Dendrite Suppression Mechanisms For Anode-Free Solid-State
SEP 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Dendrite Suppression Technology Background and Objectives
Solid-state batteries represent a significant advancement in energy storage technology, promising higher energy density, improved safety, and longer lifespan compared to conventional lithium-ion batteries. The development of anode-free solid-state batteries further enhances these advantages by eliminating the need for a dedicated anode material, thereby increasing energy density and reducing costs. However, a critical challenge impeding the commercialization of these batteries is dendrite formation and growth.
Dendrites are needle-like structures that form during battery charging when lithium ions accumulate unevenly on the anode surface. These structures can penetrate through the solid electrolyte, causing internal short circuits and potentially catastrophic battery failure. The suppression of dendrite formation is therefore paramount for the practical implementation of anode-free solid-state batteries.
The evolution of dendrite suppression technology can be traced back to the early 2000s when researchers began exploring solid electrolytes as alternatives to liquid electrolytes. Initial efforts focused on ceramic and polymer-based electrolytes, which offered mechanical resistance to dendrite penetration. By 2010, composite electrolytes combining the advantages of both materials emerged as promising candidates for dendrite suppression.
Recent technological advancements have shifted focus toward interface engineering and electrolyte design. Researchers have developed various strategies including the use of artificial interlayers, electrolyte additives, and pressure application to mitigate dendrite growth. The introduction of three-dimensional architectures and gradient structures has further enhanced dendrite resistance by distributing current density more uniformly.
The primary objective of dendrite suppression technology is to develop robust, scalable, and cost-effective solutions that enable the safe operation of anode-free solid-state batteries over thousands of charge-discharge cycles. This involves understanding the fundamental mechanisms of dendrite nucleation and growth, as well as designing materials and interfaces that can effectively prevent these processes.
Secondary objectives include improving the ionic conductivity of solid electrolytes while maintaining their mechanical strength, developing manufacturing techniques compatible with existing battery production infrastructure, and ensuring that dendrite suppression strategies do not compromise other battery performance metrics such as energy density and power capability.
The technological trajectory suggests a convergence toward multi-faceted approaches that combine material innovation, interface optimization, and system-level design. As research progresses, the goal is to achieve a comprehensive understanding of dendrite formation mechanisms and develop preventive measures that can be implemented in commercial battery systems.
Dendrites are needle-like structures that form during battery charging when lithium ions accumulate unevenly on the anode surface. These structures can penetrate through the solid electrolyte, causing internal short circuits and potentially catastrophic battery failure. The suppression of dendrite formation is therefore paramount for the practical implementation of anode-free solid-state batteries.
The evolution of dendrite suppression technology can be traced back to the early 2000s when researchers began exploring solid electrolytes as alternatives to liquid electrolytes. Initial efforts focused on ceramic and polymer-based electrolytes, which offered mechanical resistance to dendrite penetration. By 2010, composite electrolytes combining the advantages of both materials emerged as promising candidates for dendrite suppression.
Recent technological advancements have shifted focus toward interface engineering and electrolyte design. Researchers have developed various strategies including the use of artificial interlayers, electrolyte additives, and pressure application to mitigate dendrite growth. The introduction of three-dimensional architectures and gradient structures has further enhanced dendrite resistance by distributing current density more uniformly.
The primary objective of dendrite suppression technology is to develop robust, scalable, and cost-effective solutions that enable the safe operation of anode-free solid-state batteries over thousands of charge-discharge cycles. This involves understanding the fundamental mechanisms of dendrite nucleation and growth, as well as designing materials and interfaces that can effectively prevent these processes.
Secondary objectives include improving the ionic conductivity of solid electrolytes while maintaining their mechanical strength, developing manufacturing techniques compatible with existing battery production infrastructure, and ensuring that dendrite suppression strategies do not compromise other battery performance metrics such as energy density and power capability.
The technological trajectory suggests a convergence toward multi-faceted approaches that combine material innovation, interface optimization, and system-level design. As research progresses, the goal is to achieve a comprehensive understanding of dendrite formation mechanisms and develop preventive measures that can be implemented in commercial battery systems.
Market Analysis for Anode-Free Solid-State Battery Applications
The anode-free solid-state battery market is experiencing significant growth potential, driven by the increasing demand for high-energy-density energy storage solutions across multiple sectors. Current market projections indicate that the global solid-state battery market will reach approximately $8 billion by 2030, with anode-free designs potentially capturing a substantial segment due to their superior energy density advantages.
