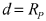Lessons From Na–Se And Na–Te For Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-S Battery Evolution and Research Objectives
Sodium-sulfur (Na-S) battery technology has evolved significantly since its inception in the 1960s by Ford Motor Company. Initially developed as high-temperature systems operating at 300-350°C, these batteries utilized molten sodium and sulfur electrodes separated by a solid beta-alumina ceramic electrolyte. This configuration delivered impressive energy density (>150 Wh/kg) and long cycle life, leading to their deployment in large-scale energy storage applications. However, the high operating temperatures posed significant safety concerns and limited their widespread adoption in portable applications.
The pursuit of room-temperature Na-S batteries began in earnest during the early 2000s, driven by the need for safer, more versatile energy storage solutions. This shift represented a paradigm change in Na-S technology, requiring fundamental redesigns of electrode materials, electrolytes, and cell architectures to enable sodium-sulfur electrochemistry at ambient temperatures. The theoretical specific capacity of sulfur (1672 mAh/g) and energy density (1230 Wh/kg) made this pursuit particularly attractive for next-generation energy storage.
Despite these promising theoretical metrics, room-temperature Na-S batteries have faced persistent challenges including poor sulfur utilization, severe polysulfide shuttling, and rapid capacity fading. These issues have limited practical energy densities to a fraction of theoretical values and restricted cycle life to typically fewer than 100 cycles in early implementations. The sodium metal anode also presents challenges related to dendrite formation and unstable solid electrolyte interphase (SEI) formation.
Recent research has increasingly focused on learning from analogous systems, particularly Na-Se and Na-Te batteries, which share similar electrochemical mechanisms but exhibit distinct advantages in certain aspects. These chalcogen-based systems demonstrate improved reaction kinetics and more stable discharge products compared to sulfur, providing valuable insights for Na-S battery development.
The primary research objectives for room-temperature Na-S batteries now center on several key areas: developing advanced carbon-sulfur composite cathodes with optimized pore structures to enhance sulfur utilization; designing functional electrolyte additives and separators to mitigate polysulfide shuttling; exploring novel sodium anode protection strategies; and understanding the fundamental reaction mechanisms through advanced characterization techniques. The ultimate goal is to achieve Na-S batteries with energy densities exceeding 300 Wh/kg, cycle life beyond 1000 cycles, and improved safety profiles.
This technology evolution represents a critical pathway toward sustainable, high-performance energy storage systems that could potentially address the limitations of current lithium-ion technologies while utilizing more abundant and cost-effective materials. The lessons from Na-Se and Na-Te systems offer promising directions for overcoming the persistent challenges in room-temperature Na-S battery development.
The pursuit of room-temperature Na-S batteries began in earnest during the early 2000s, driven by the need for safer, more versatile energy storage solutions. This shift represented a paradigm change in Na-S technology, requiring fundamental redesigns of electrode materials, electrolytes, and cell architectures to enable sodium-sulfur electrochemistry at ambient temperatures. The theoretical specific capacity of sulfur (1672 mAh/g) and energy density (1230 Wh/kg) made this pursuit particularly attractive for next-generation energy storage.
Despite these promising theoretical metrics, room-temperature Na-S batteries have faced persistent challenges including poor sulfur utilization, severe polysulfide shuttling, and rapid capacity fading. These issues have limited practical energy densities to a fraction of theoretical values and restricted cycle life to typically fewer than 100 cycles in early implementations. The sodium metal anode also presents challenges related to dendrite formation and unstable solid electrolyte interphase (SEI) formation.
Recent research has increasingly focused on learning from analogous systems, particularly Na-Se and Na-Te batteries, which share similar electrochemical mechanisms but exhibit distinct advantages in certain aspects. These chalcogen-based systems demonstrate improved reaction kinetics and more stable discharge products compared to sulfur, providing valuable insights for Na-S battery development.
The primary research objectives for room-temperature Na-S batteries now center on several key areas: developing advanced carbon-sulfur composite cathodes with optimized pore structures to enhance sulfur utilization; designing functional electrolyte additives and separators to mitigate polysulfide shuttling; exploring novel sodium anode protection strategies; and understanding the fundamental reaction mechanisms through advanced characterization techniques. The ultimate goal is to achieve Na-S batteries with energy densities exceeding 300 Wh/kg, cycle life beyond 1000 cycles, and improved safety profiles.
