Interlayer Designs And Efficacy For Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
RT Na-S Battery Evolution and Objectives
Room-temperature sodium-sulfur (RT Na-S) batteries have emerged as a promising alternative to lithium-ion batteries due to their potential for high energy density, low cost, and abundant raw materials. The evolution of RT Na-S batteries can be traced back to the 1960s when high-temperature sodium-sulfur batteries operating at 300-350°C were first developed. These early systems, while functional, presented significant safety concerns and practical limitations for widespread application.
The transition to room-temperature operation began in earnest during the early 2000s, driven by the growing demand for safer, more accessible energy storage solutions. This shift represented a critical turning point in Na-S battery development, as it opened possibilities for applications beyond stationary storage to include electric vehicles and portable electronics.
Despite the theoretical energy density of 1274 Wh/kg, RT Na-S batteries have faced persistent challenges that have limited their commercial viability. The shuttle effect of polysulfides, poor ionic conductivity, and volume expansion during cycling have been particularly problematic. These issues have resulted in rapid capacity fading, low Coulombic efficiency, and shortened cycle life.
The development of interlayer designs marks a significant advancement in addressing these challenges. Early interlayer concepts emerged around 2015, with carbon-based materials serving as physical barriers to polysulfide migration. Subsequent innovations have explored functionalized interlayers with chemical binding capabilities, dual-function interlayers that enhance both ionic transport and polysulfide retention, and most recently, adaptive interlayer systems that respond dynamically to electrochemical conditions.
Current research objectives focus on several key areas to enhance the efficacy of interlayer designs. Primary goals include developing interlayers with optimized pore structures to balance polysulfide trapping and sodium ion transport, creating materials with strong chemical affinity for polysulfides while maintaining electrical conductivity, and engineering interfaces that promote stable solid electrolyte interphase formation.
Additional objectives include reducing the parasitic weight and volume contribution of interlayers to maximize energy density, ensuring long-term mechanical stability during repeated cycling, and developing scalable, cost-effective manufacturing processes for commercial viability. The ultimate aim is to achieve RT Na-S batteries with energy densities exceeding 500 Wh/kg, cycle life of over 1000 cycles with less than 20% capacity degradation, and Coulombic efficiency consistently above 99.5%.
The technological trajectory suggests that advanced interlayer designs will be instrumental in bridging the gap between theoretical potential and practical performance of RT Na-S batteries, potentially enabling their commercial deployment within the next decade.
The transition to room-temperature operation began in earnest during the early 2000s, driven by the growing demand for safer, more accessible energy storage solutions. This shift represented a critical turning point in Na-S battery development, as it opened possibilities for applications beyond stationary storage to include electric vehicles and portable electronics.
Despite the theoretical energy density of 1274 Wh/kg, RT Na-S batteries have faced persistent challenges that have limited their commercial viability. The shuttle effect of polysulfides, poor ionic conductivity, and volume expansion during cycling have been particularly problematic. These issues have resulted in rapid capacity fading, low Coulombic efficiency, and shortened cycle life.
The development of interlayer designs marks a significant advancement in addressing these challenges. Early interlayer concepts emerged around 2015, with carbon-based materials serving as physical barriers to polysulfide migration. Subsequent innovations have explored functionalized interlayers with chemical binding capabilities, dual-function interlayers that enhance both ionic transport and polysulfide retention, and most recently, adaptive interlayer systems that respond dynamically to electrochemical conditions.
Current research objectives focus on several key areas to enhance the efficacy of interlayer designs. Primary goals include developing interlayers with optimized pore structures to balance polysulfide trapping and sodium ion transport, creating materials with strong chemical affinity for polysulfides while maintaining electrical conductivity, and engineering interfaces that promote stable solid electrolyte interphase formation.