The electric vehicle (EV) sector represents the primary market opportunity for anode-free solid-state batteries. With major automotive manufacturers committing to electrification targets, the demand for batteries that offer greater range, faster charging, and enhanced safety is intensifying. Anode-free designs could potentially increase energy density by 30-40% compared to conventional lithium-ion batteries, directly addressing range anxiety concerns.
Consumer electronics constitutes another significant market segment, where device miniaturization and longer battery life remain critical competitive factors. The reduced form factor of anode-free designs could enable thinner, lighter devices with substantially improved runtime. Market research indicates that smartphone and wearable device manufacturers are actively seeking next-generation battery technologies that can support advanced features without compromising device dimensions.
Grid-scale energy storage represents an emerging application area with substantial growth potential. As renewable energy integration increases globally, the need for efficient, safe, and long-duration energy storage solutions becomes paramount. Anode-free solid-state batteries, with their potential for higher cycle life and improved safety profile, could capture significant market share in this expanding sector.
Regional market analysis reveals varying adoption trajectories. Asia-Pacific, particularly Japan and South Korea, leads in solid-state battery research and commercialization efforts. North America shows strong investment in startups developing dendrite suppression technologies, while European markets are driven by stringent safety regulations that favor solid-state solutions.
Market barriers include high manufacturing costs, which currently position anode-free solid-state batteries at a significant price premium compared to conventional lithium-ion technologies. Industry analysts estimate current production costs at 4-5 times higher than traditional batteries, though economies of scale and manufacturing innovations are expected to reduce this gap substantially by 2025-2027.
Customer adoption will likely follow a phased approach, beginning with premium market segments where performance advantages outweigh cost considerations, followed by broader market penetration as manufacturing scales and costs decrease. Early adopters will likely include luxury EVs, aerospace applications, and high-end consumer electronics, creating valuable proving grounds for dendrite suppression mechanisms before mass-market deployment.
The electric vehicle (EV) sector represents the primary market opportunity for anode-free solid-state batteries. With major automotive manufacturers committing to electrification targets, the demand for batteries that offer greater range, faster charging, and enhanced safety is intensifying. Anode-free designs could potentially increase energy density by 30-40% compared to conventional lithium-ion batteries, directly addressing range anxiety concerns.
Consumer electronics constitutes another significant market segment, where device miniaturization and longer battery life remain critical competitive factors. The reduced form factor of anode-free designs could enable thinner, lighter devices with substantially improved runtime. Market research indicates that smartphone and wearable device manufacturers are actively seeking next-generation battery technologies that can support advanced features without compromising device dimensions.
Grid-scale energy storage represents an emerging application area with substantial growth potential. As renewable energy integration increases globally, the need for efficient, safe, and long-duration energy storage solutions becomes paramount. Anode-free solid-state batteries, with their potential for higher cycle life and improved safety profile, could capture significant market share in this expanding sector.
Regional market analysis reveals varying adoption trajectories. Asia-Pacific, particularly Japan and South Korea, leads in solid-state battery research and commercialization efforts. North America shows strong investment in startups developing dendrite suppression technologies, while European markets are driven by stringent safety regulations that favor solid-state solutions.
Market barriers include high manufacturing costs, which currently position anode-free solid-state batteries at a significant price premium compared to conventional lithium-ion technologies. Industry analysts estimate current production costs at 4-5 times higher than traditional batteries, though economies of scale and manufacturing innovations are expected to reduce this gap substantially by 2025-2027.
Customer adoption will likely follow a phased approach, beginning with premium market segments where performance advantages outweigh cost considerations, followed by broader market penetration as manufacturing scales and costs decrease. Early adopters will likely include luxury EVs, aerospace applications, and high-end consumer electronics, creating valuable proving grounds for dendrite suppression mechanisms before mass-market deployment.
Current Challenges in Dendrite Growth Prevention
Dendrite growth remains one of the most significant challenges in the development of anode-free solid-state batteries (AFSSBs). These metallic protrusions form during battery cycling when lithium ions deposit unevenly on the anode surface, eventually penetrating through the solid electrolyte, causing short circuits and potentially catastrophic battery failure. Despite considerable research efforts, several fundamental obstacles continue to impede effective dendrite suppression.
