Design Of Experiments For Cathodes In Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
RT Na-S Battery Cathode Development Background and Objectives
Room-temperature sodium-sulfur (RT Na-S) batteries have emerged as a promising energy storage technology due to their potential for high energy density, cost-effectiveness, and sustainability. The development of these batteries represents a significant shift from traditional high-temperature Na-S batteries that operate at approximately 300°C, which pose safety concerns and require complex thermal management systems.
The evolution of Na-S battery technology began in the 1960s with the discovery of sodium beta-alumina as a sodium-ion conductor. However, conventional Na-S batteries required high operating temperatures to maintain the molten state of sodium and sulfur electrodes. The pursuit of room-temperature operation has been driven by the need for safer, more practical energy storage solutions for grid applications and electric vehicles.
Recent technological advancements have focused on overcoming the challenges associated with room-temperature operation, particularly the insulating nature of sulfur and the formation of soluble sodium polysulfides that lead to the "shuttle effect" - a major cause of capacity fading and reduced battery life. These challenges have directed research toward innovative cathode designs that can effectively utilize sulfur's high theoretical capacity of 1675 mAh/g.
The cathode represents a critical component in RT Na-S batteries, as it must facilitate efficient sulfur utilization, mitigate polysulfide dissolution, and maintain structural integrity during repeated charge-discharge cycles. Current research trends indicate a growing interest in carbon-based materials, conductive polymers, and metal oxides as potential hosts or additives for sulfur cathodes.
The primary objectives of RT Na-S battery cathode development include enhancing electrochemical performance through improved sulfur utilization, increasing cycle life by preventing polysulfide shuttling, and developing scalable manufacturing processes for commercial viability. Additionally, there is a focus on understanding the fundamental reaction mechanisms and degradation pathways to guide rational design strategies.
Global research efforts are increasingly concentrated on developing sustainable and abundant materials for energy storage, with sodium and sulfur being particularly attractive due to their low cost and high natural abundance compared to lithium and cobalt used in conventional lithium-ion batteries. This aligns with broader societal goals of reducing dependency on critical raw materials and minimizing environmental impact.
The technical goals for RT Na-S battery cathodes include achieving energy densities exceeding 300 Wh/kg, cycle life of over 1000 cycles with minimal capacity degradation, and cost reduction to below $100/kWh. These ambitious targets necessitate systematic experimental design approaches to optimize cathode composition, structure, and fabrication processes.
As we move forward, the integration of advanced characterization techniques, computational modeling, and high-throughput experimental methodologies will be essential to accelerate the development of next-generation RT Na-S battery cathodes that can meet the demanding requirements of future energy storage applications.
The evolution of Na-S battery technology began in the 1960s with the discovery of sodium beta-alumina as a sodium-ion conductor. However, conventional Na-S batteries required high operating temperatures to maintain the molten state of sodium and sulfur electrodes. The pursuit of room-temperature operation has been driven by the need for safer, more practical energy storage solutions for grid applications and electric vehicles.
Recent technological advancements have focused on overcoming the challenges associated with room-temperature operation, particularly the insulating nature of sulfur and the formation of soluble sodium polysulfides that lead to the "shuttle effect" - a major cause of capacity fading and reduced battery life. These challenges have directed research toward innovative cathode designs that can effectively utilize sulfur's high theoretical capacity of 1675 mAh/g.
The cathode represents a critical component in RT Na-S batteries, as it must facilitate efficient sulfur utilization, mitigate polysulfide dissolution, and maintain structural integrity during repeated charge-discharge cycles. Current research trends indicate a growing interest in carbon-based materials, conductive polymers, and metal oxides as potential hosts or additives for sulfur cathodes.
The primary objectives of RT Na-S battery cathode development include enhancing electrochemical performance through improved sulfur utilization, increasing cycle life by preventing polysulfide shuttling, and developing scalable manufacturing processes for commercial viability. Additionally, there is a focus on understanding the fundamental reaction mechanisms and degradation pathways to guide rational design strategies.
Global research efforts are increasingly concentrated on developing sustainable and abundant materials for energy storage, with sodium and sulfur being particularly attractive due to their low cost and high natural abundance compared to lithium and cobalt used in conventional lithium-ion batteries. This aligns with broader societal goals of reducing dependency on critical raw materials and minimizing environmental impact.