Additional objectives include reducing the parasitic weight and volume contribution of interlayers to maximize energy density, ensuring long-term mechanical stability during repeated cycling, and developing scalable, cost-effective manufacturing processes for commercial viability. The ultimate aim is to achieve RT Na-S batteries with energy densities exceeding 500 Wh/kg, cycle life of over 1000 cycles with less than 20% capacity degradation, and Coulombic efficiency consistently above 99.5%.
The technological trajectory suggests that advanced interlayer designs will be instrumental in bridging the gap between theoretical potential and practical performance of RT Na-S batteries, potentially enabling their commercial deployment within the next decade.
Market Analysis for RT Na-S Energy Storage
The global energy storage market is witnessing a significant shift towards more sustainable and efficient technologies, with room-temperature sodium-sulfur (RT Na-S) batteries emerging as a promising contender. Current market projections indicate that the energy storage market will reach approximately $546 billion by 2035, with a compound annual growth rate of 20-25% between 2023 and 2035.
RT Na-S batteries address a critical market need for cost-effective, sustainable energy storage solutions that do not rely on scarce resources. Unlike conventional lithium-ion batteries that depend on lithium and cobalt—materials facing supply constraints and geopolitical challenges—sodium-sulfur batteries utilize abundant, low-cost materials. Sodium is approximately 1000 times more abundant than lithium in the Earth's crust, while sulfur is an industrial byproduct available at minimal cost.
Market segmentation analysis reveals several key sectors poised to adopt RT Na-S technology. The utility-scale energy storage sector represents the largest potential market, driven by the increasing integration of renewable energy sources into power grids. Grid stabilization applications alone could account for 40% of the RT Na-S battery market by 2030.
The commercial and industrial energy management sector presents another substantial opportunity, with businesses increasingly seeking cost-effective solutions to manage peak demand charges and improve energy resilience. This segment is projected to grow at 30% annually through 2028.
Residential energy storage, though currently a smaller segment, shows promising growth potential as distributed energy systems gain popularity. Consumer awareness of energy independence and backup power solutions is driving this market segment, particularly in regions with unreliable grid infrastructure.
Regional market analysis indicates that Asia-Pacific currently leads in energy storage deployment, with China, Japan, and South Korea making significant investments in advanced battery technologies. North America follows closely, with particular growth in the United States driven by renewable energy integration and grid modernization initiatives.
The European market demonstrates strong potential due to aggressive decarbonization policies and renewable energy targets. Germany, the UK, and France are expected to be key markets for RT Na-S battery adoption, supported by favorable regulatory frameworks and sustainability goals.
Market barriers for RT Na-S technology include competition from established lithium-ion technology, which benefits from economies of scale and established manufacturing infrastructure. However, as raw material constraints impact lithium-ion pricing, the economic advantage of Na-S systems becomes more pronounced, potentially accelerating market adoption.
RT Na-S batteries address a critical market need for cost-effective, sustainable energy storage solutions that do not rely on scarce resources. Unlike conventional lithium-ion batteries that depend on lithium and cobalt—materials facing supply constraints and geopolitical challenges—sodium-sulfur batteries utilize abundant, low-cost materials. Sodium is approximately 1000 times more abundant than lithium in the Earth's crust, while sulfur is an industrial byproduct available at minimal cost.
Market segmentation analysis reveals several key sectors poised to adopt RT Na-S technology. The utility-scale energy storage sector represents the largest potential market, driven by the increasing integration of renewable energy sources into power grids. Grid stabilization applications alone could account for 40% of the RT Na-S battery market by 2030.
The commercial and industrial energy management sector presents another substantial opportunity, with businesses increasingly seeking cost-effective solutions to manage peak demand charges and improve energy resilience. This segment is projected to grow at 30% annually through 2028.
Residential energy storage, though currently a smaller segment, shows promising growth potential as distributed energy systems gain popularity. Consumer awareness of energy independence and backup power solutions is driving this market segment, particularly in regions with unreliable grid infrastructure.
