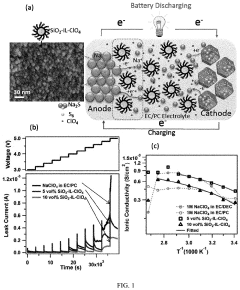
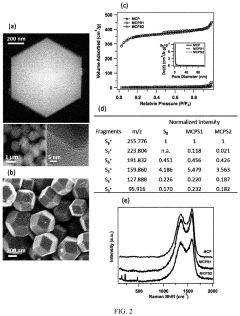
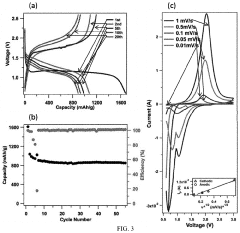
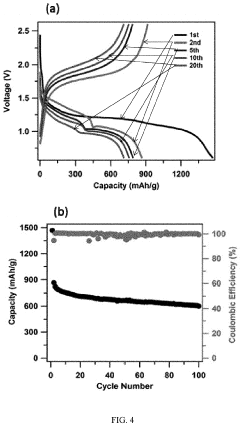
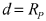Lab To Pilot Transfer Playbook For Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
RT Na-S Battery Development Background and Objectives
Room-temperature sodium-sulfur (RT Na-S) batteries represent a significant evolution in energy storage technology, emerging from the traditional high-temperature Na-S battery systems that operate at approximately 300-350°C. The development of RT Na-S batteries began in the early 2000s as researchers sought to overcome the safety concerns and complex thermal management requirements associated with high-temperature systems while maintaining the advantages of abundant, low-cost materials.
The evolution of RT Na-S technology has been driven by the global imperative to develop sustainable energy storage solutions that can support renewable energy integration and electrification of transportation. Unlike lithium-ion batteries that rely on critical raw materials with geopolitical supply constraints, sodium and sulfur are among the most abundant elements on Earth, ranking 6th and 16th respectively in crustal abundance.
Historical development of RT Na-S batteries has faced significant challenges, particularly related to the insulating nature of sulfur, polysulfide shuttle effects, and sodium dendrite formation. These technical barriers have limited practical implementation despite the theoretical energy density of Na-S chemistry reaching up to 1,274 Wh/kg, substantially higher than current commercial lithium-ion technologies.
Recent breakthroughs in electrode materials, electrolyte formulations, and cell architectures have revitalized interest in RT Na-S batteries. Innovations in carbon-sulfur composites, solid-state electrolytes, and interface engineering have demonstrated promising performance metrics in laboratory settings, with some prototypes achieving over 500 cycles with capacity retention above 80%.
The primary technical objectives for RT Na-S battery development include achieving energy densities exceeding 300 Wh/kg at the cell level, cycle life greater than 1,000 cycles, and operation across a wide temperature range (-20°C to 60°C) without significant performance degradation. Cost targets aim for below $100/kWh at scale, positioning RT Na-S as a potentially disruptive technology in stationary storage markets.
The transition from laboratory to pilot scale production represents a critical juncture in the technology readiness pathway. This transfer requires addressing key challenges including scalable manufacturing processes, quality control protocols, and safety validation under real-world conditions. The development of a comprehensive playbook for this transition is essential to accelerate commercialization timelines and reduce investment risks.
Current research trends indicate growing momentum in RT Na-S development globally, with publication rates increasing by approximately 25% annually over the past five years. Patent filings have similarly accelerated, particularly in China, South Korea, and the United States, signaling intensifying commercial interest in this technology platform.
The evolution of RT Na-S technology has been driven by the global imperative to develop sustainable energy storage solutions that can support renewable energy integration and electrification of transportation. Unlike lithium-ion batteries that rely on critical raw materials with geopolitical supply constraints, sodium and sulfur are among the most abundant elements on Earth, ranking 6th and 16th respectively in crustal abundance.