This technology evolution represents a critical pathway toward sustainable, high-performance energy storage systems that could potentially address the limitations of current lithium-ion technologies while utilizing more abundant and cost-effective materials. The lessons from Na-Se and Na-Te systems offer promising directions for overcoming the persistent challenges in room-temperature Na-S battery development.
Market Analysis for Room-Temperature Na-S Battery Applications
The global market for energy storage solutions is experiencing significant growth, with room-temperature sodium-sulfur (RT Na-S) batteries emerging as a promising alternative to conventional lithium-ion technologies. The market potential for RT Na-S batteries is primarily driven by their cost advantage, as sodium and sulfur are abundant elements with established supply chains, offering a substantial reduction in raw material costs compared to lithium-based systems.
Current market projections indicate that the stationary energy storage sector represents the most immediate opportunity for RT Na-S battery deployment. This sector is expected to grow at a CAGR of over 20% through 2030, creating a substantial addressable market for emerging battery technologies. The increasing integration of renewable energy sources into power grids worldwide necessitates efficient and economical energy storage solutions, positioning RT Na-S batteries as a competitive option.
Industrial and utility-scale applications present the most promising initial market entry points for RT Na-S technology. These sectors prioritize cost-effectiveness and longevity over energy density constraints, aligning well with the current performance profile of RT Na-S batteries. The lessons learned from sodium-selenium and sodium-tellurium systems regarding interface stability and reaction kinetics directly translate to improved commercial viability in these applications.
Regional market analysis reveals varying adoption potentials. Asia-Pacific, particularly China and India, demonstrates the highest growth potential due to aggressive renewable energy targets and substantial grid infrastructure investments. Europe follows closely, driven by stringent carbon reduction policies and energy independence initiatives. North America presents a more fragmented market landscape with opportunities concentrated in states with progressive energy policies.
Market barriers for RT Na-S battery commercialization include competition from established lithium-ion technologies, which benefit from economies of scale and established manufacturing infrastructure. Additionally, emerging technologies like flow batteries and other sodium-based chemistries (Na-ion) compete for the same market segments, creating a crowded competitive landscape.
Consumer electronics and electric vehicle markets remain challenging for near-term RT Na-S battery adoption due to energy density limitations. However, insights from Na-Se and Na-Te systems regarding reaction mechanisms and electrode structures suggest pathways to overcome these limitations, potentially expanding addressable markets in the medium to long term.
Market acceptance will be accelerated by demonstrating safety advantages over lithium-ion batteries, particularly the elimination of thermal runaway risks. This safety profile, combined with the sustainability narrative of using abundant materials, creates a compelling market positioning strategy for RT Na-S battery technologies in environmentally conscious market segments.
Current market projections indicate that the stationary energy storage sector represents the most immediate opportunity for RT Na-S battery deployment. This sector is expected to grow at a CAGR of over 20% through 2030, creating a substantial addressable market for emerging battery technologies. The increasing integration of renewable energy sources into power grids worldwide necessitates efficient and economical energy storage solutions, positioning RT Na-S batteries as a competitive option.
Industrial and utility-scale applications present the most promising initial market entry points for RT Na-S technology. These sectors prioritize cost-effectiveness and longevity over energy density constraints, aligning well with the current performance profile of RT Na-S batteries. The lessons learned from sodium-selenium and sodium-tellurium systems regarding interface stability and reaction kinetics directly translate to improved commercial viability in these applications.
Regional market analysis reveals varying adoption potentials. Asia-Pacific, particularly China and India, demonstrates the highest growth potential due to aggressive renewable energy targets and substantial grid infrastructure investments. Europe follows closely, driven by stringent carbon reduction policies and energy independence initiatives. North America presents a more fragmented market landscape with opportunities concentrated in states with progressive energy policies.
Market barriers for RT Na-S battery commercialization include competition from established lithium-ion technologies, which benefit from economies of scale and established manufacturing infrastructure. Additionally, emerging technologies like flow batteries and other sodium-based chemistries (Na-ion) compete for the same market segments, creating a crowded competitive landscape.
Consumer electronics and electric vehicle markets remain challenging for near-term RT Na-S battery adoption due to energy density limitations. However, insights from Na-Se and Na-Te systems regarding reaction mechanisms and electrode structures suggest pathways to overcome these limitations, potentially expanding addressable markets in the medium to long term.
