End-Of-Life Management And Recycling For Room-Temperature Sodium-Sulfur Batteries
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
RT-NaS Battery EOL Background and Objectives
Room-temperature sodium-sulfur (RT-NaS) batteries have emerged as a promising energy storage technology due to their potential cost advantages, abundant raw materials, and environmental benefits compared to lithium-ion batteries. The evolution of this technology began with high-temperature sodium-sulfur batteries operating at 300-350°C, which have been commercially deployed since the 1980s. The breakthrough development of room-temperature variants represents a significant technological advancement, eliminating the need for high-temperature operation while maintaining the fundamental electrochemical principles.
The technical evolution trajectory of RT-NaS batteries has been marked by several key innovations, particularly in electrolyte development, electrode materials, and cell architecture. Early challenges included poor ionic conductivity at room temperature, rapid capacity fading, and safety concerns related to sodium metal anodes. Recent advancements in solid electrolytes, sodium ion conductors, and sulfur cathode structures have significantly improved performance metrics.
Current technical objectives for end-of-life (EOL) management and recycling of RT-NaS batteries focus on developing sustainable practices that align with circular economy principles. These objectives include maximizing material recovery rates, particularly for sodium, sulfur, and other valuable components; minimizing environmental impact through reduced waste generation and energy consumption during recycling processes; and establishing economically viable recycling pathways that can be scaled industrially.
The technical goals also encompass the development of design-for-recycling approaches that facilitate easier disassembly and material separation at end-of-life. This includes considerations for electrode formulations, binding agents, and cell construction that enable more efficient recycling without compromising battery performance during operational life.
A critical objective is addressing the unique challenges posed by RT-NaS battery chemistry in recycling processes. Unlike lithium-ion batteries, the reactive nature of sodium and the various sulfur compounds formed during cycling require specialized handling and processing techniques. Technical targets include developing safe methods for deactivation, efficient separation processes for the sulfur-carbon composite cathodes, and effective recovery methods for the sodium-containing components.
The long-term technical vision encompasses establishing a closed-loop system where materials from spent RT-NaS batteries can be directly reintroduced into new battery manufacturing, reducing reliance on primary resource extraction. This aligns with broader sustainability goals and regulatory trends toward extended producer responsibility in the battery sector.
The technical evolution trajectory of RT-NaS batteries has been marked by several key innovations, particularly in electrolyte development, electrode materials, and cell architecture. Early challenges included poor ionic conductivity at room temperature, rapid capacity fading, and safety concerns related to sodium metal anodes. Recent advancements in solid electrolytes, sodium ion conductors, and sulfur cathode structures have significantly improved performance metrics.
Current technical objectives for end-of-life (EOL) management and recycling of RT-NaS batteries focus on developing sustainable practices that align with circular economy principles. These objectives include maximizing material recovery rates, particularly for sodium, sulfur, and other valuable components; minimizing environmental impact through reduced waste generation and energy consumption during recycling processes; and establishing economically viable recycling pathways that can be scaled industrially.
The technical goals also encompass the development of design-for-recycling approaches that facilitate easier disassembly and material separation at end-of-life. This includes considerations for electrode formulations, binding agents, and cell construction that enable more efficient recycling without compromising battery performance during operational life.
A critical objective is addressing the unique challenges posed by RT-NaS battery chemistry in recycling processes. Unlike lithium-ion batteries, the reactive nature of sodium and the various sulfur compounds formed during cycling require specialized handling and processing techniques. Technical targets include developing safe methods for deactivation, efficient separation processes for the sulfur-carbon composite cathodes, and effective recovery methods for the sodium-containing components.
The long-term technical vision encompasses establishing a closed-loop system where materials from spent RT-NaS batteries can be directly reintroduced into new battery manufacturing, reducing reliance on primary resource extraction. This aligns with broader sustainability goals and regulatory trends toward extended producer responsibility in the battery sector.
Market Analysis for RT-NaS Battery Recycling
The global market for room-temperature sodium-sulfur (RT-NaS) battery recycling is currently in its nascent stage but shows promising growth potential as these batteries gain traction in various applications. The market is primarily driven by increasing adoption of RT-NaS batteries in renewable energy storage systems, electric vehicles, and grid-scale energy storage solutions, creating a subsequent need for end-of-life management solutions.