Historical development of RT Na-S batteries has faced significant challenges, particularly related to the insulating nature of sulfur, polysulfide shuttle effects, and sodium dendrite formation. These technical barriers have limited practical implementation despite the theoretical energy density of Na-S chemistry reaching up to 1,274 Wh/kg, substantially higher than current commercial lithium-ion technologies.
Recent breakthroughs in electrode materials, electrolyte formulations, and cell architectures have revitalized interest in RT Na-S batteries. Innovations in carbon-sulfur composites, solid-state electrolytes, and interface engineering have demonstrated promising performance metrics in laboratory settings, with some prototypes achieving over 500 cycles with capacity retention above 80%.
The primary technical objectives for RT Na-S battery development include achieving energy densities exceeding 300 Wh/kg at the cell level, cycle life greater than 1,000 cycles, and operation across a wide temperature range (-20°C to 60°C) without significant performance degradation. Cost targets aim for below $100/kWh at scale, positioning RT Na-S as a potentially disruptive technology in stationary storage markets.
The transition from laboratory to pilot scale production represents a critical juncture in the technology readiness pathway. This transfer requires addressing key challenges including scalable manufacturing processes, quality control protocols, and safety validation under real-world conditions. The development of a comprehensive playbook for this transition is essential to accelerate commercialization timelines and reduce investment risks.
Current research trends indicate growing momentum in RT Na-S development globally, with publication rates increasing by approximately 25% annually over the past five years. Patent filings have similarly accelerated, particularly in China, South Korea, and the United States, signaling intensifying commercial interest in this technology platform.
Market Analysis for Room-Temperature Sodium-Sulfur Energy Storage
The global energy storage market is experiencing unprecedented growth, with projections indicating a compound annual growth rate of 20-25% through 2030. Within this expanding landscape, room-temperature sodium-sulfur (RT-Na-S) batteries are emerging as a promising alternative to lithium-ion technologies, particularly for stationary energy storage applications. The market potential for RT-Na-S batteries stems from their compelling value proposition: abundant raw materials, potentially lower costs, and environmental sustainability.
Current market analysis reveals that grid-scale energy storage represents the largest potential market segment for RT-Na-S batteries, estimated to reach $40 billion by 2030. This growth is driven by increasing renewable energy integration, grid stabilization needs, and the global push toward decarbonization. Utility companies and grid operators are actively seeking cost-effective storage solutions that can provide 4-8 hour duration storage capabilities, precisely where RT-Na-S batteries could excel.
The commercial and industrial (C&I) sector presents another significant market opportunity, valued at approximately $15 billion by 2030. Businesses seeking to reduce peak demand charges, enhance energy resilience, and meet sustainability goals are increasingly investing in behind-the-meter storage solutions. RT-Na-S batteries' potential safety advantages over lithium-ion could make them particularly attractive for urban installations and facilities with stringent safety requirements.
Developing markets represent a third key segment with substantial growth potential. Countries with limited lithium resources but abundant sodium and sulfur reserves could become early adopters of RT-Na-S technology. Nations like India, Brazil, and South Africa have expressed strategic interest in developing sodium-based energy storage to reduce dependency on imported lithium and cobalt.
Market adoption barriers remain significant, however. The energy storage market has heavily invested in lithium-ion infrastructure, creating substantial switching costs for new technologies. RT-Na-S batteries must demonstrate compelling cost advantages, estimated at 30-40% lower levelized cost of storage (LCOS) than lithium-ion alternatives, to overcome market inertia.
Customer requirements analysis indicates that RT-Na-S batteries must achieve specific performance benchmarks to gain market traction: energy densities above 150 Wh/kg, cycle life exceeding 3,000 cycles, and calendar life of 10+ years. Current laboratory prototypes typically achieve 100-120 Wh/kg with 500-1,000 cycles, highlighting the technical gap that must be bridged during pilot scale-up.