The technical goals for RT Na-S battery cathodes include achieving energy densities exceeding 300 Wh/kg, cycle life of over 1000 cycles with minimal capacity degradation, and cost reduction to below $100/kWh. These ambitious targets necessitate systematic experimental design approaches to optimize cathode composition, structure, and fabrication processes.
As we move forward, the integration of advanced characterization techniques, computational modeling, and high-throughput experimental methodologies will be essential to accelerate the development of next-generation RT Na-S battery cathodes that can meet the demanding requirements of future energy storage applications.
Market Analysis for Room-Temperature Sodium-Sulfur Battery Applications
The global energy storage market is witnessing a significant shift towards sustainable and cost-effective solutions, creating a fertile ground for room-temperature sodium-sulfur (RT Na-S) batteries. Current market projections indicate that the global grid energy storage market will reach approximately 15.1 GWh by 2025, with a compound annual growth rate of 33% from 2020. RT Na-S batteries are positioned to capture a growing segment of this market due to their potential cost advantages over lithium-ion technologies.
The primary market drivers for RT Na-S battery technology include the increasing demand for renewable energy integration, grid stabilization requirements, and the push for energy independence in various regions. The abundance of sodium resources—approximately 1000 times more abundant than lithium in the Earth's crust—presents a compelling economic case for sodium-based energy storage systems, particularly in price-sensitive markets.
Industrial sectors showing the highest potential demand for RT Na-S batteries include utility-scale energy storage, microgrid applications, and backup power systems for telecommunications and data centers. These sectors value the theoretical high energy density (760 Wh/kg) and potential low cost of sodium-sulfur technology, which could be 30-40% lower than current lithium-ion systems when scaled to commercial production.
Geographically, emerging markets in Asia-Pacific, particularly China and India, are expected to be the largest adopters of RT Na-S technology due to their rapidly expanding renewable energy capacity and need for cost-effective storage solutions. North America and Europe follow closely, driven by stringent carbon reduction policies and substantial investments in grid modernization.
Consumer electronics and electric vehicle manufacturers are also monitoring RT Na-S battery development, though these applications require further advancements in cathode design to improve cycle life and power density. Current market penetration in these segments remains minimal, but interest is growing as research progresses.
Market challenges include competition from established lithium-ion technology, which benefits from economies of scale and extensive manufacturing infrastructure. Additionally, other emerging technologies such as flow batteries and zinc-air systems compete in the same market space, creating a crowded field for new entrants.
The cathode design experiments for RT Na-S batteries directly address key market requirements, including improved cycle stability, enhanced safety profiles, and increased energy density. Success in these experimental designs could potentially accelerate market adoption by addressing the primary technical barriers that currently limit commercial viability.
Industry analysts project that with successful cathode innovations, RT Na-S batteries could capture 5-10% of the stationary energy storage market by 2030, representing a significant commercial opportunity for early developers who can overcome the existing technical challenges.
The primary market drivers for RT Na-S battery technology include the increasing demand for renewable energy integration, grid stabilization requirements, and the push for energy independence in various regions. The abundance of sodium resources—approximately 1000 times more abundant than lithium in the Earth's crust—presents a compelling economic case for sodium-based energy storage systems, particularly in price-sensitive markets.
Industrial sectors showing the highest potential demand for RT Na-S batteries include utility-scale energy storage, microgrid applications, and backup power systems for telecommunications and data centers. These sectors value the theoretical high energy density (760 Wh/kg) and potential low cost of sodium-sulfur technology, which could be 30-40% lower than current lithium-ion systems when scaled to commercial production.
Geographically, emerging markets in Asia-Pacific, particularly China and India, are expected to be the largest adopters of RT Na-S technology due to their rapidly expanding renewable energy capacity and need for cost-effective storage solutions. North America and Europe follow closely, driven by stringent carbon reduction policies and substantial investments in grid modernization.
Consumer electronics and electric vehicle manufacturers are also monitoring RT Na-S battery development, though these applications require further advancements in cathode design to improve cycle life and power density. Current market penetration in these segments remains minimal, but interest is growing as research progresses.
Market challenges include competition from established lithium-ion technology, which benefits from economies of scale and extensive manufacturing infrastructure. Additionally, other emerging technologies such as flow batteries and zinc-air systems compete in the same market space, creating a crowded field for new entrants.