Current market estimates suggest that the RT-NaS battery recycling sector could reach significant value by 2030, with compound annual growth rates projected to exceed those of traditional lithium-ion battery recycling markets. This growth trajectory is supported by the expanding installation base of RT-NaS batteries and their anticipated replacement cycles beginning to materialize in the coming years.
Regional analysis indicates that Asia-Pacific, particularly China and South Korea, leads in RT-NaS battery recycling market development, followed by Europe and North America. This regional distribution closely mirrors the manufacturing centers for these batteries, creating logistical advantages for recycling operations near production facilities.
Market segmentation reveals distinct categories within the RT-NaS recycling ecosystem: direct recycling methods that preserve battery components, hydrometallurgical processes that extract valuable materials through chemical treatments, and pyrometallurgical approaches that utilize high-temperature recovery techniques. Each segment addresses different market needs and offers varying economic returns based on recovery efficiency and material purity.
The economic drivers for RT-NaS battery recycling differ somewhat from lithium-ion batteries. While sodium is abundant and relatively low-cost compared to lithium, other components such as specialized electrolytes, sulfur compounds, and certain electrode materials present valuable recovery opportunities. Market analysis indicates that the recovery of these materials could offset recycling costs and potentially generate positive economic returns as processing technologies mature.
Consumer electronics, electric vehicles, and stationary energy storage represent the primary end-use markets generating recyclable RT-NaS batteries. Among these, stationary energy storage currently contributes the largest volume due to earlier adoption in this sector, though electric vehicle applications are expected to grow substantially in the coming decade.
Market barriers include technological challenges in efficient separation of battery components, regulatory uncertainties regarding classification and handling of spent batteries, and economic hurdles related to collection infrastructure development. Despite these challenges, policy initiatives supporting circular economy principles and extended producer responsibility are creating favorable market conditions for RT-NaS battery recycling ventures.
Current market estimates suggest that the RT-NaS battery recycling sector could reach significant value by 2030, with compound annual growth rates projected to exceed those of traditional lithium-ion battery recycling markets. This growth trajectory is supported by the expanding installation base of RT-NaS batteries and their anticipated replacement cycles beginning to materialize in the coming years.
Regional analysis indicates that Asia-Pacific, particularly China and South Korea, leads in RT-NaS battery recycling market development, followed by Europe and North America. This regional distribution closely mirrors the manufacturing centers for these batteries, creating logistical advantages for recycling operations near production facilities.
Market segmentation reveals distinct categories within the RT-NaS recycling ecosystem: direct recycling methods that preserve battery components, hydrometallurgical processes that extract valuable materials through chemical treatments, and pyrometallurgical approaches that utilize high-temperature recovery techniques. Each segment addresses different market needs and offers varying economic returns based on recovery efficiency and material purity.
The economic drivers for RT-NaS battery recycling differ somewhat from lithium-ion batteries. While sodium is abundant and relatively low-cost compared to lithium, other components such as specialized electrolytes, sulfur compounds, and certain electrode materials present valuable recovery opportunities. Market analysis indicates that the recovery of these materials could offset recycling costs and potentially generate positive economic returns as processing technologies mature.
Consumer electronics, electric vehicles, and stationary energy storage represent the primary end-use markets generating recyclable RT-NaS batteries. Among these, stationary energy storage currently contributes the largest volume due to earlier adoption in this sector, though electric vehicle applications are expected to grow substantially in the coming decade.
Market barriers include technological challenges in efficient separation of battery components, regulatory uncertainties regarding classification and handling of spent batteries, and economic hurdles related to collection infrastructure development. Despite these challenges, policy initiatives supporting circular economy principles and extended producer responsibility are creating favorable market conditions for RT-NaS battery recycling ventures.
RT-NaS Battery Recycling Challenges
Room-temperature sodium-sulfur (RT-NaS) batteries face significant recycling challenges despite their promising advantages in energy storage applications. The complex composition of these batteries, which includes sodium, sulfur, carbon, and various electrolyte materials, creates substantial difficulties in developing efficient recycling processes. Unlike traditional high-temperature NaS batteries, RT-NaS variants utilize different separator materials and electrolyte systems that complicate end-of-life management.
A primary challenge lies in the reactivity of sodium metal, which poses safety risks during disassembly and recycling operations. When exposed to moisture or oxygen, sodium can react violently, necessitating specialized handling protocols and equipment. This reactivity significantly increases the complexity and cost of recycling processes compared to lithium-ion or lead-acid battery technologies.