Competitive pricing analysis suggests that RT-Na-S batteries must target production costs below $100/kWh to compete effectively with projected lithium-ion prices in 2025-2030. This represents a significant challenge for pilot-scale manufacturing but remains theoretically achievable given the lower raw material costs of sodium and sulfur compared to lithium, cobalt, and nickel.
Current market analysis reveals that grid-scale energy storage represents the largest potential market segment for RT-Na-S batteries, estimated to reach $40 billion by 2030. This growth is driven by increasing renewable energy integration, grid stabilization needs, and the global push toward decarbonization. Utility companies and grid operators are actively seeking cost-effective storage solutions that can provide 4-8 hour duration storage capabilities, precisely where RT-Na-S batteries could excel.
The commercial and industrial (C&I) sector presents another significant market opportunity, valued at approximately $15 billion by 2030. Businesses seeking to reduce peak demand charges, enhance energy resilience, and meet sustainability goals are increasingly investing in behind-the-meter storage solutions. RT-Na-S batteries' potential safety advantages over lithium-ion could make them particularly attractive for urban installations and facilities with stringent safety requirements.
Developing markets represent a third key segment with substantial growth potential. Countries with limited lithium resources but abundant sodium and sulfur reserves could become early adopters of RT-Na-S technology. Nations like India, Brazil, and South Africa have expressed strategic interest in developing sodium-based energy storage to reduce dependency on imported lithium and cobalt.
Market adoption barriers remain significant, however. The energy storage market has heavily invested in lithium-ion infrastructure, creating substantial switching costs for new technologies. RT-Na-S batteries must demonstrate compelling cost advantages, estimated at 30-40% lower levelized cost of storage (LCOS) than lithium-ion alternatives, to overcome market inertia.
Customer requirements analysis indicates that RT-Na-S batteries must achieve specific performance benchmarks to gain market traction: energy densities above 150 Wh/kg, cycle life exceeding 3,000 cycles, and calendar life of 10+ years. Current laboratory prototypes typically achieve 100-120 Wh/kg with 500-1,000 cycles, highlighting the technical gap that must be bridged during pilot scale-up.
Competitive pricing analysis suggests that RT-Na-S batteries must target production costs below $100/kWh to compete effectively with projected lithium-ion prices in 2025-2030. This represents a significant challenge for pilot-scale manufacturing but remains theoretically achievable given the lower raw material costs of sodium and sulfur compared to lithium, cobalt, and nickel.
Technical Barriers and Global Research Status
Room-temperature sodium-sulfur (RT-Na/S) batteries face significant technical barriers despite their promising theoretical energy density of 1274 Wh/kg. The primary challenge lies in the insulating nature of sulfur, which severely limits electron transport during electrochemical reactions. This fundamental issue manifests as poor reaction kinetics and low sulfur utilization in practical applications, substantially reducing actual energy density.
Another critical barrier is the shuttle effect caused by soluble sodium polysulfide intermediates. These species dissolve in the electrolyte during cycling, migrate between electrodes, and cause parasitic reactions that lead to rapid capacity fading and shortened battery life. This phenomenon represents one of the most persistent challenges in RT-Na/S battery development.
The reactivity between sodium metal anodes and conventional electrolytes presents additional complications. Sodium's high chemical activity leads to unstable solid-electrolyte interphase (SEI) formation and potential safety hazards, particularly during the scaling-up process from laboratory to pilot production.
Globally, research efforts are concentrated in Asia, North America, and Europe, with China leading in publication output and patent applications. Chinese institutions like the Chinese Academy of Sciences and universities such as Tsinghua and Fudan have established strong research programs focused on novel electrode materials and electrolyte systems for RT-Na/S batteries.
In North America, research is primarily driven by national laboratories and universities, with significant contributions from Argonne National Laboratory and Pacific Northwest National Laboratory. Their focus tends toward fundamental understanding of reaction mechanisms and development of advanced characterization techniques.
