium-ion conducting polymer membrane that serves as both separator and electrolyte, significantly improving the battery's cycle stability. Their integrated system design includes modular battery packs with sophisticated thermal management that can be scaled from residential to utility applications. BASF's room-temperature Na-S batteries demonstrate round-trip efficiencies of approximately 85-90% and are designed for applications requiring 4-8 hour discharge durations, making them particularly suitable for daily cycling in grid storage applications.
Strengths: World-class materials science expertise; extensive manufacturing capabilities for scaling production; strong intellectual property portfolio in battery materials; established relationships with utility companies. Weaknesses: Less experience in complete battery system integration compared to dedicated battery manufacturers; relatively new entrant to the grid storage market specifically with sodium-sulfur technology.
Core Na-S Battery Materials and Chemistry Innovations
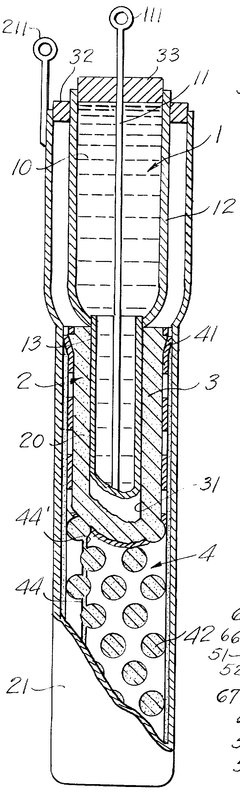
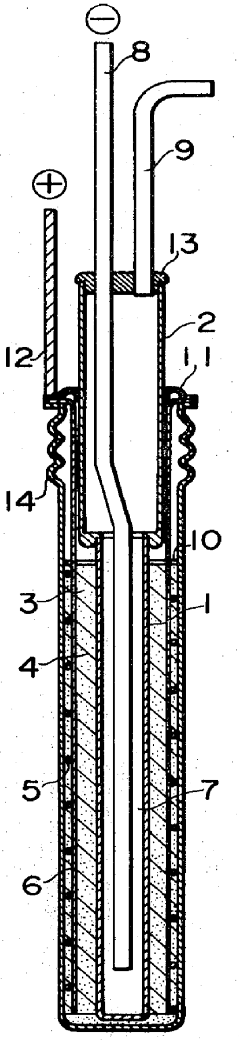
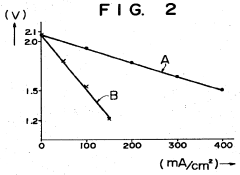
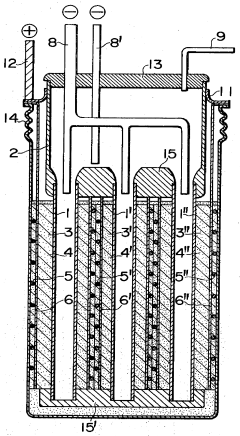
Sodium-sulfur storage battery
PatentInactiveUS3883367A
Innovation
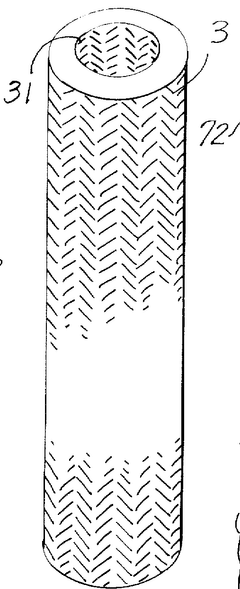
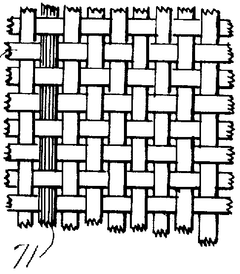
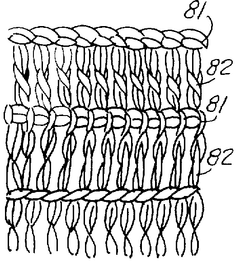
- A porous current collector made of knitted or woven carbon or graphite yarns with high elastic modulus and tensile strength, where carbon monofilaments are oriented to enhance crystal alignment, providing improved corrosion resistance, mechanical strength, and ease of Na2Sx movement, allowing for efficient charge-discharge operations.