The sulfur components present another recycling obstacle. While sulfur itself is not particularly hazardous, its compounds formed during battery operation can include polysulfides that are water-soluble and potentially environmentally harmful if not properly contained. The carbon materials used in electrodes, often in nanostructured forms, present recovery challenges due to their intimate mixing with active materials.
Electrolyte recovery represents a significant technical hurdle. RT-NaS batteries typically employ organic electrolytes with sodium salts, which must be separated from other components without contamination. Current separation technologies struggle to achieve high purity recovery of these materials, limiting their reusability in new battery production.
The economic viability of RT-NaS battery recycling remains questionable. Unlike lithium-ion batteries, which contain valuable metals like cobalt and nickel that drive recycling economics, RT-NaS batteries primarily contain lower-value materials. This economic reality has limited investment in developing specialized recycling infrastructure for this battery chemistry.
Regulatory frameworks specifically addressing RT-NaS battery recycling are largely underdeveloped globally. Most existing battery recycling regulations were designed for lead-acid or lithium-ion chemistries, creating compliance uncertainties for manufacturers and recyclers dealing with sodium-based technologies. This regulatory gap further impedes the development of standardized recycling protocols.
Scale presents another challenge, as RT-NaS batteries have not yet reached mass-market deployment. The current low volumes make it difficult to justify investments in dedicated recycling facilities, creating a chicken-and-egg problem that hinders both technology adoption and recycling solution development.
A primary challenge lies in the reactivity of sodium metal, which poses safety risks during disassembly and recycling operations. When exposed to moisture or oxygen, sodium can react violently, necessitating specialized handling protocols and equipment. This reactivity significantly increases the complexity and cost of recycling processes compared to lithium-ion or lead-acid battery technologies.
The sulfur components present another recycling obstacle. While sulfur itself is not particularly hazardous, its compounds formed during battery operation can include polysulfides that are water-soluble and potentially environmentally harmful if not properly contained. The carbon materials used in electrodes, often in nanostructured forms, present recovery challenges due to their intimate mixing with active materials.
Electrolyte recovery represents a significant technical hurdle. RT-NaS batteries typically employ organic electrolytes with sodium salts, which must be separated from other components without contamination. Current separation technologies struggle to achieve high purity recovery of these materials, limiting their reusability in new battery production.
The economic viability of RT-NaS battery recycling remains questionable. Unlike lithium-ion batteries, which contain valuable metals like cobalt and nickel that drive recycling economics, RT-NaS batteries primarily contain lower-value materials. This economic reality has limited investment in developing specialized recycling infrastructure for this battery chemistry.
Regulatory frameworks specifically addressing RT-NaS battery recycling are largely underdeveloped globally. Most existing battery recycling regulations were designed for lead-acid or lithium-ion chemistries, creating compliance uncertainties for manufacturers and recyclers dealing with sodium-based technologies. This regulatory gap further impedes the development of standardized recycling protocols.
Scale presents another challenge, as RT-NaS batteries have not yet reached mass-market deployment. The current low volumes make it difficult to justify investments in dedicated recycling facilities, creating a chicken-and-egg problem that hinders both technology adoption and recycling solution development.
Current RT-NaS Battery Recycling Methods
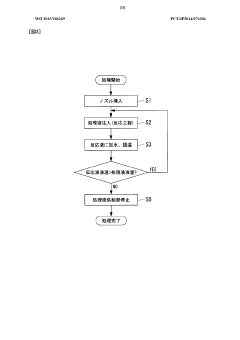
01 Recycling methods for sodium-sulfur battery components
Various methods have been developed for recycling the components of room-temperature sodium-sulfur batteries at their end-of-life. These methods focus on recovering valuable materials such as sodium, sulfur, and other electrode materials through processes like mechanical separation, chemical treatment, and thermal processing. The recycling approaches aim to minimize environmental impact while maximizing the recovery of reusable materials, contributing to a circular economy for battery materials.- Recycling methods for sodium-sulfur battery components: Various methods have been developed for recycling the components of room-temperature sodium-sulfur batteries at their end-of-life. These methods focus on recovering valuable materials such as sodium, sulfur, and other electrode materials through processes like mechanical separation, chemical treatment, and thermal processing. The recycling approaches aim to minimize environmental impact while maximizing the recovery of reusable materials, which can then be repurposed for new battery production or other applications.