Regional market analysis indicates that Asia-Pacific currently leads in energy storage deployment, with China, Japan, and South Korea making significant investments in advanced battery technologies. North America follows closely, with particular growth in the United States driven by renewable energy integration and grid modernization initiatives.
The European market demonstrates strong potential due to aggressive decarbonization policies and renewable energy targets. Germany, the UK, and France are expected to be key markets for RT Na-S battery adoption, supported by favorable regulatory frameworks and sustainability goals.
Market barriers for RT Na-S technology include competition from established lithium-ion technology, which benefits from economies of scale and established manufacturing infrastructure. However, as raw material constraints impact lithium-ion pricing, the economic advantage of Na-S systems becomes more pronounced, potentially accelerating market adoption.
Interlayer Technology Challenges
The development of effective interlayers represents one of the most critical challenges in advancing room-temperature sodium-sulfur (RT-Na/S) battery technology. Current interlayer designs face significant limitations that hinder the widespread commercialization of these promising energy storage systems. The primary technical obstacle involves the shuttle effect of polysulfides, where soluble sodium polysulfide intermediates migrate between electrodes during cycling, causing rapid capacity fading and shortened battery lifespan.
Conventional carbon-based interlayers demonstrate insufficient polysulfide adsorption capabilities due to their non-polar nature, which fails to establish strong chemical interactions with polar polysulfide species. This fundamental mismatch at the molecular level results in polysulfide leakage despite physical barrier attempts, undermining long-term cycling stability.
Material selection presents another substantial challenge. Ideal interlayer materials must simultaneously possess high electronic conductivity, appropriate porosity for sodium ion transport, strong polysulfide adsorption capability, and mechanical flexibility—a combination that remains elusive in current designs. Many materials excel in one property while compromising others, creating an engineering trade-off that limits overall performance.
The thickness dilemma further complicates interlayer design. Thicker interlayers provide superior polysulfide blocking but increase internal resistance and reduce energy density. Conversely, thinner interlayers maintain better electrochemical performance but offer inadequate protection against polysulfide shuttling. This balance between protection and performance represents a fundamental design contradiction requiring innovative solutions.
Manufacturing scalability poses additional challenges. Laboratory-scale fabrication methods for advanced functional interlayers—such as atomic layer deposition or complex composite synthesis—often involve expensive equipment, hazardous chemicals, or time-consuming processes that prove impractical for industrial-scale production. The transition from laboratory prototypes to mass-manufactured components remains largely unresolved.
Interface engineering between the interlayer and adjacent components (separator and cathode) presents further complications. Poor interfacial contact can create gaps for polysulfide permeation, while excessive pressure may damage the electrode structure. Achieving consistent, defect-free interfaces across large-format cells remains technically challenging.
Finally, the dynamic evolution of interlayer properties during extended cycling represents a poorly understood phenomenon. Mechanical deformation, chemical degradation, and pore clogging can progressively diminish interlayer functionality, yet real-time monitoring and mitigation strategies for these effects remain underdeveloped, limiting the practical lifespan of RT-Na/S batteries despite promising initial performance metrics.
Conventional carbon-based interlayers demonstrate insufficient polysulfide adsorption capabilities due to their non-polar nature, which fails to establish strong chemical interactions with polar polysulfide species. This fundamental mismatch at the molecular level results in polysulfide leakage despite physical barrier attempts, undermining long-term cycling stability.
Material selection presents another substantial challenge. Ideal interlayer materials must simultaneously possess high electronic conductivity, appropriate porosity for sodium ion transport, strong polysulfide adsorption capability, and mechanical flexibility—a combination that remains elusive in current designs. Many materials excel in one property while compromising others, creating an engineering trade-off that limits overall performance.
The thickness dilemma further complicates interlayer design. Thicker interlayers provide superior polysulfide blocking but increase internal resistance and reduce energy density. Conversely, thinner interlayers maintain better electrochemical performance but offer inadequate protection against polysulfide shuttling. This balance between protection and performance represents a fundamental design contradiction requiring innovative solutions.