Sodium-sulfur storage battery
PatentInactiveUS3770502A
Innovation
- The battery design incorporates a solid electrolyte made of β-Al2O3 with a graphite sheet acting as an electrode matrix for sulfur, joined using a sulfur-resistant solder glass, and features a bellows structure in the container to absorb stress from sulfur phase transition, along with multiple solid electrolytes for enhanced mechanical strength and conductivity.
Economic Feasibility and Cost Analysis
The economic feasibility of room-temperature sodium-sulfur (RT Na-S) batteries for grid storage value stacking represents a critical consideration for market adoption. Current cost analyses indicate that RT Na-S batteries offer significant potential for cost reduction compared to lithium-ion alternatives, with material costs estimated at $83-125/kWh and projected system-level costs of $150-200/kWh at scale. This positions them favorably against the current $200-300/kWh range for grid-scale lithium-ion systems.
The value stacking capability of RT Na-S batteries creates multiple revenue streams that enhance their economic viability. By providing services such as energy arbitrage, frequency regulation, capacity value, and transmission and distribution deferral simultaneously, these batteries can achieve payback periods of 5-7 years under current market conditions, with potential for further improvement as markets evolve to better compensate storage services.
Manufacturing scalability presents both opportunities and challenges. The abundant nature of sodium and sulfur as raw materials mitigates supply chain risks that plague lithium-ion technologies. However, current production processes require optimization to achieve economies of scale. Analysis suggests that reaching a production volume of 1 GWh annually could reduce manufacturing costs by approximately 30%, bringing system costs below $150/kWh.
Lifecycle cost assessment reveals favorable total cost of ownership metrics. With demonstrated cycle life approaching 2,000 cycles at 80% depth of discharge and theoretical potential for 4,000+ cycles through advanced electrolyte formulations, the levelized cost of storage (LCOS) could reach $0.10-0.15/kWh for energy applications. This compares favorably with lithium-ion systems at similar scales.
Regulatory and market factors significantly impact economic feasibility. Markets with established capacity mechanisms, ancillary service compensation, and time-of-use rate structures provide more favorable economics for RT Na-S deployment. Sensitivity analysis indicates that a carbon price of $30-50/ton would further enhance competitiveness against fossil fuel peaking plants.
Investment requirements for commercial deployment remain substantial but achievable. Initial capital expenditure for manufacturing facilities capable of producing 100 MWh annually is estimated at $50-75 million, with additional R&D investments of $15-25 million needed to address remaining technical challenges in electrode design and electrolyte stability.
The value stacking capability of RT Na-S batteries creates multiple revenue streams that enhance their economic viability. By providing services such as energy arbitrage, frequency regulation, capacity value, and transmission and distribution deferral simultaneously, these batteries can achieve payback periods of 5-7 years under current market conditions, with potential for further improvement as markets evolve to better compensate storage services.
Manufacturing scalability presents both opportunities and challenges. The abundant nature of sodium and sulfur as raw materials mitigates supply chain risks that plague lithium-ion technologies. However, current production processes require optimization to achieve economies of scale. Analysis suggests that reaching a production volume of 1 GWh annually could reduce manufacturing costs by approximately 30%, bringing system costs below $150/kWh.
Lifecycle cost assessment reveals favorable total cost of ownership metrics. With demonstrated cycle life approaching 2,000 cycles at 80% depth of discharge and theoretical potential for 4,000+ cycles through advanced electrolyte formulations, the levelized cost of storage (LCOS) could reach $0.10-0.15/kWh for energy applications. This compares favorably with lithium-ion systems at similar scales.
Regulatory and market factors significantly impact economic feasibility. Markets with established capacity mechanisms, ancillary service compensation, and time-of-use rate structures provide more favorable economics for RT Na-S deployment. Sensitivity analysis indicates that a carbon price of $30-50/ton would further enhance competitiveness against fossil fuel peaking plants.
Investment requirements for commercial deployment remain substantial but achievable. Initial capital expenditure for manufacturing facilities capable of producing 100 MWh annually is estimated at $50-75 million, with additional R&D investments of $15-25 million needed to address remaining technical challenges in electrode design and electrolyte stability.