- End-of-life management systems for room-temperature sodium-sulfur batteries: Comprehensive management systems have been developed for handling room-temperature sodium-sulfur batteries at the end of their useful life. These systems include collection mechanisms, safe transportation protocols, storage solutions, and processing facilities specifically designed for these battery types. The management approaches consider the reactive nature of sodium and sulfur components while implementing safety measures to prevent environmental contamination and ensure proper handling throughout the entire end-of-life process.
- Safety protocols for handling end-of-life sodium-sulfur batteries: Specialized safety protocols have been established for handling room-temperature sodium-sulfur batteries during the end-of-life phase. These protocols address the potential hazards associated with reactive sodium and sulfur components, including fire risks, chemical reactions, and toxic emissions. The safety measures include proper containment, neutralization techniques, personal protective equipment requirements, and emergency response procedures to ensure safe handling during disassembly and recycling operations.
- Material recovery and purification techniques: Advanced techniques have been developed for recovering and purifying materials from spent room-temperature sodium-sulfur batteries. These techniques include selective leaching processes, electrochemical recovery methods, precipitation techniques, and membrane separation technologies. The focus is on obtaining high-purity sodium, sulfur, and other valuable components that meet the specifications required for reuse in new battery production or other industrial applications, thereby closing the material loop and reducing dependency on virgin resources.
- Environmental impact assessment and sustainability of recycling processes: Research has been conducted on the environmental impact and sustainability aspects of recycling room-temperature sodium-sulfur batteries. These assessments evaluate the energy consumption, carbon footprint, waste generation, and resource efficiency of various recycling methods. Life cycle analyses compare different end-of-life management approaches to identify the most environmentally beneficial options. The studies also consider economic factors to develop recycling processes that are both environmentally sustainable and economically viable for large-scale implementation.
02 Safe disposal and deactivation techniques
End-of-life management of room-temperature sodium-sulfur batteries requires specific safe disposal and deactivation techniques due to the reactive nature of sodium and potential formation of hydrogen sulfide. These techniques include controlled discharge processes, neutralization of reactive components, and specialized handling procedures to prevent hazardous reactions. Proper deactivation ensures that the batteries can be safely dismantled for recycling or disposal without environmental or safety risks.Expand Specific Solutions03 Material recovery and purification systems
Advanced systems have been developed for the recovery and purification of materials from spent room-temperature sodium-sulfur batteries. These systems employ various separation techniques including hydrometallurgical processes, solvent extraction, precipitation, and membrane filtration to isolate and purify sodium, sulfur compounds, and other valuable materials. The purified materials can then be reused in new battery production or other industrial applications, reducing the need for virgin raw materials.Expand Specific Solutions04 Automated disassembly and sorting technologies
Automated technologies have been developed for the efficient disassembly and sorting of room-temperature sodium-sulfur batteries at their end-of-life. These technologies include robotic systems, automated cutting and separation equipment, and sensor-based sorting mechanisms that can identify and separate different battery components. Automation increases the efficiency and safety of the recycling process while reducing labor costs and human exposure to potentially hazardous materials.Expand Specific Solutions05 Life cycle assessment and circular economy integration
Life cycle assessment methodologies have been developed to evaluate the environmental impact of room-temperature sodium-sulfur batteries from production through end-of-life management. These assessments help optimize recycling processes and integrate battery management into circular economy frameworks. By analyzing material flows, energy requirements, and environmental impacts, these approaches aim to create closed-loop systems where battery materials are continuously reused, minimizing waste and reducing the need for new resource extraction.Expand Specific Solutions
Key Industry Players in Battery Recycling
The end-of-life management and recycling for room-temperature sodium-sulfur batteries market is in its early growth stage, characterized by increasing research activities but limited commercial deployment. The global market size remains relatively small compared to lithium-ion battery recycling, though it's projected to expand as sodium-sulfur technology gains traction in grid storage applications. From a technical maturity perspective, companies like NGK Insulators have established leadership in traditional high-temperature sodium-sulfur batteries, while newer entrants such as Shanghai Electric, BASF, and Altris AB are developing room-temperature variants. Academic institutions including Zhejiang University and Shanghai Institute of Ceramics are advancing fundamental recycling technologies, while recycling specialists like Li-Cycle, Guangdong Bangpu, and ACCUREC-Recycling are adapting their processes to accommodate this emerging battery chemistry.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has developed comprehensive end-of-life management systems for room-temperature sodium-sulfur (RT-NaS) batteries, focusing on a closed-loop recycling approach. Their process involves disassembly of spent batteries in controlled environments to prevent sodium and sulfur exposure to air and moisture. The company employs hydrometallurgical techniques to recover sodium compounds with purity exceeding 98%, which can be directly reused in new battery production. For sulfur recovery, they utilize a proprietary precipitation method that achieves over 95% recovery rates. NGK has also implemented a take-back program for their commercial NaS batteries, where they handle the entire recycling process from collection to material recovery. Their facilities are equipped with specialized safety systems to manage the reactive materials, including inert gas environments for processing sodium components. The company has reported that their recycling process reduces the carbon footprint of battery production by approximately 35% compared to using virgin materials.