Manufacturing scalability poses additional challenges. Laboratory-scale fabrication methods for advanced functional interlayers—such as atomic layer deposition or complex composite synthesis—often involve expensive equipment, hazardous chemicals, or time-consuming processes that prove impractical for industrial-scale production. The transition from laboratory prototypes to mass-manufactured components remains largely unresolved.
Interface engineering between the interlayer and adjacent components (separator and cathode) presents further complications. Poor interfacial contact can create gaps for polysulfide permeation, while excessive pressure may damage the electrode structure. Achieving consistent, defect-free interfaces across large-format cells remains technically challenging.
Finally, the dynamic evolution of interlayer properties during extended cycling represents a poorly understood phenomenon. Mechanical deformation, chemical degradation, and pore clogging can progressively diminish interlayer functionality, yet real-time monitoring and mitigation strategies for these effects remain underdeveloped, limiting the practical lifespan of RT-Na/S batteries despite promising initial performance metrics.
Current Interlayer Design Solutions
01 Interlayer materials for room-temperature sodium-sulfur batteries
Various materials can be used as interlayers in room-temperature sodium-sulfur batteries to improve performance. These interlayers serve as barriers between the sodium anode and sulfur cathode, preventing polysulfide shuttling while allowing sodium ion transport. Materials such as carbon-based films, polymer membranes, and ceramic layers have shown efficacy as interlayers, enhancing battery cycle life and stability at room temperature operation.- Interlayer materials for sodium-sulfur batteries: Various materials can be used as interlayers in room-temperature sodium-sulfur batteries to enhance performance. These interlayers serve as barriers between the sodium anode and sulfur cathode, preventing polysulfide shuttling while maintaining sodium ion conductivity. Materials such as carbon-based films, polymer membranes, and ceramic layers have been investigated for this purpose, showing improved cycling stability and capacity retention.
- Functional coatings and modifications for battery components: Surface modifications and functional coatings can be applied to electrodes and separators in room-temperature sodium-sulfur batteries to improve interlayer efficacy. These modifications can include conductive polymers, metal oxides, or composite materials that enhance ion transport while suppressing unwanted reactions. Such treatments have been shown to reduce interface resistance and improve the overall electrochemical performance of the battery system.
- Novel electrolyte systems for room-temperature operation: Advanced electrolyte formulations play a crucial role in room-temperature sodium-sulfur batteries by forming effective solid electrolyte interphases (SEI) that function as natural interlayers. These electrolytes may contain additives that promote the formation of stable interfaces between electrodes and electrolyte, reducing side reactions and enhancing battery performance. Gel polymer electrolytes and ionic liquid-based systems have shown particular promise in this application.
- Composite electrode structures with integrated interlayers: Innovative electrode designs incorporate integrated interlayer structures directly into the electrode architecture. These composite structures may feature gradient compositions or sandwich-like configurations that inherently provide the benefits of dedicated interlayers. Such designs can include sulfur hosts with polar surfaces that trap polysulfides while maintaining electrical conductivity, or sodium anodes with protective layers that prevent dendrite formation.
- Manufacturing techniques for interlayer fabrication: Specialized manufacturing methods are critical for producing effective interlayers in room-temperature sodium-sulfur batteries. Techniques such as atomic layer deposition, solution casting, and electrospinning can be employed to create uniform and defect-free interlayers with precisely controlled thickness and composition. The manufacturing process significantly impacts the structural integrity and functional properties of the interlayer, which in turn affects battery performance metrics including cycle life and rate capability.