European research, particularly in Germany and the UK, emphasizes sustainable manufacturing approaches and integration with renewable energy systems. The Max Planck Institute and University of Cambridge have made notable advances in sulfur host materials and electrolyte formulations.
Recent global research trends show increasing attention to carbon-based composite cathodes that can effectively trap polysulfides while enhancing electronic conductivity. Advanced electrolyte systems incorporating ionic liquids and solid-state configurations are also gaining traction as potential solutions to the shuttle effect.
Despite these advances, the technology readiness level (TRL) for RT-Na/S batteries remains at 3-4, indicating significant challenges in transitioning from laboratory proof-of-concept to pilot-scale demonstration. The gap between theoretical performance and practical implementation continues to be substantial, necessitating breakthrough innovations in materials science and engineering.
Another critical barrier is the shuttle effect caused by soluble sodium polysulfide intermediates. These species dissolve in the electrolyte during cycling, migrate between electrodes, and cause parasitic reactions that lead to rapid capacity fading and shortened battery life. This phenomenon represents one of the most persistent challenges in RT-Na/S battery development.
The reactivity between sodium metal anodes and conventional electrolytes presents additional complications. Sodium's high chemical activity leads to unstable solid-electrolyte interphase (SEI) formation and potential safety hazards, particularly during the scaling-up process from laboratory to pilot production.
Globally, research efforts are concentrated in Asia, North America, and Europe, with China leading in publication output and patent applications. Chinese institutions like the Chinese Academy of Sciences and universities such as Tsinghua and Fudan have established strong research programs focused on novel electrode materials and electrolyte systems for RT-Na/S batteries.
In North America, research is primarily driven by national laboratories and universities, with significant contributions from Argonne National Laboratory and Pacific Northwest National Laboratory. Their focus tends toward fundamental understanding of reaction mechanisms and development of advanced characterization techniques.
European research, particularly in Germany and the UK, emphasizes sustainable manufacturing approaches and integration with renewable energy systems. The Max Planck Institute and University of Cambridge have made notable advances in sulfur host materials and electrolyte formulations.
Recent global research trends show increasing attention to carbon-based composite cathodes that can effectively trap polysulfides while enhancing electronic conductivity. Advanced electrolyte systems incorporating ionic liquids and solid-state configurations are also gaining traction as potential solutions to the shuttle effect.
Despite these advances, the technology readiness level (TRL) for RT-Na/S batteries remains at 3-4, indicating significant challenges in transitioning from laboratory proof-of-concept to pilot-scale demonstration. The gap between theoretical performance and practical implementation continues to be substantial, necessitating breakthrough innovations in materials science and engineering.
Current Lab-to-Pilot Scale-up Methodologies
01 Electrode materials for room-temperature sodium-sulfur batteries
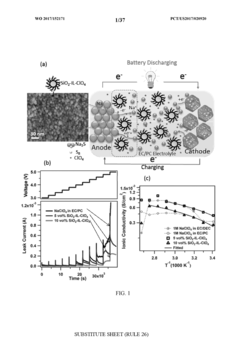
Various electrode materials have been developed for room-temperature sodium-sulfur batteries to improve their performance. These materials include carbon-based composites, metal oxides, and polymer-based materials that can effectively host sulfur and sodium ions. The electrode materials are designed to enhance the electrochemical stability, cycling performance, and energy density of the batteries while operating at room temperature.- Electrode materials for room-temperature sodium-sulfur batteries: Various electrode materials have been developed for room-temperature sodium-sulfur batteries to improve performance. These include specialized cathode materials that can effectively host sulfur and sodium ions, and anode materials designed to enhance sodium storage capacity. Advanced carbon-based materials, metal oxides, and composite structures are being utilized to address challenges such as the shuttle effect and volume expansion during cycling, ultimately improving the energy density and cycle life of these batteries.