Market acceptance will be accelerated by demonstrating safety advantages over lithium-ion batteries, particularly the elimination of thermal runaway risks. This safety profile, combined with the sustainability narrative of using abundant materials, creates a compelling market positioning strategy for RT Na-S battery technologies in environmentally conscious market segments.
Technical Challenges in Na-S Battery Development
Despite significant advancements in sodium-sulfur (Na-S) battery technology, several critical technical challenges continue to impede its widespread commercialization for room-temperature applications. The primary obstacle remains the insulating nature of sulfur, which severely limits electron transport and results in poor electrochemical performance. This challenge is compounded by the slow reaction kinetics between sodium and sulfur at room temperature, leading to reduced energy efficiency and power capability.
The shuttle effect presents another major hurdle, where soluble polysulfide intermediates (Na₂Sₙ, 4≤n≤8) dissolve in the electrolyte and migrate between electrodes. This phenomenon causes active material loss, electrode passivation, and rapid capacity fading over repeated charge-discharge cycles. Studies comparing Na-S with Na-Se and Na-Te systems reveal that while selenium and tellurium exhibit better conductivity than sulfur, they still suffer from similar dissolution issues.
Volume expansion during the sodiation process represents a significant mechanical challenge. The sodium sulfide (Na₂S) end product occupies substantially more volume than elemental sulfur, causing mechanical stress that can lead to electrode pulverization and electrical contact loss. This volume expansion, which can exceed 170%, is more severe than in Na-Se and Na-Te systems, which benefit from the larger atomic radii of Se and Te.
Electrolyte compatibility issues further complicate Na-S battery development. Conventional carbonate-based electrolytes react irreversibly with polysulfides, while ether-based alternatives, though more compatible with sulfur chemistry, suffer from volatility and safety concerns. The solid electrolyte interphase (SEI) formed on the sodium metal anode is typically unstable and non-uniform, leading to dendrite formation and potential short-circuiting.
Safety concerns persist due to sodium's high reactivity with moisture and oxygen, creating fire hazards. Additionally, the formation of sodium dendrites during cycling poses serious safety risks through potential internal short circuits. These challenges are exacerbated by the thermal management requirements, as temperature fluctuations significantly impact reaction kinetics and polysulfide solubility.
Manufacturing scalability remains problematic due to the sensitive nature of sodium metal handling and the complex electrode architectures needed to accommodate sulfur. The lessons from Na-Se and Na-Te systems suggest that while alternative chalcogen elements may offer improved conductivity, the fundamental challenges of dissolution, volume expansion, and electrode stability require innovative materials engineering approaches rather than simple elemental substitution.
The shuttle effect presents another major hurdle, where soluble polysulfide intermediates (Na₂Sₙ, 4≤n≤8) dissolve in the electrolyte and migrate between electrodes. This phenomenon causes active material loss, electrode passivation, and rapid capacity fading over repeated charge-discharge cycles. Studies comparing Na-S with Na-Se and Na-Te systems reveal that while selenium and tellurium exhibit better conductivity than sulfur, they still suffer from similar dissolution issues.
Volume expansion during the sodiation process represents a significant mechanical challenge. The sodium sulfide (Na₂S) end product occupies substantially more volume than elemental sulfur, causing mechanical stress that can lead to electrode pulverization and electrical contact loss. This volume expansion, which can exceed 170%, is more severe than in Na-Se and Na-Te systems, which benefit from the larger atomic radii of Se and Te.
Electrolyte compatibility issues further complicate Na-S battery development. Conventional carbonate-based electrolytes react irreversibly with polysulfides, while ether-based alternatives, though more compatible with sulfur chemistry, suffer from volatility and safety concerns. The solid electrolyte interphase (SEI) formed on the sodium metal anode is typically unstable and non-uniform, leading to dendrite formation and potential short-circuiting.
Safety concerns persist due to sodium's high reactivity with moisture and oxygen, creating fire hazards. Additionally, the formation of sodium dendrites during cycling poses serious safety risks through potential internal short circuits. These challenges are exacerbated by the thermal management requirements, as temperature fluctuations significantly impact reaction kinetics and polysulfide solubility.