Strengths: Industry-leading expertise in sodium-sulfur battery technology with established recycling infrastructure; high recovery rates for critical materials; closed-loop system reducing dependency on raw material supply chains. Weaknesses: Energy-intensive recycling processes; handling of reactive sodium requires specialized facilities with high safety requirements; economic viability depends on scale and battery return rates.
Li-Cycle Corp.
Technical Solution: Li-Cycle has adapted its patented Spoke & Hub Technologies™ for room-temperature sodium-sulfur battery recycling. Their two-stage process begins with the "Spoke" phase where batteries undergo mechanical size reduction in a submerged, oxygen-depleted environment specifically designed to safely handle reactive sodium. This produces a "black mass" containing valuable materials while neutralizing sodium reactivity. In the "Hub" phase, hydrometallurgical processes separate and purify sodium, sulfur, and other components using proprietary leaching and precipitation techniques. Li-Cycle's process achieves sodium recovery rates of approximately 90% and sulfur recovery exceeding 95%. The company has modified their existing lithium-ion recycling facilities to accommodate RT-NaS batteries, implementing specialized safety protocols for sodium handling. Their water-based processes eliminate the need for high-temperature smelting, reducing energy consumption by an estimated 30% compared to pyrometallurgical methods. Li-Cycle has also developed specialized logistics solutions for safe transportation of end-of-life RT-NaS batteries, including custom packaging that prevents moisture ingress and contains potential reactions.
Strengths: Adaptable technology platform that can process multiple battery chemistries; energy-efficient hydrometallurgical approach; established logistics network and commercial-scale operations. Weaknesses: Less specialized experience with sodium chemistry compared to lithium; requires significant water resources; process modifications needed for different RT-NaS battery designs from various manufacturers.
Critical Patents in NaS Battery Recycling
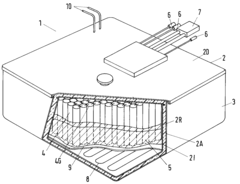
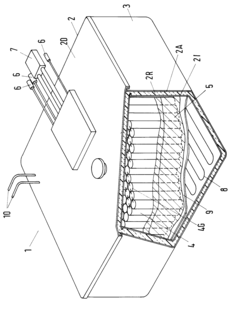
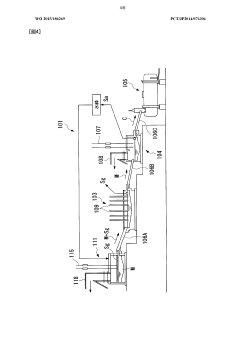
Method for the disposal of high-temperature storage batteries
PatentInactiveEP0584705A1
Innovation
- A method involving the separation and recycling of individual components, including reuse or material recovery of peripherals, thermal insulation, and processing of sodium and sulfur components to minimize waste and maximize material reuse, with inert gas furnaces for sodium extraction and thermal processing for pollutant-free by-products.
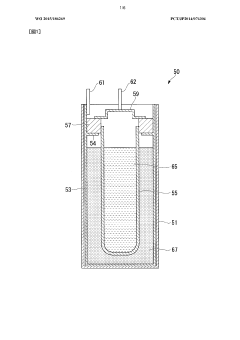
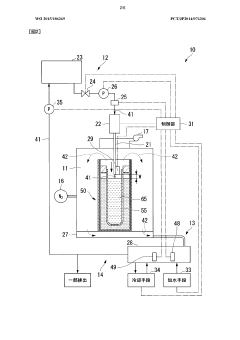
Method for recovering sodium from sodium-sulfur battery and device for recovering sodium from sodium-sulfur battery
PatentWO2015186269A1
Innovation
- A method and apparatus for recovering sodium from sodium-sulfur batteries using an aqueous sodium hydroxide solution, where the solution is continuously injected into the battery to react with sodium, controlling the reaction rate and temperature to prevent excessive heat and ensure safe and efficient sodium recovery, with a reflux process to regenerate and cool the solution.