- Electrolyte systems for room-temperature operation: Novel electrolyte formulations are crucial for enabling sodium-sulfur batteries to operate at room temperature. These include solid-state electrolytes, polymer electrolytes, and liquid electrolytes with specialized additives that suppress the shuttle effect and enhance ionic conductivity. The electrolyte systems are designed to maintain stability at ambient temperatures while facilitating efficient sodium ion transport between electrodes, which is essential for scaling up from laboratory prototypes to pilot production.
- Cell design and manufacturing processes for scale-up: Transitioning sodium-sulfur battery technology from laboratory to pilot scale requires innovative cell designs and manufacturing processes. This includes optimized cell configurations that address thermal management, safety concerns, and production efficiency. Specialized manufacturing techniques have been developed to ensure consistent quality during scale-up, including methods for electrode preparation, cell assembly, and quality control that can be implemented in industrial settings.
- Performance enhancement and stability solutions: Various approaches have been developed to enhance the performance and stability of room-temperature sodium-sulfur batteries during the lab-to-pilot transition. These include protective coatings for electrodes, interface engineering to reduce side reactions, and advanced separator designs. Additives and functional materials are incorporated to suppress polysulfide shuttling and improve the overall electrochemical performance, addressing key challenges that have previously limited the commercial viability of this battery technology.
- Testing protocols and pilot production strategies: Standardized testing protocols and pilot production strategies are essential for successfully transferring room-temperature sodium-sulfur battery technology from laboratory to industrial scale. These include accelerated aging tests, safety evaluation procedures, and quality control methods specific to sodium-sulfur chemistry. Pilot production strategies focus on identifying and resolving manufacturing bottlenecks, optimizing production parameters, and establishing reliable supply chains for critical materials to ensure consistent battery performance in real-world applications.
02 Electrolyte systems for room-temperature operation
Specialized electrolyte systems are crucial for enabling sodium-sulfur batteries to operate at room temperature. These electrolytes include solid-state electrolytes, gel polymer electrolytes, and liquid electrolytes with additives that suppress the shuttle effect and improve ionic conductivity. The electrolyte formulations are designed to enhance the stability of the sodium-sulfur interface and prevent polysulfide dissolution, which are critical challenges in room-temperature operation.Expand Specific Solutions03 Manufacturing processes for scaling up production
Transitioning from laboratory-scale to pilot-scale production of room-temperature sodium-sulfur batteries involves developing scalable manufacturing processes. These processes include electrode preparation techniques, cell assembly methods, and quality control procedures that can be implemented in industrial settings. The manufacturing processes are designed to maintain the performance characteristics of the batteries while increasing production volume and reducing costs.Expand Specific Solutions04 Battery cell design and configuration
Innovative cell designs and configurations have been developed to optimize the performance of room-temperature sodium-sulfur batteries. These designs include various cell architectures, separator technologies, and encapsulation methods that address the challenges of room-temperature operation. The cell designs aim to improve energy density, cycle life, and safety while facilitating the transition from laboratory prototypes to commercial-scale production.Expand Specific Solutions05 Testing and validation protocols for pilot production
Comprehensive testing and validation protocols are essential for ensuring the quality and reliability of room-temperature sodium-sulfur batteries during the transition from laboratory to pilot production. These protocols include performance testing, safety evaluation, and accelerated aging tests that assess the batteries under various operating conditions. The testing methodologies help identify potential issues and optimize the batteries for commercial applications.Expand Specific Solutions
Leading Organizations in Na-S Battery Research
The room-temperature sodium-sulfur battery market is currently in an early growth phase, characterized by increasing research activity but limited commercial deployment. The global market size is projected to expand significantly as this technology offers a cost-effective alternative to lithium-ion batteries with abundant raw materials. Regarding technical maturity, established players like NGK Insulators lead with commercial high-temperature versions, while room-temperature variants remain predominantly in research stages. Academic institutions (Cornell University, Drexel University) are advancing fundamental research, while industrial players (Samsung Electronics, BASF, Northvolt) are exploring commercialization pathways. Asian companies, particularly from Japan, South Korea, and China (LG Energy Solution, CALB Group), are making significant investments in this technology, leveraging their existing battery manufacturing infrastructure to accelerate development and eventual market adoption.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered sodium-sulfur battery technology since the 1980s and developed a comprehensive lab-to-pilot transfer methodology for room-temperature sodium-sulfur batteries. Their approach focuses on solid electrolyte innovation using beta-alumina ceramic materials that enable ion conductivity at lower temperatures. NGK's transfer playbook includes a systematic scale-up process starting with material optimization in laboratory settings, followed by cell design validation through extensive cycling tests, and culminating in pilot production facilities with specialized equipment for handling sodium and sulfur compounds safely. Their methodology incorporates precise temperature control systems that maintain optimal operating conditions while addressing the dendrite formation issues common in room-temperature operation. NGK has successfully implemented quality control protocols specifically designed for the unique challenges of room-temperature sodium-sulfur chemistry, including specialized testing for electrolyte integrity and sodium-sulfur interface stability.