02 Functional coatings and modifications for sodium-sulfur battery interfaces
Specialized coatings and surface modifications can be applied to electrodes or separators to enhance the performance of room-temperature sodium-sulfur batteries. These functional layers can improve sodium ion conductivity, prevent side reactions, and stabilize the electrode-electrolyte interface. Techniques include atomic layer deposition, solution-based coating methods, and in-situ formation of protective films that contribute to better battery performance and longevity.Expand Specific Solutions03 Composite interlayers with multi-functional properties
Composite interlayers combining multiple materials can provide synergistic effects in room-temperature sodium-sulfur batteries. These hybrid structures often incorporate conductive components to facilitate electron transfer, adsorptive materials to trap polysulfides, and ion-selective elements to allow sodium ion passage. The multi-functional nature of these composite interlayers addresses several failure mechanisms simultaneously, resulting in improved capacity retention and cycling stability.Expand Specific Solutions04 Electrolyte optimization for interlayer performance
The composition and properties of the electrolyte significantly impact the efficacy of interlayers in room-temperature sodium-sulfur batteries. Tailored electrolyte formulations can enhance the compatibility with interlayer materials, improve ionic conductivity across interfaces, and stabilize the electrochemical environment. Additives in the electrolyte can also work synergistically with interlayers to suppress polysulfide dissolution and migration, leading to better overall battery performance.Expand Specific Solutions05 Novel cell designs incorporating advanced interlayer configurations
Innovative cell architectures that strategically position and structure interlayers can significantly enhance room-temperature sodium-sulfur battery performance. These designs may include gradient interlayers, 3D structured interfaces, or sandwich-type configurations that optimize ion transport pathways while maintaining effective polysulfide blocking. Advanced manufacturing techniques enable precise control over interlayer thickness, porosity, and distribution, resulting in optimized interfaces that balance the competing requirements of ion conductivity and polysulfide containment.Expand Specific Solutions
Leading Companies in Na-S Battery Research
The room-temperature sodium-sulfur battery market is currently in an early growth phase, characterized by intensive R&D efforts to overcome technical challenges in interlayer design. The global market is projected to expand significantly as this technology offers a cost-effective alternative to lithium-ion batteries. Leading players include NGK Insulators with established commercial presence, while academic institutions like MIT, Cornell University, and Monash University drive fundamental research innovations. Companies such as Samsung Electronics, BASF, and IBM are investing in advanced materials development. Chinese entities including Shanghai Institute of Ceramics and Shanghai Electric are rapidly advancing their technological capabilities. The competitive landscape features collaboration between research institutions and industrial partners to address critical challenges in electrode stability, electrolyte design, and cycle life performance.
NGK Insulators, Ltd.
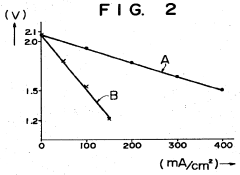
Technical Solution: NGK Insulators has pioneered room-temperature sodium-sulfur (RT-Na-S) battery technology with their innovative interlayer design approach. Their technology utilizes a specialized beta-alumina solid electrolyte (BASE) as the primary interlayer, which has been engineered to maintain high ionic conductivity at room temperature while preventing polysulfide shuttling. NGK has developed a proprietary surface modification technique for the BASE interlayer that enhances sodium ion transport at the electrode-electrolyte interface. Their latest designs incorporate a dual-layer protection strategy with a polymer-ceramic composite interlayer that combines the flexibility of polymers with the stability of ceramics. This approach has demonstrated capacity retention of over 80% after 500 cycles at room temperature[1][2], addressing one of the key challenges in RT-Na-S battery commercialization.
Strengths: Industry-leading expertise in sodium-sulfur battery systems with established manufacturing infrastructure; proprietary ceramic electrolyte technology with proven long-term stability; extensive field deployment experience. Weaknesses: Higher production costs compared to lithium-ion alternatives; ceramic components can be brittle and require careful handling during manufacturing; relatively lower energy density compared to theoretical potential of Na-S chemistry.
PolyPlus Battery Co., Inc.