The cathode design experiments for RT Na-S batteries directly address key market requirements, including improved cycle stability, enhanced safety profiles, and increased energy density. Success in these experimental designs could potentially accelerate market adoption by addressing the primary technical barriers that currently limit commercial viability.
Industry analysts project that with successful cathode innovations, RT Na-S batteries could capture 5-10% of the stationary energy storage market by 2030, representing a significant commercial opportunity for early developers who can overcome the existing technical challenges.
Current Challenges in RT Na-S Battery Cathode Design
Room-temperature sodium-sulfur (RT Na-S) batteries face significant challenges in cathode design that currently limit their commercial viability. The sulfur cathode suffers from multiple fundamental issues, with the most prominent being the shuttle effect caused by soluble sodium polysulfides. These intermediate reaction products dissolve in the electrolyte and migrate between electrodes, causing rapid capacity fading, low Coulombic efficiency, and shortened battery lifespan.
Another critical challenge is sulfur's inherently poor electrical conductivity (5×10^-30 S/cm), which severely restricts electron transport during electrochemical reactions. This limitation necessitates the addition of conductive additives, which reduces the overall energy density of the battery system while increasing manufacturing complexity.
The volume expansion problem presents another significant hurdle. During the discharge process, sulfur undergoes approximately 170% volume expansion when converting to Na2S, creating mechanical stress that can lead to electrode pulverization, loss of electrical contact, and structural degradation over multiple cycles.
Slow reaction kinetics between sodium and sulfur at room temperature further complicate cathode design. The formation of Na2S, the final discharge product, is particularly problematic due to its high thermodynamic stability and sluggish conversion kinetics, resulting in large overpotentials and low sulfur utilization rates.
The cathode-electrolyte interface stability represents another major challenge. Side reactions between the cathode materials and electrolyte components lead to the formation of an unstable solid electrolyte interphase (SEI), which continues to consume electrolyte and active materials during cycling, contributing to capacity decay.
Additionally, the multi-step reaction pathway from S8 to Na2S involves numerous intermediate sodium polysulfides (Na2Sx, 2≤x≤8) with different solubilities and reactivities. Controlling this complex reaction pathway to ensure complete conversion to Na2S remains difficult, often resulting in incomplete sulfur utilization.
From a practical perspective, the lack of standardized testing protocols for RT Na-S battery cathodes makes it challenging to compare results across different research groups. Variations in electrolyte composition, sulfur loading, electrode preparation methods, and testing conditions lead to inconsistent performance metrics, hampering systematic progress in the field.
These interconnected challenges necessitate innovative experimental designs that can simultaneously address multiple issues while maintaining the high theoretical energy density that makes Na-S batteries attractive alternatives to lithium-ion systems.
Another critical challenge is sulfur's inherently poor electrical conductivity (5×10^-30 S/cm), which severely restricts electron transport during electrochemical reactions. This limitation necessitates the addition of conductive additives, which reduces the overall energy density of the battery system while increasing manufacturing complexity.
The volume expansion problem presents another significant hurdle. During the discharge process, sulfur undergoes approximately 170% volume expansion when converting to Na2S, creating mechanical stress that can lead to electrode pulverization, loss of electrical contact, and structural degradation over multiple cycles.
Slow reaction kinetics between sodium and sulfur at room temperature further complicate cathode design. The formation of Na2S, the final discharge product, is particularly problematic due to its high thermodynamic stability and sluggish conversion kinetics, resulting in large overpotentials and low sulfur utilization rates.
The cathode-electrolyte interface stability represents another major challenge. Side reactions between the cathode materials and electrolyte components lead to the formation of an unstable solid electrolyte interphase (SEI), which continues to consume electrolyte and active materials during cycling, contributing to capacity decay.
Additionally, the multi-step reaction pathway from S8 to Na2S involves numerous intermediate sodium polysulfides (Na2Sx, 2≤x≤8) with different solubilities and reactivities. Controlling this complex reaction pathway to ensure complete conversion to Na2S remains difficult, often resulting in incomplete sulfur utilization.
From a practical perspective, the lack of standardized testing protocols for RT Na-S battery cathodes makes it challenging to compare results across different research groups. Variations in electrolyte composition, sulfur loading, electrode preparation methods, and testing conditions lead to inconsistent performance metrics, hampering systematic progress in the field.