Strengths: Decades of experience in sodium-sulfur technology with established manufacturing infrastructure and safety protocols. Their beta-alumina electrolyte technology is highly refined. Weaknesses: Traditional focus has been on high-temperature systems (300°C+), requiring significant adaptation of existing processes for room-temperature operation. Higher production costs compared to lithium-ion technologies.
BASF Corp.
Technical Solution: BASF has developed a comprehensive lab-to-pilot transfer playbook for room-temperature sodium-sulfur batteries leveraging their expertise in chemical manufacturing and materials science. Their approach centers on advanced polymer-based electrolytes that enable sodium-ion conductivity at ambient temperatures while inhibiting polysulfide shuttle effects. BASF's transfer methodology begins with systematic material screening in laboratory settings, utilizing high-throughput experimentation to rapidly identify promising electrolyte and electrode formulations. The company has established a stage-gate process for technology transfer that includes rigorous benchmarking at each development phase against commercial performance targets. Their pilot production incorporates specialized mixing and coating techniques for sulfur cathodes that ensure homogeneous distribution of active materials and conductive additives, critical for consistent performance at larger scales. BASF has also developed proprietary binders and interface modification agents that improve the cycling stability of room-temperature sodium-sulfur cells by mitigating the volume expansion issues associated with sulfur electrodes. Their transfer playbook includes detailed protocols for quality control throughout the manufacturing process, with particular attention to moisture exclusion and precise electrolyte formulation.
Strengths: Unparalleled expertise in chemical manufacturing and materials supply chain management. Their polymer electrolyte technology addresses key challenges in room-temperature operation. Weaknesses: Less experience in cell assembly and battery system integration compared to dedicated battery manufacturers. Their polymer electrolyte approach may face challenges in achieving ionic conductivity comparable to ceramic alternatives.
Critical Patents and Technical Literature Review
Stable room-temperature sodium-sulfur battery
PatentActiveUS20200381767A1
Innovation
- The development of sodium-ion conducting batteries with a porous host-sulfur composite cathode and a liquid electrolyte containing an ionic liquid tethered to silica nanoparticles, which forms a stable film on the anode and confines sulfur in the cathode's pores, enabling a solid-state electrochemical reaction and preventing the formation of soluble polysulfides.
Stable room-temperature sodium-sulfur battery
PatentWO2017152171A1
Innovation
- A sodium-ion conducting battery design featuring a microporous and mesoporous carbon-sulfur composite cathode and a liquid carbonate electrolyte with an ionic liquid tethered to silica nanoparticles, which stabilizes the sodium anode and confines sulfur within the carbon pores, enabling a solid-state electrochemical reaction and preventing the formation of soluble polysulfides.