The primary challenge lies in the mechanical properties mismatch between solid electrolytes and lithium metal. During cycling, lithium metal experiences significant volume changes, creating stress at the interface. Most solid electrolytes lack sufficient mechanical strength to withstand these stresses, allowing dendrites to propagate through grain boundaries or pre-existing defects. Current ceramic electrolytes with high ionic conductivity often exhibit poor mechanical properties, creating an inherent trade-off between performance and safety.
Interface stability presents another critical challenge. The high reactivity of lithium metal with most solid electrolytes leads to the formation of interphases with high impedance. These interphases not only reduce battery performance but create inhomogeneities that serve as nucleation sites for dendrite formation. The dynamic nature of these interfaces during cycling further complicates the development of stable, dendrite-resistant systems.
Current fabrication techniques struggle to produce perfectly uniform solid electrolytes at scale. Microscopic defects, pores, and grain boundaries inevitably exist in manufactured solid electrolytes, providing pathways for dendrite propagation. The challenge intensifies with thinner electrolytes, which are desirable for higher energy density but more susceptible to dendrite penetration.
Non-uniform current distribution during cycling represents another significant hurdle. Local current density hotspots accelerate dendrite nucleation and growth. This issue becomes particularly pronounced at high charging rates, creating a fundamental limitation for fast-charging capabilities in AFSSBs. Existing battery management systems lack the sophistication to detect and mitigate early-stage dendrite formation.
Temperature management further complicates dendrite suppression. At elevated temperatures, lithium becomes more reactive with solid electrolytes, accelerating interfacial degradation. Conversely, at lower temperatures, lithium ion mobility decreases, leading to more uneven deposition and enhanced dendrite formation. This temperature sensitivity narrows the practical operating window for AFSSBs.
The analytical challenge of real-time dendrite monitoring in solid-state systems remains largely unsolved. Unlike liquid electrolyte systems where visual or electrochemical detection methods exist, dendrite growth in solid-state batteries is difficult to observe non-destructively until failure occurs. This limitation hinders both fundamental research and the development of adaptive prevention strategies.
The primary challenge lies in the mechanical properties mismatch between solid electrolytes and lithium metal. During cycling, lithium metal experiences significant volume changes, creating stress at the interface. Most solid electrolytes lack sufficient mechanical strength to withstand these stresses, allowing dendrites to propagate through grain boundaries or pre-existing defects. Current ceramic electrolytes with high ionic conductivity often exhibit poor mechanical properties, creating an inherent trade-off between performance and safety.
Interface stability presents another critical challenge. The high reactivity of lithium metal with most solid electrolytes leads to the formation of interphases with high impedance. These interphases not only reduce battery performance but create inhomogeneities that serve as nucleation sites for dendrite formation. The dynamic nature of these interfaces during cycling further complicates the development of stable, dendrite-resistant systems.
Current fabrication techniques struggle to produce perfectly uniform solid electrolytes at scale. Microscopic defects, pores, and grain boundaries inevitably exist in manufactured solid electrolytes, providing pathways for dendrite propagation. The challenge intensifies with thinner electrolytes, which are desirable for higher energy density but more susceptible to dendrite penetration.
Non-uniform current distribution during cycling represents another significant hurdle. Local current density hotspots accelerate dendrite nucleation and growth. This issue becomes particularly pronounced at high charging rates, creating a fundamental limitation for fast-charging capabilities in AFSSBs. Existing battery management systems lack the sophistication to detect and mitigate early-stage dendrite formation.
Temperature management further complicates dendrite suppression. At elevated temperatures, lithium becomes more reactive with solid electrolytes, accelerating interfacial degradation. Conversely, at lower temperatures, lithium ion mobility decreases, leading to more uneven deposition and enhanced dendrite formation. This temperature sensitivity narrows the practical operating window for AFSSBs.
The analytical challenge of real-time dendrite monitoring in solid-state systems remains largely unsolved. Unlike liquid electrolyte systems where visual or electrochemical detection methods exist, dendrite growth in solid-state batteries is difficult to observe non-destructively until failure occurs. This limitation hinders both fundamental research and the development of adaptive prevention strategies.