These interconnected challenges necessitate innovative experimental designs that can simultaneously address multiple issues while maintaining the high theoretical energy density that makes Na-S batteries attractive alternatives to lithium-ion systems.
Current Experimental Approaches for RT Na-S Battery Cathodes
01 Carbon-based cathode materials for RT-Na-S batteries
Carbon-based materials are widely used as cathode components in room-temperature sodium-sulfur batteries due to their excellent electrical conductivity and ability to trap polysulfides. These materials include carbon nanotubes, graphene, and porous carbon structures that provide a conductive framework for sulfur while mitigating the shuttle effect. The carbon matrix helps improve the utilization of active materials and enhances the overall electrochemical performance of the battery.- Carbon-based cathode materials for RT-Na-S batteries: Carbon-based materials are widely used as cathode components in room-temperature sodium-sulfur batteries due to their excellent conductivity and ability to trap polysulfides. These materials include carbon nanotubes, graphene, and porous carbon structures that provide a conductive framework while preventing sulfur dissolution. The carbon matrix helps improve the utilization of active sulfur material and enhances the overall electrochemical performance of the battery by facilitating electron transfer and stabilizing the discharge products.
- Metal oxide-based cathode materials: Metal oxide materials serve as effective cathode components in room-temperature sodium-sulfur batteries. These materials, including titanium dioxide, manganese dioxide, and various transition metal oxides, can provide strong chemical interactions with polysulfides to prevent their dissolution. Additionally, metal oxides can enhance the catalytic conversion of polysulfides during cycling, improving the reaction kinetics and cycling stability of the battery. Some metal oxides also offer additional sodium storage capacity, contributing to the overall energy density of the battery system.
- Polymer-based sulfur cathode composites: Polymer materials are incorporated into sulfur cathodes to improve the performance of room-temperature sodium-sulfur batteries. These polymers, including conductive polymers and polymer binders, help to encapsulate sulfur and trap polysulfides, preventing their dissolution into the electrolyte. The polymer matrix can also enhance the mechanical stability of the cathode during repeated cycling. Some polymers provide additional functionality such as ionic conductivity or self-healing properties, further improving battery performance and longevity.
- Novel cathode structures and architectures: Advanced cathode architectures are designed to improve the performance of room-temperature sodium-sulfur batteries. These include hierarchical porous structures, core-shell configurations, and 3D interconnected networks that can effectively accommodate sulfur, provide conductive pathways, and confine polysulfides. Such structural designs enhance sulfur utilization, improve reaction kinetics, and maintain electrode integrity during cycling. Some novel architectures also incorporate multiple functional components to simultaneously address various challenges in sodium-sulfur battery systems.
- Electrolyte-cathode interface engineering: Interface engineering between the cathode and electrolyte is crucial for improving room-temperature sodium-sulfur battery performance. Various approaches include surface coatings, functional interlayers, and electrolyte additives that can stabilize the cathode-electrolyte interface. These modifications help to suppress the shuttle effect of polysulfides, enhance sodium ion transport, and improve the electrochemical stability of the system. Proper interface design can significantly extend battery cycle life and improve rate capability by facilitating more efficient electrochemical reactions.