Environmental Impact and Sustainability Assessment
Room-temperature sodium-sulfur (RT Na-S) batteries represent a significant advancement in sustainable energy storage technology, offering a more environmentally friendly alternative to conventional lithium-ion batteries. The environmental impact assessment of these batteries reveals several key advantages in terms of resource utilization and ecological footprint.
The raw materials for RT Na-S batteries—sodium and sulfur—are abundantly available in the earth's crust and oceans, reducing the environmental pressures associated with resource extraction. Unlike lithium, which is concentrated in specific geographical regions, sodium is widely distributed globally, minimizing the environmental degradation linked to intensive mining operations. Sulfur, often a byproduct of petroleum refining, can be repurposed for battery production, contributing to waste reduction and circular economy principles.
Life cycle assessments indicate that RT Na-S batteries have a lower carbon footprint compared to lithium-ion alternatives. The manufacturing process requires less energy and produces fewer greenhouse gas emissions, particularly when powered by renewable energy sources. This advantage becomes more pronounced when considering the entire battery lifecycle, from raw material extraction to end-of-life management.
Water consumption and land use impacts are also reduced with RT Na-S technology. The extraction of sodium from seawater or salt deposits generally requires less water than lithium extraction from brine pools, which can deplete local water resources in arid regions. Additionally, the reduced need for rare earth elements and heavy metals in RT Na-S batteries decreases the risk of soil and water contamination associated with mining these materials.
End-of-life considerations further enhance the sustainability profile of RT Na-S batteries. The components are more readily recyclable than those in conventional batteries, with higher recovery rates for both sodium and sulfur. Recycling processes for these materials are generally less energy-intensive and produce fewer toxic byproducts, supporting closed-loop material flows and reducing landfill waste.
When deployed in grid storage applications, RT Na-S batteries enable greater integration of renewable energy sources, indirectly reducing emissions from fossil fuel power generation. This value stacking approach—combining energy storage with renewable energy systems—amplifies the environmental benefits beyond the direct impacts of the battery technology itself.
However, challenges remain in optimizing the environmental performance of RT Na-S batteries. Current electrolyte formulations may contain organic solvents with potential environmental hazards, and manufacturing processes still require refinement to minimize waste and energy consumption. Ongoing research focuses on developing greener electrolytes and more efficient production methods to further enhance the sustainability credentials of this promising technology.
The raw materials for RT Na-S batteries—sodium and sulfur—are abundantly available in the earth's crust and oceans, reducing the environmental pressures associated with resource extraction. Unlike lithium, which is concentrated in specific geographical regions, sodium is widely distributed globally, minimizing the environmental degradation linked to intensive mining operations. Sulfur, often a byproduct of petroleum refining, can be repurposed for battery production, contributing to waste reduction and circular economy principles.
Life cycle assessments indicate that RT Na-S batteries have a lower carbon footprint compared to lithium-ion alternatives. The manufacturing process requires less energy and produces fewer greenhouse gas emissions, particularly when powered by renewable energy sources. This advantage becomes more pronounced when considering the entire battery lifecycle, from raw material extraction to end-of-life management.
Water consumption and land use impacts are also reduced with RT Na-S technology. The extraction of sodium from seawater or salt deposits generally requires less water than lithium extraction from brine pools, which can deplete local water resources in arid regions. Additionally, the reduced need for rare earth elements and heavy metals in RT Na-S batteries decreases the risk of soil and water contamination associated with mining these materials.
End-of-life considerations further enhance the sustainability profile of RT Na-S batteries. The components are more readily recyclable than those in conventional batteries, with higher recovery rates for both sodium and sulfur. Recycling processes for these materials are generally less energy-intensive and produce fewer toxic byproducts, supporting closed-loop material flows and reducing landfill waste.
When deployed in grid storage applications, RT Na-S batteries enable greater integration of renewable energy sources, indirectly reducing emissions from fossil fuel power generation. This value stacking approach—combining energy storage with renewable energy systems—amplifies the environmental benefits beyond the direct impacts of the battery technology itself.
However, challenges remain in optimizing the environmental performance of RT Na-S batteries. Current electrolyte formulations may contain organic solvents with potential environmental hazards, and manufacturing processes still require refinement to minimize waste and energy consumption. Ongoing research focuses on developing greener electrolytes and more efficient production methods to further enhance the sustainability credentials of this promising technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!