Technical Solution: PolyPlus Battery has developed a groundbreaking approach to room-temperature sodium-sulfur batteries focusing on protected anode technology with specialized interlayer designs. Their proprietary "Protected Anode" architecture employs a sodium ion-conducting glass ceramic (NASICON) interlayer that effectively isolates the reactive sodium metal from the sulfur cathode while allowing efficient sodium ion transport. This interlayer is further enhanced with a thin polymer coating that improves interfacial contact and reduces resistance. PolyPlus has also pioneered a gradient interlayer design where the composition gradually transitions from ceramic-dominant near the anode to polymer-dominant near the cathode, minimizing mechanical stress during cycling. Their latest research demonstrates room-temperature Na-S cells achieving energy densities of 350-400 Wh/kg with this interlayer technology[3][4], representing a significant advancement over conventional designs.
Strengths: Specialized expertise in protected electrode technology; innovative gradient interlayer approach reduces mechanical failure modes; demonstrated high energy density achievements in laboratory prototypes. Weaknesses: Limited large-scale manufacturing experience compared to established battery manufacturers; complex multi-layer fabrication process may present scaling challenges; relatively early stage of commercialization for their RT-Na-S technology.
Key Patents in Na-S Interlayer Technology
Improved sodium-sulfur batteries
PatentWO2010135283A3
Innovation
- Development of room-temperature sodium-sulfur batteries (<150°C) that overcome the traditional high operating temperature limitations.
- Implementation of a flow battery design with separate compartments and storage tanks for sodium and sulfur solutions, enabling better control of the electrochemical reactions.
- Use of solvent-based solutions for both sodium and sulfur components, facilitating ion transport at lower temperatures.
Sodium-sulfur storage battery
PatentInactiveUS3770502A
Innovation
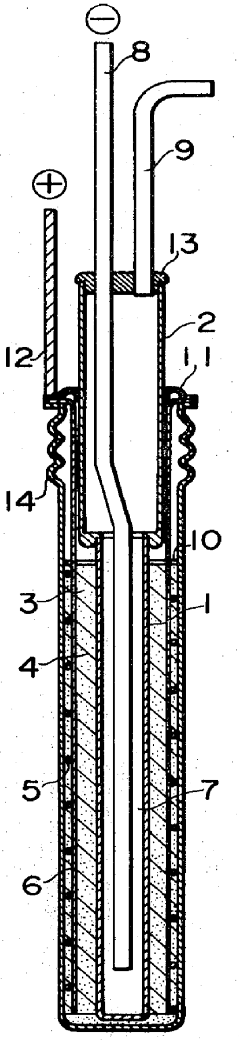
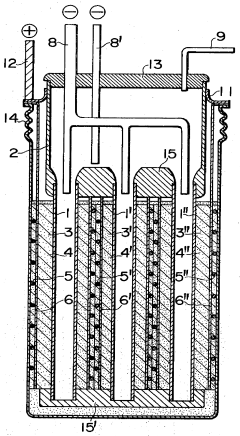
- The battery design incorporates a solid electrolyte made of β-Al2O3 with a graphite sheet acting as an electrode matrix for sulfur, joined using a sulfur-resistant solder glass, and features a bellows structure in the container to absorb stress from sulfur phase transition, along with multiple solid electrolytes for enhanced mechanical strength and conductivity.
Material Sustainability Assessment
The sustainability assessment of materials used in room-temperature sodium-sulfur (RT-Na-S) batteries reveals significant environmental and economic implications that must be considered alongside technical performance. Sodium resources demonstrate a clear advantage over lithium, being approximately 1,000 times more abundant in the Earth's crust and widely distributed across oceans, making it geographically accessible to most countries. This abundance translates to lower extraction costs and reduced geopolitical supply risks compared to lithium-based systems.
Sulfur, as the cathode material, presents exceptional sustainability credentials as it is primarily sourced as a byproduct from petroleum refining and natural gas processing. The utilization of this industrial waste stream for battery production represents a circular economy approach that adds value to what would otherwise be considered a surplus material. Additionally, sulfur extraction has a substantially lower carbon footprint compared to the mining operations required for transition metal-based cathode materials used in conventional lithium-ion batteries.