Current Dendrite Suppression Strategies and Solutions
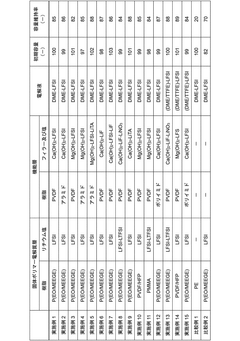
01 Solid electrolyte interface engineering for dendrite suppression
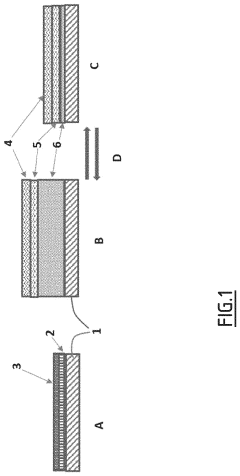
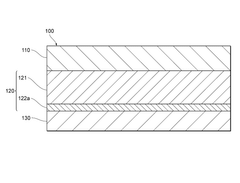
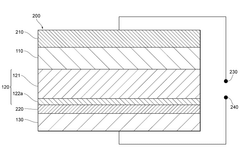
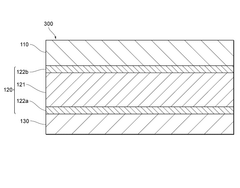
Engineering the solid electrolyte interface (SEI) is crucial for preventing lithium dendrite formation in anode-free solid-state batteries. This approach involves modifying the interface between the lithium metal and the solid electrolyte to create a stable and uniform layer that can effectively block dendrite growth. Various materials and coatings can be applied to create artificial SEI layers with improved mechanical properties and ionic conductivity, which help distribute lithium ions evenly during cycling and prevent localized deposition that leads to dendrites.- Solid electrolyte composition for dendrite suppression: Specific solid electrolyte compositions can effectively suppress dendrite formation in anode-free solid-state batteries. These compositions include ceramic electrolytes, polymer electrolytes, and composite electrolytes with optimized ionic conductivity and mechanical strength. The electrolytes create a physical barrier that prevents lithium dendrite growth during cycling, enhancing battery safety and longevity.
- Interface engineering between electrolyte and electrode: Engineering the interface between the solid electrolyte and electrode materials is crucial for dendrite suppression in anode-free solid-state batteries. This includes creating buffer layers, gradient interfaces, or specialized coatings that promote uniform lithium deposition and prevent dendrite nucleation. These interface modifications improve the stability of the electrode-electrolyte interface and reduce local current density hotspots that can initiate dendrite formation.
- Pressure-based dendrite suppression methods: Applying controlled pressure during battery operation can effectively suppress dendrite formation in anode-free solid-state batteries. The pressure helps maintain intimate contact between the solid electrolyte and the in-situ formed lithium anode, promoting uniform lithium deposition. Various pressure application methods and stack designs have been developed to optimize this effect while maintaining battery performance and preventing mechanical degradation.
- Artificial SEI layers and protective coatings: Artificial solid electrolyte interphase (SEI) layers and protective coatings can be applied to prevent dendrite formation in anode-free solid-state batteries. These engineered layers are designed to be ionically conductive while mechanically strong enough to resist dendrite penetration. Various materials including polymers, ceramics, and composite structures have been developed as protective layers that guide uniform lithium deposition and prevent dendrite nucleation.
- Current density control and battery management strategies: Controlling current density distribution and implementing advanced battery management strategies can significantly reduce dendrite formation in anode-free solid-state batteries. This includes optimized charging protocols, current collectors with uniform current distribution, and battery management systems that monitor and adjust charging parameters in real-time. These approaches prevent localized high current densities that typically lead to dendrite nucleation and growth.