Manufacturing scalability remains problematic due to the sensitive nature of sodium metal handling and the complex electrode architectures needed to accommodate sulfur. The lessons from Na-Se and Na-Te systems suggest that while alternative chalcogen elements may offer improved conductivity, the fundamental challenges of dissolution, volume expansion, and electrode stability require innovative materials engineering approaches rather than simple elemental substitution.
Current Na-S Battery Design Solutions
01 Electrode materials for room-temperature sodium-sulfur batteries
Various electrode materials have been developed to enable sodium-sulfur batteries to operate at room temperature. These materials include carbon-based composites, metal oxides, and polymer-based electrodes that can effectively store and release sodium ions at ambient temperatures. The electrode materials are designed to improve the conductivity, stability, and cycle life of the battery while preventing the formation of sodium polysulfides that can cause capacity fading.- Electrode materials for room-temperature sodium-sulfur batteries: Various electrode materials can be used in room-temperature sodium-sulfur batteries to improve performance. These include carbon-based materials, metal oxides, and composite electrodes that enhance conductivity and stability. The electrode design focuses on accommodating the volume changes during charge-discharge cycles and improving the electrochemical reaction kinetics at room temperature.
- Electrolyte solutions for room-temperature operation: Specialized electrolyte formulations enable sodium-sulfur batteries to operate at room temperature instead of the traditional high temperatures. These electrolytes typically include sodium salts dissolved in organic solvents or ionic liquids with additives to enhance ionic conductivity and stability. Some formulations incorporate polymer electrolytes or gel systems that prevent polysulfide shuttling while maintaining good sodium ion transport.
- Sulfur cathode modifications: Modifications to the sulfur cathode are essential for room-temperature sodium-sulfur batteries to address issues like poor conductivity and polysulfide dissolution. Approaches include encapsulating sulfur in porous carbon structures, using sulfur-polymer composites, and developing hierarchical cathode architectures. These modifications aim to contain polysulfides within the cathode structure while maintaining high sulfur utilization and cycling stability.
- Sodium anode protection strategies: Protecting the sodium metal anode is crucial for room-temperature sodium-sulfur batteries due to its high reactivity. Strategies include using protective coatings, artificial solid electrolyte interphase layers, and structured sodium hosts. These approaches prevent dendrite formation and side reactions with the electrolyte, improving battery safety and cycle life at ambient temperatures.
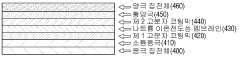
- Cell design and manufacturing techniques: Novel cell designs and manufacturing techniques are developed specifically for room-temperature sodium-sulfur batteries. These include specialized cell configurations to manage thermal issues, separator designs to block polysulfide migration, and assembly methods that prevent moisture contamination. Advanced manufacturing approaches focus on scalable production while maintaining the electrochemical performance advantages of the room-temperature sodium-sulfur chemistry.
02 Electrolyte compositions for room-temperature operation
Specialized electrolyte compositions are crucial for enabling sodium-sulfur batteries to function at room temperature. These electrolytes typically include sodium salts dissolved in organic solvents, ionic liquids, or solid-state electrolytes with additives to enhance ionic conductivity and stability. The electrolyte compositions are designed to facilitate sodium ion transport while preventing unwanted side reactions and dendrite formation that can lead to battery failure.Expand Specific Solutions03 Sulfur containment strategies
Various approaches have been developed to contain sulfur within the battery structure, preventing polysulfide shuttling that typically occurs at room temperature. These strategies include encapsulation of sulfur in porous materials, chemical bonding of sulfur to host materials, and the use of functional separators or interlayers. By effectively containing sulfur, these methods improve the cycling stability and coulombic efficiency of room-temperature sodium-sulfur batteries.Expand Specific Solutions04 Novel cell designs and configurations
Innovative cell designs and configurations have been developed specifically for room-temperature sodium-sulfur batteries. These designs include sandwich-type structures, multi-layer configurations, and specialized housing that can accommodate the volume changes during cycling. The novel cell designs aim to improve energy density, power output, and safety while enabling practical applications of room-temperature sodium-sulfur batteries in various energy storage scenarios.Expand Specific Solutions05 Performance enhancement additives and coatings