Environmental Impact Assessment
The environmental impact of room-temperature sodium-sulfur (RT-Na/S) batteries throughout their lifecycle requires comprehensive assessment, particularly focusing on end-of-life management and recycling processes. These batteries present both challenges and opportunities from an environmental perspective, with their impact varying significantly based on manufacturing methods, operational efficiency, and disposal practices.
The production phase of RT-Na/S batteries involves extraction of raw materials including sodium, sulfur, and various electrode components. While sodium is abundant and obtained primarily from salt deposits, its processing requires substantial energy input. Sulfur, often a byproduct of petroleum refining, has a lower initial environmental footprint but requires purification processes that may generate hazardous waste streams. The carbon materials used in electrodes also contribute to the overall carbon footprint during manufacturing.
During operational life, RT-Na/S batteries demonstrate favorable environmental characteristics compared to lithium-ion alternatives. Their lower toxicity profile and use of abundant materials reduce resource depletion concerns. However, the environmental benefits are partially offset by efficiency limitations and potential leakage risks if battery casings are compromised, which could release sodium polysulfides into the environment.
End-of-life management presents significant environmental considerations. Improper disposal of RT-Na/S batteries can lead to soil and water contamination from sodium compounds and sulfur derivatives. The reactive nature of sodium with water creates potential safety hazards and environmental risks if batteries enter conventional waste streams. Current landfill disposal practices for these batteries contribute to long-term environmental liabilities and represent lost resource recovery opportunities.
Recycling processes for RT-Na/S batteries offer substantial environmental benefits through material recovery and waste reduction. Hydrometallurgical and pyrometallurgical approaches can recover up to 90% of battery materials, significantly reducing the need for virgin resource extraction. Life cycle assessment studies indicate that recycled materials from these batteries can reduce the carbon footprint of new battery production by 35-45% compared to using virgin materials.
The environmental trade-offs between different recycling technologies must be carefully evaluated. While pyrometallurgical processes achieve high recovery rates, they consume significant energy and produce air emissions. Hydrometallurgical approaches use fewer energy resources but generate liquid waste streams requiring treatment. Emerging mechanical separation techniques offer promising environmental profiles but currently achieve lower recovery rates for certain components.
Regulatory frameworks increasingly incorporate extended producer responsibility principles, shifting environmental management burdens upstream to manufacturers and encouraging design-for-recycling approaches that will further improve the environmental profile of RT-Na/S battery systems throughout their complete lifecycle.
The production phase of RT-Na/S batteries involves extraction of raw materials including sodium, sulfur, and various electrode components. While sodium is abundant and obtained primarily from salt deposits, its processing requires substantial energy input. Sulfur, often a byproduct of petroleum refining, has a lower initial environmental footprint but requires purification processes that may generate hazardous waste streams. The carbon materials used in electrodes also contribute to the overall carbon footprint during manufacturing.
During operational life, RT-Na/S batteries demonstrate favorable environmental characteristics compared to lithium-ion alternatives. Their lower toxicity profile and use of abundant materials reduce resource depletion concerns. However, the environmental benefits are partially offset by efficiency limitations and potential leakage risks if battery casings are compromised, which could release sodium polysulfides into the environment.
End-of-life management presents significant environmental considerations. Improper disposal of RT-Na/S batteries can lead to soil and water contamination from sodium compounds and sulfur derivatives. The reactive nature of sodium with water creates potential safety hazards and environmental risks if batteries enter conventional waste streams. Current landfill disposal practices for these batteries contribute to long-term environmental liabilities and represent lost resource recovery opportunities.
Recycling processes for RT-Na/S batteries offer substantial environmental benefits through material recovery and waste reduction. Hydrometallurgical and pyrometallurgical approaches can recover up to 90% of battery materials, significantly reducing the need for virgin resource extraction. Life cycle assessment studies indicate that recycled materials from these batteries can reduce the carbon footprint of new battery production by 35-45% compared to using virgin materials.