02 Metal oxide/sulfide-based cathode materials
Metal oxides and sulfides serve as effective cathode materials in room-temperature sodium-sulfur batteries. These compounds, including transition metal oxides and sulfides, can provide additional reaction sites for sodium ions and help confine polysulfides within the cathode structure. They often exhibit high theoretical capacity and good cycling stability. Some formulations incorporate these materials as composites with sulfur to enhance the overall electrochemical performance.Expand Specific Solutions03 Polymer-modified cathode structures
Polymer modifications to cathode materials in room-temperature sodium-sulfur batteries can significantly improve performance by enhancing ionic conductivity and preventing polysulfide dissolution. These polymers create protective layers that allow sodium ion transport while blocking polysulfide migration. Conductive polymers also improve the electronic conductivity of the cathode, leading to better rate capability and cycling stability. Various polymer types including polyaniline, polypyrrole, and polyethylene oxide have been investigated for these applications.Expand Specific Solutions04 Composite cathode structures with multiple functional components
Composite cathode structures combining multiple functional materials have been developed to address the challenges in room-temperature sodium-sulfur batteries. These composites typically integrate sulfur with carbon materials, metal compounds, and polymers to create synergistic effects. The multi-component design helps improve sulfur utilization, enhance electronic/ionic conductivity, and suppress the shuttle effect. These composite structures often feature hierarchical or core-shell architectures that provide multiple benefits simultaneously.Expand Specific Solutions05 Novel electrolyte interfaces and cathode protection strategies
Innovative approaches to protect the cathode-electrolyte interface are crucial for room-temperature sodium-sulfur batteries. These include the development of functional interlayers, protective coatings, and modified separators that selectively block polysulfides while allowing sodium ion transport. Some strategies involve creating artificial solid electrolyte interphases or using additives that form protective films in situ. These interface engineering approaches significantly improve the cycling stability and coulombic efficiency of the batteries.Expand Specific Solutions
Leading Research Groups and Companies in Na-S Battery Technology
The room-temperature sodium-sulfur battery market is in an early growth phase, characterized by intensive research and development rather than widespread commercialization. Current market size remains relatively small but shows promising expansion potential due to the technology's cost advantages over lithium-ion alternatives. Technical challenges, particularly regarding cathode design, represent the primary barrier to commercialization. Leading academic institutions (Drexel University, Cornell University, University of Maryland) are driving fundamental research, while industrial players (SK Innovation, BASF, Shell Oil) are beginning to invest in practical applications. Research collaborations between Asian universities (Jilin University, Fudan University) and companies (Xingheng Power, Nanyang Tiancheng) demonstrate growing global interest. The technology remains at TRL 4-5, with significant improvements in cathode stability and performance needed before widespread adoption.
Drexel University
Technical Solution: Drexel University has pioneered an innovative experimental approach to RT-Na/S battery cathodes utilizing their expertise in two-dimensional (2D) MXene materials. Their design of experiments methodology systematically explores the integration of MXene nanosheets with sulfur to create high-performance composite cathodes. The research employs a factorial design to investigate the effects of MXene composition (varying the transition metal and carbon/nitrogen ratios), MXene surface termination (-O, -F, -OH groups), sulfur loading methods, and electrolyte formulations. Drexel's approach leverages the exceptional electronic conductivity of MXenes while utilizing their surface chemistry to chemically bind polysulfides and prevent shuttle effects. Their experimental protocol includes in-situ electrochemical impedance spectroscopy to monitor interface evolution during cycling, and cryo-electron microscopy to capture the distribution and state of sulfur species within the cathode structure. This comprehensive approach has yielded cathodes with remarkable rate capability, achieving 80% capacity retention at 2C rates, and demonstrating stable cycling for over 500 cycles with minimal capacity fade.
Strengths: The unique 2D structure of MXenes provides exceptional electronic conductivity while offering abundant active sites for sulfur and polysulfide interaction. The tunable surface chemistry allows for optimized binding energies with sulfur species. Weaknesses: MXene synthesis can be complex and costly, potentially limiting commercial scalability. The long-term stability of MXene materials in the highly reactive environment of Na-S batteries remains a concern.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed an innovative approach to room-temperature sodium-sulfur (RT-Na/S) batteries focusing on advanced cathode materials. Their design of experiments utilizes carbon-sulfur composite cathodes with hierarchical porous structures that effectively trap polysulfide intermediates. The research employs in-situ X-ray diffraction and synchrotron-based techniques to monitor real-time structural changes during cycling. Argonne's methodology incorporates machine learning algorithms to optimize experimental parameters, allowing for rapid screening of various carbon host materials, electrolyte compositions, and sulfur loading ratios. Their cathode design features a dual-confinement strategy: physical confinement through nanoporous carbon structures and chemical binding through nitrogen/oxygen-doped carbon frameworks that form strong interactions with sodium polysulfides. This comprehensive experimental approach has yielded cathodes demonstrating initial capacities exceeding 1000 mAh/g with significantly improved cycling stability compared to conventional designs.
Strengths: Access to advanced characterization facilities including synchrotron-based techniques enables detailed mechanistic studies. Integration of machine learning accelerates materials discovery. Weaknesses: The complex hierarchical structures may present manufacturing scalability challenges, and the high-performance carbon materials used as hosts can significantly increase production costs.