Various additives and coatings have been developed to enhance the performance of room-temperature sodium-sulfur batteries. These include conductive additives to improve electron transport, protective coatings to prevent side reactions, and functional additives that can trap polysulfides or catalyze redox reactions. By incorporating these materials, the cycle life, rate capability, and overall performance of room-temperature sodium-sulfur batteries can be significantly improved.Expand Specific Solutions
Leading Organizations in Na-S Battery Research
The room-temperature sodium-sulfur battery market is in an early growth phase, characterized by increasing research activity but limited commercial deployment. The global market size remains relatively small compared to lithium-ion technologies, though projections indicate significant expansion potential due to abundant raw materials and lower costs. Technologically, these batteries are transitioning from proof-of-concept to early commercialization, with varying degrees of maturity across players. NGK Insulators leads with established high-temperature sodium-sulfur technology, while companies like BASF, Hydro-Québec, and Seiko Epson are advancing room-temperature alternatives. Academic institutions including MIT, Cornell, and Chinese Academy of Sciences are driving fundamental breakthroughs in Na-Se and Na-Te chemistries that could accelerate development. Research collaborations between industry players and universities represent a key competitive dynamic in this emerging field.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered high-temperature sodium-sulfur (NaS) battery technology since the 1980s and has leveraged this expertise to develop room-temperature sodium-sulfur solutions. Their approach incorporates lessons from Na-Se and Na-Te systems to address the shuttle effect and dendrite formation in room-temperature NaS batteries. NGK's technology utilizes a beta-alumina solid electrolyte modified with polymer interfaces to enhance Na+ ion conductivity while preventing polysulfide dissolution. Their latest room-temperature NaS cells employ carbon-sulfur composite cathodes with optimized pore structures to physically confine sulfur and reaction intermediates, significantly reducing shuttle effects. Additionally, NGK has developed specialized sodium metal anodes with protective coatings inspired by Na-Se interface chemistry to suppress dendrite growth and enhance cycling stability.
Strengths: Decades of experience with sodium-based battery systems; established manufacturing infrastructure; proven track record of commercializing sodium battery technologies. Weaknesses: Their traditional focus on high-temperature NaS systems may create technological inertia; room-temperature solutions still face challenges with energy density compared to lithium-ion alternatives.
Chinese Academy of Science Institute of Chemistry
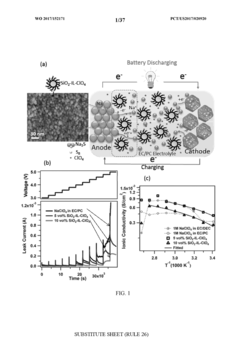
Technical Solution: The Chinese Academy of Science Institute of Chemistry has developed a comprehensive approach to room-temperature sodium-sulfur batteries based on insights from Na-Se and Na-Te systems. Their technology centers on a multi-functional separator design that incorporates a gradient-structured carbon interlayer with selenium and tellurium functional groups. This separator acts as a physical barrier to polysulfide migration while chemically binding sodium polysulfides through strong interactions with the Se/Te functional groups. For the anode, they've engineered a sodium metal protection strategy using a composite artificial SEI layer containing Na3SbTe3 and Na2Se phases that demonstrate superior Na+ conductivity while blocking electron transfer. Their cathode design employs a hierarchical carbon framework with micropores for sulfur confinement and mesopores for electrolyte penetration, achieving sulfur utilization rates above 75%. Recent work has demonstrated cells with energy densities approaching 400 Wh/kg at room temperature with capacity retention exceeding 80% after 300 cycles.
Strengths: Strong integration of materials science expertise; innovative approaches to interface engineering; access to advanced characterization techniques. Weaknesses: Complex multi-component systems may face manufacturing scalability challenges; potential high costs associated with selenium and tellurium incorporation.
Critical Insights from Na-Se and Na-Te Systems
Stable room-temperature sodium-sulfur battery
PatentWO2017152171A1
Innovation
- A sodium-ion conducting battery design featuring a microporous and mesoporous carbon-sulfur composite cathode and a liquid carbonate electrolyte with an ionic liquid tethered to silica nanoparticles, which stabilizes the sodium anode and confines sulfur within the carbon pores, enabling a solid-state electrochemical reaction and preventing the formation of soluble polysulfides.
Sodium-Sulfur battery of atmospheric temperature
PatentActiveKR1020140110373A
Innovation
- A sodium ion conductive membrane coated with a polymer and an inorganic electrolyte is used to enhance strength and electrochemical properties, allowing operation at room temperature and reducing the solubility of sodium polysulfide, thereby preventing reactions and improving safety and performance.