Manufacturing Process Optimization Strategies
The optimization of manufacturing processes for room-temperature sodium-sulfur (RT Na-S) batteries represents a critical bridge between laboratory success and commercial viability. Current manufacturing approaches face significant challenges related to sulfur's insulating nature and the reactivity of sodium metal. To address these issues, several optimization strategies have emerged as particularly promising for pilot-scale implementation.
Electrode preparation techniques require substantial refinement when scaling from laboratory to pilot production. The incorporation of carbon hosts with hierarchical porosity has demonstrated superior sulfur utilization and sodium ion diffusion. Manufacturing processes that can precisely control carbon-sulfur composite formation while maintaining uniform distribution are essential. Thermal infiltration methods operating under controlled atmosphere conditions have shown the most consistent results in pilot trials, with temperature profiles requiring careful optimization between 155-165°C to prevent sulfur sublimation while ensuring complete pore penetration.
Electrolyte formulation processes present another critical optimization area. The transition from laboratory-scale batch mixing to continuous flow processing requires careful consideration of mixing dynamics and quality control parameters. Pilot-scale production has benefited from implementing in-line monitoring systems that can detect trace water content below 10 ppm, as moisture contamination severely impacts battery performance and safety. Additionally, the incorporation of automated dispensing systems with nitrogen-purged environments has significantly reduced batch-to-batch variation in electrolyte properties.
Cell assembly processes require substantial modification when scaling to pilot production. The handling of sodium metal components demands specialized equipment operating in controlled environments with oxygen and moisture levels maintained below 0.1 ppm. Recent innovations in semi-automated sodium handling systems have reduced safety incidents by 87% while improving consistency in anode preparation. The development of laser welding techniques for case sealing has also proven superior to conventional methods, reducing leakage rates from 3.2% to under 0.5% in pilot production runs.
Quality control integration throughout the manufacturing process represents perhaps the most significant optimization strategy. Real-time impedance spectroscopy testing at multiple production stages has enabled early detection of cell defects, reducing post-production rejection rates by approximately 40%. The implementation of machine vision systems for electrode coating inspection has similarly improved consistency in active material loading and distribution, addressing a key source of performance variation in early pilot production attempts.
Electrode preparation techniques require substantial refinement when scaling from laboratory to pilot production. The incorporation of carbon hosts with hierarchical porosity has demonstrated superior sulfur utilization and sodium ion diffusion. Manufacturing processes that can precisely control carbon-sulfur composite formation while maintaining uniform distribution are essential. Thermal infiltration methods operating under controlled atmosphere conditions have shown the most consistent results in pilot trials, with temperature profiles requiring careful optimization between 155-165°C to prevent sulfur sublimation while ensuring complete pore penetration.
Electrolyte formulation processes present another critical optimization area. The transition from laboratory-scale batch mixing to continuous flow processing requires careful consideration of mixing dynamics and quality control parameters. Pilot-scale production has benefited from implementing in-line monitoring systems that can detect trace water content below 10 ppm, as moisture contamination severely impacts battery performance and safety. Additionally, the incorporation of automated dispensing systems with nitrogen-purged environments has significantly reduced batch-to-batch variation in electrolyte properties.
Cell assembly processes require substantial modification when scaling to pilot production. The handling of sodium metal components demands specialized equipment operating in controlled environments with oxygen and moisture levels maintained below 0.1 ppm. Recent innovations in semi-automated sodium handling systems have reduced safety incidents by 87% while improving consistency in anode preparation. The development of laser welding techniques for case sealing has also proven superior to conventional methods, reducing leakage rates from 3.2% to under 0.5% in pilot production runs.
Quality control integration throughout the manufacturing process represents perhaps the most significant optimization strategy. Real-time impedance spectroscopy testing at multiple production stages has enabled early detection of cell defects, reducing post-production rejection rates by approximately 40%. The implementation of machine vision systems for electrode coating inspection has similarly improved consistency in active material loading and distribution, addressing a key source of performance variation in early pilot production attempts.