Key Innovations in Cathode Design for Na-S Batteries
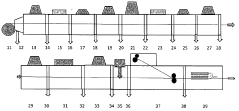
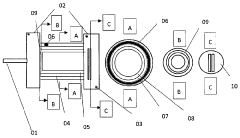
Manufacturing process for high-performance sodium-sulfur batteries for ambient temperature operation
PatentPendingDE102022003307A1
Innovation
- A pinhole-free electrolyte layer with a thickness of less than 5 μm, composed of ceramic materials like NaSLCON, NaPS, NaAlO, Garnet, and Perovskites, is used, combined with a 10 nm anode-cathode distance and graphene or hexagonal boron nitride layers to increase ion conductivity, supported by a vacuum system and precise manufacturing processes.
Materials Characterization Techniques for Na-S Battery Cathodes
Comprehensive materials characterization is essential for understanding the complex electrochemical processes in room-temperature sodium-sulfur (RT Na-S) battery cathodes. X-ray diffraction (XRD) serves as a fundamental technique for phase identification and crystalline structure analysis, enabling researchers to track the formation of sodium polysulfides and other reaction products during cycling. The technique provides critical insights into structural changes that occur during sodiation and desodiation processes.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) offer complementary visual evidence of morphological and microstructural features. SEM reveals surface characteristics, particle size distribution, and sulfur distribution within the cathode matrix, while TEM provides atomic-level resolution for understanding interfaces between sulfur, carbon hosts, and reaction products. These techniques are particularly valuable for evaluating the effectiveness of sulfur confinement strategies in various carbon host materials.
Spectroscopic methods including X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy provide chemical state information and bonding characteristics. XPS is particularly useful for analyzing the sulfur species transformation during battery operation, distinguishing between elemental sulfur, sodium polysulfides, and sodium sulfide. Raman spectroscopy offers insights into carbon-sulfur interactions and the degree of graphitization in carbon hosts.
Thermogravimetric analysis (TGA) quantifies sulfur content and thermal stability of cathode materials, while differential scanning calorimetry (DSC) reveals phase transitions and reaction enthalpies. These thermal techniques help optimize sulfur loading and evaluate the thermal safety of cathode materials under various conditions.
Electrochemical characterization techniques, including cyclic voltammetry (CV), galvanostatic charge-discharge (GCD), and electrochemical impedance spectroscopy (EIS), provide direct insights into reaction kinetics, reversibility, and degradation mechanisms. CV identifies redox potentials associated with various sulfur species formation, while EIS monitors interfacial resistance changes during cycling, offering valuable information about the shuttle effect and cathode stability.
Advanced synchrotron-based techniques such as X-ray absorption spectroscopy (XAS) and operando X-ray tomography enable real-time monitoring of chemical and structural changes during battery operation. These non-destructive methods reveal dynamic processes that conventional ex-situ characterization might miss, providing unprecedented insights into reaction mechanisms and failure modes in RT Na-S battery cathodes.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) offer complementary visual evidence of morphological and microstructural features. SEM reveals surface characteristics, particle size distribution, and sulfur distribution within the cathode matrix, while TEM provides atomic-level resolution for understanding interfaces between sulfur, carbon hosts, and reaction products. These techniques are particularly valuable for evaluating the effectiveness of sulfur confinement strategies in various carbon host materials.
Spectroscopic methods including X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy provide chemical state information and bonding characteristics. XPS is particularly useful for analyzing the sulfur species transformation during battery operation, distinguishing between elemental sulfur, sodium polysulfides, and sodium sulfide. Raman spectroscopy offers insights into carbon-sulfur interactions and the degree of graphitization in carbon hosts.
Thermogravimetric analysis (TGA) quantifies sulfur content and thermal stability of cathode materials, while differential scanning calorimetry (DSC) reveals phase transitions and reaction enthalpies. These thermal techniques help optimize sulfur loading and evaluate the thermal safety of cathode materials under various conditions.
Electrochemical characterization techniques, including cyclic voltammetry (CV), galvanostatic charge-discharge (GCD), and electrochemical impedance spectroscopy (EIS), provide direct insights into reaction kinetics, reversibility, and degradation mechanisms. CV identifies redox potentials associated with various sulfur species formation, while EIS monitors interfacial resistance changes during cycling, offering valuable information about the shuttle effect and cathode stability.