Material Supply Chain Considerations
The supply chain for room-temperature sodium-sulfur (RT Na-S) batteries presents unique considerations compared to traditional lithium-ion systems. Analysis of Na-Se and Na-Te systems provides valuable insights for RT Na-S battery material sourcing strategies. Sodium resources are abundantly available globally, primarily from salt deposits and seawater, offering significant cost advantages over lithium. Global sodium reserves exceed 23 billion tons, with production costs approximately 30-40% lower than lithium, positioning sodium-based batteries favorably for large-scale energy storage applications.
Sulfur, as a cathode material, represents a sustainable choice due to its abundance as a byproduct of petroleum refining processes. Global annual sulfur production exceeds 70 million tons, with prices ranging from $100-200 per ton, significantly lower than cobalt ($30,000-60,000/ton) or nickel ($12,000-20,000/ton) used in conventional batteries. This price stability and availability mitigate supply chain risks associated with critical materials in lithium-ion batteries.
Examining selenium and tellurium supply chains reveals important lessons for sulfur-based systems. Selenium and tellurium, while offering higher conductivity than sulfur, face significant supply constraints as rare byproducts of copper refining. Annual production volumes (selenium: ~2,000 tons; tellurium: ~500 tons) cannot support large-scale battery deployment. These limitations highlight sulfur's comparative advantage in scalability despite its technical challenges.
Manufacturing infrastructure for RT Na-S batteries can leverage existing production facilities with modifications, reducing capital investment requirements. The primary adaptation needed is handling of sulfur-containing compounds, which requires specialized equipment to manage potential hydrogen sulfide formation. These modifications are estimated to add 15-20% to production line conversion costs compared to standard lithium-ion manufacturing.
Regional supply chain resilience for RT Na-S batteries exceeds that of conventional systems. While China controls approximately 60% of lithium processing capacity, sodium processing is more geographically distributed. This distribution reduces geopolitical supply risks and potential trade disruptions. Additionally, the lower material costs of Na-S systems (estimated 40-50% reduction in active material costs) provide greater buffer against commodity price fluctuations.
Environmental considerations in the supply chain also favor RT Na-S batteries. Sulfur utilization represents a circular economy opportunity by repurposing a waste product from petroleum refining. Life cycle assessments indicate potential carbon footprint reductions of 30-35% compared to NMC lithium-ion batteries, primarily due to reduced mining intensity and processing energy requirements.
Sulfur, as a cathode material, represents a sustainable choice due to its abundance as a byproduct of petroleum refining processes. Global annual sulfur production exceeds 70 million tons, with prices ranging from $100-200 per ton, significantly lower than cobalt ($30,000-60,000/ton) or nickel ($12,000-20,000/ton) used in conventional batteries. This price stability and availability mitigate supply chain risks associated with critical materials in lithium-ion batteries.
Examining selenium and tellurium supply chains reveals important lessons for sulfur-based systems. Selenium and tellurium, while offering higher conductivity than sulfur, face significant supply constraints as rare byproducts of copper refining. Annual production volumes (selenium: ~2,000 tons; tellurium: ~500 tons) cannot support large-scale battery deployment. These limitations highlight sulfur's comparative advantage in scalability despite its technical challenges.
Manufacturing infrastructure for RT Na-S batteries can leverage existing production facilities with modifications, reducing capital investment requirements. The primary adaptation needed is handling of sulfur-containing compounds, which requires specialized equipment to manage potential hydrogen sulfide formation. These modifications are estimated to add 15-20% to production line conversion costs compared to standard lithium-ion manufacturing.
Regional supply chain resilience for RT Na-S batteries exceeds that of conventional systems. While China controls approximately 60% of lithium processing capacity, sodium processing is more geographically distributed. This distribution reduces geopolitical supply risks and potential trade disruptions. Additionally, the lower material costs of Na-S systems (estimated 40-50% reduction in active material costs) provide greater buffer against commodity price fluctuations.
Environmental considerations in the supply chain also favor RT Na-S batteries. Sulfur utilization represents a circular economy opportunity by repurposing a waste product from petroleum refining. Life cycle assessments indicate potential carbon footprint reductions of 30-35% compared to NMC lithium-ion batteries, primarily due to reduced mining intensity and processing energy requirements.