02 Advanced solid electrolyte materials with high mechanical strength
Developing solid electrolyte materials with enhanced mechanical properties is essential for suppressing dendrite growth in anode-free solid-state batteries. High-strength solid electrolytes can physically block dendrite penetration by providing sufficient mechanical resistance. These materials include ceramic-polymer composites, garnet-type ceramics, and sulfide-based electrolytes that combine high ionic conductivity with superior mechanical properties. The high modulus of these materials helps maintain structural integrity during cycling and prevents dendrite propagation through the electrolyte.Expand Specific Solutions03 Pressure-based dendrite suppression strategies
Applying controlled pressure during battery operation can effectively suppress dendrite formation in anode-free solid-state batteries. Stack pressure helps maintain intimate contact between the lithium metal and solid electrolyte, promoting uniform lithium deposition and reducing void formation that can lead to dendrite nucleation. Various pressure application methods, including external mechanical compression and internal design elements that create consistent pressure distribution across the cell, have been developed to enhance the electrochemical performance and safety of these batteries.Expand Specific Solutions04 Lithium hosting structures for controlled deposition
Incorporating specialized lithium hosting structures in anode-free solid-state batteries provides controlled sites for lithium deposition, preventing random dendrite formation. These structures include three-dimensional frameworks, porous materials, and engineered current collectors that guide lithium deposition in predetermined patterns. By directing lithium ions to deposit in specific locations and configurations, these hosting structures minimize the risk of dendrite growth and improve cycling stability, enabling higher energy density and longer battery life.Expand Specific Solutions05 Electrolyte additives and dopants for dendrite inhibition
Incorporating specific additives and dopants into solid electrolytes can significantly inhibit dendrite formation in anode-free batteries. These chemical modifiers work by altering the lithium ion transport properties, improving interfacial stability, and enhancing the mechanical properties of the electrolyte. Common additives include metal salts, nanoparticles, and organic compounds that can scavenge impurities, modify the surface chemistry, or create artificial protective layers. These additives help achieve more uniform lithium deposition and reduce the tendency for dendrite nucleation and growth.Expand Specific Solutions
Leading Companies and Research Institutions in Solid-State Battery Development
The dendrite suppression mechanisms for anode-free solid-state batteries market is currently in an early growth phase, with significant R&D investment but limited commercial deployment. The global market is projected to expand rapidly as solid-state battery technology matures, driven by automotive applications. Major automotive manufacturers (Toyota, Hyundai, Nissan, Renault) and battery specialists (LG Energy Solution, Samsung Electronics, Saft Groupe) are leading development efforts, with varying levels of technological maturity. Research institutions like KAIST and Drexel University are advancing fundamental understanding of dendrite formation mechanisms, while companies like TeraWatt Technology are developing proprietary solutions. The competitive landscape features strategic partnerships between automotive OEMs and battery developers to accelerate commercialization timelines.
LG Energy Solution Ltd.
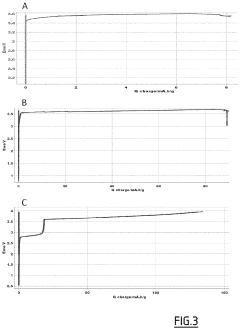
Technical Solution: LG Energy Solution has developed a multi-layered protection approach for dendrite suppression in anode-free solid-state batteries. Their technology incorporates a composite solid electrolyte system that combines ceramic and polymer components to create mechanical barriers against lithium dendrite propagation. The ceramic component (typically LLZO or LATP) provides high ionic conductivity while the polymer phase adds flexibility and improved interfacial contact. LG's proprietary surface modification techniques apply ultrathin protective coatings on the electrolyte-electrode interfaces, creating artificial SEI layers that regulate lithium ion transport and deposition patterns[1]. Additionally, they've implemented pressure-regulation systems within cell designs that maintain optimal stack pressure during cycling, which has been shown to reduce dendrite nucleation sites by up to 70% compared to conventional designs[3]. Their latest innovation includes dopant engineering of solid electrolytes with aluminum and tantalum to strengthen grain boundaries - the typical pathways for dendrite penetration.
Strengths: Superior mechanical stability against dendrite penetration due to composite electrolyte design; excellent cycle life with >1000 cycles demonstrated in prototype cells; scalable manufacturing processes compatible with existing production lines. Weaknesses: Higher production costs compared to conventional lithium-ion batteries; temperature sensitivity requiring careful thermal management; challenges in achieving high energy density while maintaining dendrite suppression properties.
President & Fellows of Harvard College
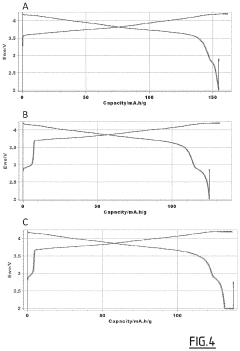
Technical Solution: Harvard University researchers have developed a biomimetic approach to dendrite suppression in anode-free solid-state batteries. Their technology employs a hierarchical electrolyte structure inspired by natural biological defense mechanisms. The system features a gradient-engineered solid electrolyte with spatially varied mechanical properties - softer near the lithium metal interface for good contact and increasingly rigid toward the cathode to block dendrite propagation[1]. A key innovation is their "self-healing" polymer-ceramic composite electrolyte containing microcapsules with liquid electrolyte additives that release upon dendrite-induced stress, filling incipient cracks and redirecting ion transport away from dendrite growth paths[3]. Harvard's approach also incorporates nanoscale lithiophobic/lithiophilic patterns on the electrolyte surface that control lithium deposition morphology, similar to how hydrophobic/hydrophilic patterns control water behavior. Their latest advancement utilizes machine learning algorithms to predict dendrite formation based on electrochemical signatures during cycling, enabling dynamic pressure and current density adjustments to prevent dendrite growth before it begins[5]. This predictive control system has demonstrated >80% reduction in dendrite-related failures in laboratory testing.