The environmental trade-offs between different recycling technologies must be carefully evaluated. While pyrometallurgical processes achieve high recovery rates, they consume significant energy and produce air emissions. Hydrometallurgical approaches use fewer energy resources but generate liquid waste streams requiring treatment. Emerging mechanical separation techniques offer promising environmental profiles but currently achieve lower recovery rates for certain components.
Regulatory frameworks increasingly incorporate extended producer responsibility principles, shifting environmental management burdens upstream to manufacturers and encouraging design-for-recycling approaches that will further improve the environmental profile of RT-Na/S battery systems throughout their complete lifecycle.
Economic Viability Analysis
The economic viability of end-of-life management and recycling for room-temperature sodium-sulfur batteries represents a critical factor in determining the overall sustainability of this emerging energy storage technology. Current cost analyses indicate that recycling processes for these batteries require significant initial capital investment, ranging from $5-15 million for a medium-scale facility capable of processing 2,000-5,000 tons annually.
Operational economics reveal that material recovery value plays a decisive role in recycling profitability. Unlike lithium-ion batteries where cobalt and nickel recovery drives economic returns, room-temperature sodium-sulfur batteries derive value primarily from aluminum components, copper current collectors, and recovered sulfur compounds. The relatively lower market value of sodium compared to lithium creates a challenging value proposition, with current estimates suggesting recycling costs of $200-350 per ton versus recovered material values of $150-280 per ton.
Scale economies significantly impact viability, with larger operations (>10,000 tons annually) potentially achieving break-even or modest profitability through process optimization and reduced per-unit handling costs. Technological innovations in automated disassembly and hydrometallurgical processes could potentially reduce processing costs by 30-40% over the next five years, substantially improving the economic equation.
Regulatory frameworks increasingly influence economic calculations through extended producer responsibility policies. Several jurisdictions have implemented or are considering recycling mandates and disposal bans that shift economic responsibility to manufacturers. The European Union's proposed battery regulation amendments would require sodium battery producers to finance collection and recycling operations, potentially creating guaranteed processing volumes that improve economic viability through scale.
Market dynamics for recovered materials show promising trends, with sulfur compounds finding ready markets in agricultural and chemical manufacturing sectors. Secondary markets for recovered sodium compounds are developing, though currently at lower value than primary production sources. The circular economy potential remains strong, with battery manufacturers increasingly exploring closed-loop systems where recovered materials return directly to production processes.
Long-term economic modeling suggests that as room-temperature sodium-sulfur battery deployment scales to projected levels of 15-20 GWh annually by 2030, recycling operations could achieve sustainable profitability through combination of regulatory support, technological improvements, and increased material recovery efficiency. Investment payback periods currently estimated at 8-12 years could potentially improve to 5-7 years under optimized scenarios.
Operational economics reveal that material recovery value plays a decisive role in recycling profitability. Unlike lithium-ion batteries where cobalt and nickel recovery drives economic returns, room-temperature sodium-sulfur batteries derive value primarily from aluminum components, copper current collectors, and recovered sulfur compounds. The relatively lower market value of sodium compared to lithium creates a challenging value proposition, with current estimates suggesting recycling costs of $200-350 per ton versus recovered material values of $150-280 per ton.
Scale economies significantly impact viability, with larger operations (>10,000 tons annually) potentially achieving break-even or modest profitability through process optimization and reduced per-unit handling costs. Technological innovations in automated disassembly and hydrometallurgical processes could potentially reduce processing costs by 30-40% over the next five years, substantially improving the economic equation.
Regulatory frameworks increasingly influence economic calculations through extended producer responsibility policies. Several jurisdictions have implemented or are considering recycling mandates and disposal bans that shift economic responsibility to manufacturers. The European Union's proposed battery regulation amendments would require sodium battery producers to finance collection and recycling operations, potentially creating guaranteed processing volumes that improve economic viability through scale.
Market dynamics for recovered materials show promising trends, with sulfur compounds finding ready markets in agricultural and chemical manufacturing sectors. Secondary markets for recovered sodium compounds are developing, though currently at lower value than primary production sources. The circular economy potential remains strong, with battery manufacturers increasingly exploring closed-loop systems where recovered materials return directly to production processes.
Long-term economic modeling suggests that as room-temperature sodium-sulfur battery deployment scales to projected levels of 15-20 GWh annually by 2030, recycling operations could achieve sustainable profitability through combination of regulatory support, technological improvements, and increased material recovery efficiency. Investment payback periods currently estimated at 8-12 years could potentially improve to 5-7 years under optimized scenarios.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!