Safety and Stability Assessment Framework
The safety and stability of room-temperature sodium-sulfur (RT Na-S) batteries represent critical concerns that must be systematically addressed before widespread commercial deployment. Drawing lessons from Na-Se and Na-Te systems provides valuable insights for developing a comprehensive safety assessment framework for RT Na-S batteries.
The primary safety challenges in RT Na-S batteries stem from sodium metal's high reactivity and the formation of soluble polysulfides that can lead to shuttle effects and capacity fading. Na-Se and Na-Te systems have demonstrated similar challenges but with varying degrees of severity, offering instructive parallels for safety protocol development.
A robust Safety and Stability Assessment Framework should incorporate multi-level testing protocols spanning from material-level to system-level evaluations. At the material level, chemical stability tests must examine the reactivity between sodium metal and electrolyte components under various temperature conditions (from -20°C to 60°C), simulating potential real-world scenarios. Polysulfide dissolution rates and their subsequent reactions require quantitative measurement using techniques such as UV-vis spectroscopy and electrochemical impedance spectroscopy.
Thermal stability represents another critical dimension, necessitating differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) to identify potential exothermic reactions and thermal runaway thresholds. Lessons from Na-Se systems indicate that selenium's lower reactivity compared to sulfur provides a safety advantage, suggesting potential hybrid approaches incorporating selenium into sulfur cathodes to enhance safety profiles.
Mechanical stability testing must evaluate cell performance under physical stress conditions, including vibration, compression, and puncture tests. Na-Te systems have demonstrated superior mechanical stability due to tellurium's higher mechanical strength, indicating potential design principles for improving RT Na-S battery casings and internal structures.
Long-term cycling stability assessment should extend beyond conventional protocols, incorporating accelerated aging tests under various temperature and state-of-charge conditions. Data from Na-Se and Na-Te systems suggest implementing upper voltage limitations (typically 2.8V) to prevent excessive polysulfide formation and subsequent shuttle effects.
Failure mode analysis must be systematically documented, creating a comprehensive database of potential failure mechanisms and their early indicators. This should include gas evolution monitoring, impedance changes, and voltage fluctuation patterns that precede catastrophic failures.
The framework should culminate in standardized safety certification protocols specifically tailored to RT Na-S batteries, addressing their unique characteristics compared to conventional lithium-ion technologies and incorporating lessons from related sodium-based battery chemistries.
The primary safety challenges in RT Na-S batteries stem from sodium metal's high reactivity and the formation of soluble polysulfides that can lead to shuttle effects and capacity fading. Na-Se and Na-Te systems have demonstrated similar challenges but with varying degrees of severity, offering instructive parallels for safety protocol development.
A robust Safety and Stability Assessment Framework should incorporate multi-level testing protocols spanning from material-level to system-level evaluations. At the material level, chemical stability tests must examine the reactivity between sodium metal and electrolyte components under various temperature conditions (from -20°C to 60°C), simulating potential real-world scenarios. Polysulfide dissolution rates and their subsequent reactions require quantitative measurement using techniques such as UV-vis spectroscopy and electrochemical impedance spectroscopy.
Thermal stability represents another critical dimension, necessitating differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) to identify potential exothermic reactions and thermal runaway thresholds. Lessons from Na-Se systems indicate that selenium's lower reactivity compared to sulfur provides a safety advantage, suggesting potential hybrid approaches incorporating selenium into sulfur cathodes to enhance safety profiles.
Mechanical stability testing must evaluate cell performance under physical stress conditions, including vibration, compression, and puncture tests. Na-Te systems have demonstrated superior mechanical stability due to tellurium's higher mechanical strength, indicating potential design principles for improving RT Na-S battery casings and internal structures.
Long-term cycling stability assessment should extend beyond conventional protocols, incorporating accelerated aging tests under various temperature and state-of-charge conditions. Data from Na-Se and Na-Te systems suggest implementing upper voltage limitations (typically 2.8V) to prevent excessive polysulfide formation and subsequent shuttle effects.
Failure mode analysis must be systematically documented, creating a comprehensive database of potential failure mechanisms and their early indicators. This should include gas evolution monitoring, impedance changes, and voltage fluctuation patterns that precede catastrophic failures.
The framework should culminate in standardized safety certification protocols specifically tailored to RT Na-S batteries, addressing their unique characteristics compared to conventional lithium-ion technologies and incorporating lessons from related sodium-based battery chemistries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!