The interlayer materials being developed for RT-Na-S batteries vary considerably in their sustainability profiles. Carbon-based interlayers derived from biomass or waste sources offer promising environmental benefits, while those requiring rare elements or energy-intensive synthesis processes present sustainability challenges. Polymer-based interlayers must be evaluated for biodegradability and potential toxicity, particularly as battery recycling infrastructure develops.
Life cycle assessment (LCA) studies indicate that RT-Na-S batteries with optimized interlayer designs could potentially reduce the global warming potential by 25-40% compared to conventional lithium-ion technologies. However, these benefits are contingent upon developing efficient manufacturing processes and establishing effective end-of-life management systems. Current recycling technologies for sodium-sulfur systems remain underdeveloped, presenting an area requiring significant innovation.
Water consumption during material processing represents another critical sustainability factor. Preliminary analyses suggest that sodium extraction from seawater could require substantially less freshwater than lithium extraction from brines, though industrial-scale processes need further optimization. The potential for sodium polysulfide dissolution presents both recycling opportunities and environmental risks that must be carefully managed.
Economic sustainability analysis reveals that material costs for RT-Na-S batteries could be 30-50% lower than lithium-ion equivalents at scale, though this advantage may be partially offset by current manufacturing complexity and lower energy density. As interlayer design advances, the cost-performance ratio is expected to improve, potentially enabling broader market adoption across stationary storage applications where weight constraints are less critical.
Sulfur, as the cathode material, presents exceptional sustainability credentials as it is primarily sourced as a byproduct from petroleum refining and natural gas processing. The utilization of this industrial waste stream for battery production represents a circular economy approach that adds value to what would otherwise be considered a surplus material. Additionally, sulfur extraction has a substantially lower carbon footprint compared to the mining operations required for transition metal-based cathode materials used in conventional lithium-ion batteries.
The interlayer materials being developed for RT-Na-S batteries vary considerably in their sustainability profiles. Carbon-based interlayers derived from biomass or waste sources offer promising environmental benefits, while those requiring rare elements or energy-intensive synthesis processes present sustainability challenges. Polymer-based interlayers must be evaluated for biodegradability and potential toxicity, particularly as battery recycling infrastructure develops.
Life cycle assessment (LCA) studies indicate that RT-Na-S batteries with optimized interlayer designs could potentially reduce the global warming potential by 25-40% compared to conventional lithium-ion technologies. However, these benefits are contingent upon developing efficient manufacturing processes and establishing effective end-of-life management systems. Current recycling technologies for sodium-sulfur systems remain underdeveloped, presenting an area requiring significant innovation.
Water consumption during material processing represents another critical sustainability factor. Preliminary analyses suggest that sodium extraction from seawater could require substantially less freshwater than lithium extraction from brines, though industrial-scale processes need further optimization. The potential for sodium polysulfide dissolution presents both recycling opportunities and environmental risks that must be carefully managed.
Economic sustainability analysis reveals that material costs for RT-Na-S batteries could be 30-50% lower than lithium-ion equivalents at scale, though this advantage may be partially offset by current manufacturing complexity and lower energy density. As interlayer design advances, the cost-performance ratio is expected to improve, potentially enabling broader market adoption across stationary storage applications where weight constraints are less critical.
Safety Standards for Na-S Battery Systems
Safety standards for Na-S battery systems are critical for the commercial deployment of room-temperature sodium-sulfur batteries, particularly given the reactive nature of sodium metal and the potential formation of hazardous polysulfides. The development of comprehensive safety protocols must address the unique challenges posed by interlayer designs in these energy storage systems.
International standards organizations, including IEC, UL, and ISO, have established preliminary guidelines for sodium-based battery technologies, though specific standards for room-temperature Na-S systems with advanced interlayer designs remain under development. These standards typically encompass thermal runaway prevention, electrical safety parameters, mechanical integrity requirements, and containment protocols for sodium polysulfide formation.