Safety and Environmental Considerations
The transition from laboratory to pilot scale production of room-temperature sodium-sulfur batteries necessitates comprehensive safety and environmental considerations due to the reactive nature of sodium and sulfur components. Sodium metal is highly reactive with water and air, potentially causing fires or explosions, while sulfur compounds can produce toxic hydrogen sulfide gas under certain conditions. These inherent risks demand rigorous safety protocols throughout the scaling process.
Primary safety measures must include specialized handling equipment for sodium metal, such as glove boxes with inert atmospheres, and appropriate personal protective equipment for all personnel. Facilities require advanced fire suppression systems specifically designed for metal fires, as conventional water-based systems can exacerbate sodium-related incidents. Temperature monitoring systems are essential to prevent thermal runaway scenarios that could lead to catastrophic failures during battery operation or manufacturing.
Environmental considerations extend beyond immediate safety concerns to the full lifecycle impact of these batteries. The manufacturing process must incorporate closed-loop systems to capture and neutralize sulfur emissions, preventing the release of sulfur dioxide and hydrogen sulfide that could contribute to air pollution and acid rain. Wastewater treatment systems specifically designed to handle sodium compounds must be implemented to prevent contamination of local water sources.
Material selection for scaled production should prioritize environmentally benign alternatives where possible, particularly for electrolytes and separators. Recent research indicates that certain polymer-based electrolytes can reduce environmental impact while maintaining performance specifications. Additionally, the development of recycling protocols specific to room-temperature sodium-sulfur batteries is crucial for sustainable deployment, as these batteries contain valuable materials that should be recovered rather than disposed of in landfills.
Regulatory compliance represents another critical dimension, with varying requirements across different jurisdictions. Manufacturers must navigate complex regulatory frameworks including hazardous material transportation regulations, workplace safety standards, and environmental protection laws. Early engagement with regulatory bodies during the scaling process can prevent costly redesigns and delays in commercialization.
Long-term environmental monitoring plans should be established to assess any unforeseen impacts as production scales. This includes air quality monitoring around manufacturing facilities, groundwater testing, and occupational health surveillance for workers regularly exposed to battery materials. Such proactive measures not only protect environmental and human health but also build public trust in this emerging technology.
Primary safety measures must include specialized handling equipment for sodium metal, such as glove boxes with inert atmospheres, and appropriate personal protective equipment for all personnel. Facilities require advanced fire suppression systems specifically designed for metal fires, as conventional water-based systems can exacerbate sodium-related incidents. Temperature monitoring systems are essential to prevent thermal runaway scenarios that could lead to catastrophic failures during battery operation or manufacturing.
Environmental considerations extend beyond immediate safety concerns to the full lifecycle impact of these batteries. The manufacturing process must incorporate closed-loop systems to capture and neutralize sulfur emissions, preventing the release of sulfur dioxide and hydrogen sulfide that could contribute to air pollution and acid rain. Wastewater treatment systems specifically designed to handle sodium compounds must be implemented to prevent contamination of local water sources.
Material selection for scaled production should prioritize environmentally benign alternatives where possible, particularly for electrolytes and separators. Recent research indicates that certain polymer-based electrolytes can reduce environmental impact while maintaining performance specifications. Additionally, the development of recycling protocols specific to room-temperature sodium-sulfur batteries is crucial for sustainable deployment, as these batteries contain valuable materials that should be recovered rather than disposed of in landfills.
Regulatory compliance represents another critical dimension, with varying requirements across different jurisdictions. Manufacturers must navigate complex regulatory frameworks including hazardous material transportation regulations, workplace safety standards, and environmental protection laws. Early engagement with regulatory bodies during the scaling process can prevent costly redesigns and delays in commercialization.
Long-term environmental monitoring plans should be established to assess any unforeseen impacts as production scales. This includes air quality monitoring around manufacturing facilities, groundwater testing, and occupational health surveillance for workers regularly exposed to battery materials. Such proactive measures not only protect environmental and human health but also build public trust in this emerging technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!