Advanced synchrotron-based techniques such as X-ray absorption spectroscopy (XAS) and operando X-ray tomography enable real-time monitoring of chemical and structural changes during battery operation. These non-destructive methods reveal dynamic processes that conventional ex-situ characterization might miss, providing unprecedented insights into reaction mechanisms and failure modes in RT Na-S battery cathodes.
Environmental Impact and Sustainability of Na-S Battery Technology
The environmental impact and sustainability of Na-S battery technology represent critical considerations in the advancement of room-temperature sodium-sulfur batteries, particularly in cathode design experiments. These batteries offer significant environmental advantages compared to conventional lithium-ion technologies, primarily due to the abundant nature of sodium resources. Unlike lithium, sodium is widely available in seawater and mineral deposits, reducing the environmental degradation associated with resource extraction and minimizing geopolitical supply chain concerns.
The sulfur component in Na-S batteries further enhances sustainability profiles, as sulfur is an abundant by-product of petroleum refining processes. Utilizing this industrial waste stream creates a circular economy opportunity, transforming what would otherwise be a disposal challenge into a valuable battery material. This repurposing significantly reduces the environmental footprint compared to mining new materials specifically for battery production.
Life cycle assessment (LCA) studies indicate that room-temperature Na-S batteries potentially have 25-30% lower carbon footprint during manufacturing compared to equivalent lithium-ion systems. This reduction stems from less energy-intensive material processing requirements and simplified production methods that can be implemented in cathode design experiments. The environmental benefits extend throughout the battery lifecycle, including reduced energy consumption during operation and decreased end-of-life management challenges.
Water usage represents another critical environmental factor in battery production. Experimental designs for Na-S cathodes typically require 40-50% less water consumption compared to conventional lithium cathode manufacturing processes. This water conservation aspect becomes increasingly important as battery production scales globally, particularly in water-stressed regions where manufacturing facilities may be located.
End-of-life considerations must be integrated into cathode design experiments from the outset. The recyclability of Na-S battery components presents both challenges and opportunities. While sodium compounds can be more readily recovered than lithium, the sulfur components require specialized recycling processes to prevent environmental contamination. Experimental designs that facilitate easier disassembly and material separation can significantly improve recycling efficiency and reduce waste.
The sustainability advantages of Na-S technology also extend to grid-scale applications, where these batteries can facilitate greater renewable energy integration. By providing cost-effective energy storage solutions, Na-S batteries can accelerate the transition away from fossil fuel dependence, multiplying their environmental benefits beyond the direct impacts of the battery technology itself. Cathode design experiments should therefore consider not only immediate performance metrics but also long-term environmental implications across the entire technology lifecycle.
The sulfur component in Na-S batteries further enhances sustainability profiles, as sulfur is an abundant by-product of petroleum refining processes. Utilizing this industrial waste stream creates a circular economy opportunity, transforming what would otherwise be a disposal challenge into a valuable battery material. This repurposing significantly reduces the environmental footprint compared to mining new materials specifically for battery production.
Life cycle assessment (LCA) studies indicate that room-temperature Na-S batteries potentially have 25-30% lower carbon footprint during manufacturing compared to equivalent lithium-ion systems. This reduction stems from less energy-intensive material processing requirements and simplified production methods that can be implemented in cathode design experiments. The environmental benefits extend throughout the battery lifecycle, including reduced energy consumption during operation and decreased end-of-life management challenges.
Water usage represents another critical environmental factor in battery production. Experimental designs for Na-S cathodes typically require 40-50% less water consumption compared to conventional lithium cathode manufacturing processes. This water conservation aspect becomes increasingly important as battery production scales globally, particularly in water-stressed regions where manufacturing facilities may be located.
End-of-life considerations must be integrated into cathode design experiments from the outset. The recyclability of Na-S battery components presents both challenges and opportunities. While sodium compounds can be more readily recovered than lithium, the sulfur components require specialized recycling processes to prevent environmental contamination. Experimental designs that facilitate easier disassembly and material separation can significantly improve recycling efficiency and reduce waste.
The sustainability advantages of Na-S technology also extend to grid-scale applications, where these batteries can facilitate greater renewable energy integration. By providing cost-effective energy storage solutions, Na-S batteries can accelerate the transition away from fossil fuel dependence, multiplying their environmental benefits beyond the direct impacts of the battery technology itself. Cathode design experiments should therefore consider not only immediate performance metrics but also long-term environmental implications across the entire technology lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!