Thermal management represents a primary safety concern, as uncontrolled reactions between sodium and sulfur can generate significant heat. Safety standards mandate temperature monitoring systems, thermal insulation requirements, and emergency shutdown protocols. For interlayer designs specifically, standards require demonstration of thermal stability across operating temperature ranges and under abuse conditions.
Electrical safety standards address issues of short-circuiting, particularly relevant to interlayer designs that aim to prevent sodium dendrite penetration. These standards specify minimum dielectric strength requirements, maximum allowable leakage currents, and insulation resistance parameters. Testing protocols typically include cycling under various electrical load conditions to verify interlayer integrity over extended operation.
Mechanical safety considerations focus on structural integrity during normal operation and under abuse conditions. Standards require resistance to vibration, shock, puncture, and crush testing. Interlayer materials must maintain their protective functions under these mechanical stresses, with particular attention to preventing sodium-sulfur direct contact.
Chemical safety standards address the containment of reaction products and prevention of hazardous gas formation. Polysulfide shuttle effects, which can degrade battery performance and potentially create safety hazards, must be effectively managed by interlayer designs. Standards specify maximum allowable gas emissions and containment requirements for all battery components.
Transportation regulations present additional compliance requirements for Na-S batteries. UN 38.3 testing procedures for lithium batteries have been adapted for sodium-based systems, covering altitude simulation, thermal testing, vibration, shock, external short circuit, impact, overcharge, and forced discharge tests. Interlayer designs must demonstrate compliance with these transportation safety requirements to enable commercial distribution.
Emerging safety certification processes are increasingly incorporating accelerated aging tests to verify long-term safety performance of interlayer materials, ensuring that degradation mechanisms do not compromise safety features over the battery's operational lifetime.
International standards organizations, including IEC, UL, and ISO, have established preliminary guidelines for sodium-based battery technologies, though specific standards for room-temperature Na-S systems with advanced interlayer designs remain under development. These standards typically encompass thermal runaway prevention, electrical safety parameters, mechanical integrity requirements, and containment protocols for sodium polysulfide formation.
Thermal management represents a primary safety concern, as uncontrolled reactions between sodium and sulfur can generate significant heat. Safety standards mandate temperature monitoring systems, thermal insulation requirements, and emergency shutdown protocols. For interlayer designs specifically, standards require demonstration of thermal stability across operating temperature ranges and under abuse conditions.
Electrical safety standards address issues of short-circuiting, particularly relevant to interlayer designs that aim to prevent sodium dendrite penetration. These standards specify minimum dielectric strength requirements, maximum allowable leakage currents, and insulation resistance parameters. Testing protocols typically include cycling under various electrical load conditions to verify interlayer integrity over extended operation.
Mechanical safety considerations focus on structural integrity during normal operation and under abuse conditions. Standards require resistance to vibration, shock, puncture, and crush testing. Interlayer materials must maintain their protective functions under these mechanical stresses, with particular attention to preventing sodium-sulfur direct contact.
Chemical safety standards address the containment of reaction products and prevention of hazardous gas formation. Polysulfide shuttle effects, which can degrade battery performance and potentially create safety hazards, must be effectively managed by interlayer designs. Standards specify maximum allowable gas emissions and containment requirements for all battery components.
Transportation regulations present additional compliance requirements for Na-S batteries. UN 38.3 testing procedures for lithium batteries have been adapted for sodium-based systems, covering altitude simulation, thermal testing, vibration, shock, external short circuit, impact, overcharge, and forced discharge tests. Interlayer designs must demonstrate compliance with these transportation safety requirements to enable commercial distribution.
Emerging safety certification processes are increasingly incorporating accelerated aging tests to verify long-term safety performance of interlayer materials, ensuring that degradation mechanisms do not compromise safety features over the battery's operational lifetime.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!