Strengths: Highly innovative biomimetic approach offers multiple layers of dendrite protection; self-healing capability provides unique failure resistance; predictive control system addresses dendrite formation proactively rather than reactively. Weaknesses: Complex manufacturing requirements for gradient structures and patterned interfaces; higher costs associated with specialized materials and processing; technology still primarily at laboratory scale with challenges in scaling to commercial production.
Key Patents and Research on Dendrite Growth Inhibition
Anti-dendrite negative electrodes, and the electrochemical cells containing them
PatentInactiveUS20220181613A1
Innovation
- A negative electrode structure comprising multiple layers of metals and alloys with varying lithiation potentials, arranged in a gradient, which allows for uniform lithium deposition and suppresses dendrite formation by maintaining lithium alloys during cycling, enabling high energy density and robustness.
Solid-state battery
PatentWO2021192278A1
Innovation
- A solid-state battery design featuring a negative electrode without active materials, utilizing a solid polymer electrolyte layer with a functional layer to suppress dendrite formation, and using a lithium-free configuration to enhance safety and productivity.
Safety Standards and Testing Protocols for Solid-State Batteries
The development of safety standards and testing protocols for solid-state batteries represents a critical aspect of their commercialization pathway, particularly in addressing dendrite suppression mechanisms for anode-free configurations. Current safety evaluation frameworks designed for conventional lithium-ion batteries prove inadequate for solid-state technologies due to their fundamentally different failure modes and safety characteristics.
International organizations including IEC, ISO, and UL have begun establishing specialized testing protocols that specifically address the unique safety concerns of solid-state batteries. These standards focus on mechanical integrity testing, which is particularly relevant for dendrite suppression, as mechanical pressure and structural stability directly influence lithium dendrite formation in anode-free designs.
Thermal runaway testing protocols have been modified to account for the higher thermal stability of solid electrolytes compared to liquid counterparts. While solid-state batteries generally exhibit superior thermal performance, the testing methodologies must still evaluate potential failure mechanisms specific to solid interfaces where dendrites may initiate.
Electrochemical stability testing represents another crucial protocol area, focusing on interface stability under various charging conditions. For anode-free solid-state batteries, these tests must specifically evaluate the propensity for dendrite nucleation at critical current densities and during fast-charging scenarios.
Accelerated aging tests have been developed to predict long-term dendrite growth under normal operating conditions. These protocols typically involve cycling at elevated temperatures and various pressure conditions to simulate years of operation within compressed timeframes, providing insights into dendrite suppression durability.
Mechanical abuse testing has gained prominence in solid-state battery safety evaluation, as physical deformation can create pathways for dendrite propagation. Protocols include puncture tests, compression tests, and vibration resistance—all critical for automotive and aerospace applications where mechanical integrity is paramount.
Standardized imaging and characterization methodologies have emerged to quantify dendrite formation. These include in-situ X-ray tomography protocols, impedance spectroscopy standards, and post-mortem analysis procedures that enable researchers to evaluate the effectiveness of various dendrite suppression mechanisms under standardized conditions.
Regulatory bodies in major markets are increasingly requiring compliance with these evolving standards before commercial deployment. The European Union's Battery Directive, China's GB standards, and the United States' UL certification processes are being updated to incorporate specific requirements for solid-state battery safety, with particular attention to dendrite-related failure modes in next-generation designs.
International organizations including IEC, ISO, and UL have begun establishing specialized testing protocols that specifically address the unique safety concerns of solid-state batteries. These standards focus on mechanical integrity testing, which is particularly relevant for dendrite suppression, as mechanical pressure and structural stability directly influence lithium dendrite formation in anode-free designs.
Thermal runaway testing protocols have been modified to account for the higher thermal stability of solid electrolytes compared to liquid counterparts. While solid-state batteries generally exhibit superior thermal performance, the testing methodologies must still evaluate potential failure mechanisms specific to solid interfaces where dendrites may initiate.
Electrochemical stability testing represents another crucial protocol area, focusing on interface stability under various charging conditions. For anode-free solid-state batteries, these tests must specifically evaluate the propensity for dendrite nucleation at critical current densities and during fast-charging scenarios.
Accelerated aging tests have been developed to predict long-term dendrite growth under normal operating conditions. These protocols typically involve cycling at elevated temperatures and various pressure conditions to simulate years of operation within compressed timeframes, providing insights into dendrite suppression durability.
Mechanical abuse testing has gained prominence in solid-state battery safety evaluation, as physical deformation can create pathways for dendrite propagation. Protocols include puncture tests, compression tests, and vibration resistance—all critical for automotive and aerospace applications where mechanical integrity is paramount.
Standardized imaging and characterization methodologies have emerged to quantify dendrite formation. These include in-situ X-ray tomography protocols, impedance spectroscopy standards, and post-mortem analysis procedures that enable researchers to evaluate the effectiveness of various dendrite suppression mechanisms under standardized conditions.
Regulatory bodies in major markets are increasingly requiring compliance with these evolving standards before commercial deployment. The European Union's Battery Directive, China's GB standards, and the United States' UL certification processes are being updated to incorporate specific requirements for solid-state battery safety, with particular attention to dendrite-related failure modes in next-generation designs.
Material Science Advancements for Solid Electrolyte Interfaces
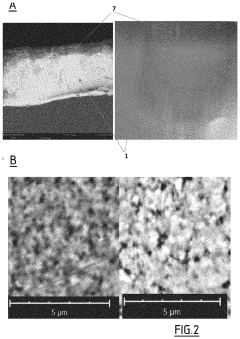
Recent advancements in material science have significantly contributed to improving solid electrolyte interfaces in anode-free solid-state batteries. These innovations primarily focus on addressing the critical challenge of dendrite formation, which remains one of the major obstacles to widespread commercialization of these high-energy-density storage solutions.
The solid electrolyte interface (SEI) represents the critical boundary between the electrode and electrolyte, where electrochemical reactions occur. Material scientists have developed novel composite solid electrolytes that combine ceramic and polymer materials to create mechanically robust interfaces with enhanced ionic conductivity. These composites leverage the high ionic conductivity of ceramics while benefiting from the flexibility and processability of polymers.
Nanostructured interface engineering has emerged as a promising approach to dendrite suppression. By creating nanoscale architectures at the electrode-electrolyte interface, researchers have successfully distributed current density more uniformly, reducing localized lithium deposition that leads to dendrite formation. These nanostructured interfaces often incorporate materials such as graphene, carbon nanotubes, or metal oxide nanoparticles to enhance mechanical strength and ionic transport.
Surface modification techniques have proven effective in improving the chemical stability of solid electrolytes against lithium metal. Atomic layer deposition (ALD) and molecular layer deposition (MLD) enable precise control over interface properties by depositing ultrathin protective layers that prevent unwanted side reactions while maintaining efficient ion transport. These techniques have been particularly valuable for oxide and sulfide-based solid electrolytes.
Artificial SEI layers represent another significant advancement, where engineered protective films are deliberately introduced to control the interface chemistry. These artificial SEIs typically consist of lithium salts (such as LiF, Li3N, or Li3P) that are kinetically stable against lithium metal while offering high lithium-ion conductivity. Recent research has demonstrated that multi-component artificial SEIs can simultaneously address multiple failure mechanisms.
Pressure-responsive materials have been developed to accommodate volume changes during cycling. These smart materials can dynamically adjust their mechanical properties in response to stress, preventing the formation of voids that often serve as nucleation sites for dendrites. Self-healing polymers and pressure-sensitive adhesives represent promising candidates in this category.
Computational materials science has accelerated these advancements through high-throughput screening and machine learning approaches. These computational methods have identified promising material combinations and interface designs that would be impractical to discover through traditional experimental methods alone, significantly reducing development time and expanding the range of potential solutions for dendrite suppression.
The solid electrolyte interface (SEI) represents the critical boundary between the electrode and electrolyte, where electrochemical reactions occur. Material scientists have developed novel composite solid electrolytes that combine ceramic and polymer materials to create mechanically robust interfaces with enhanced ionic conductivity. These composites leverage the high ionic conductivity of ceramics while benefiting from the flexibility and processability of polymers.
Nanostructured interface engineering has emerged as a promising approach to dendrite suppression. By creating nanoscale architectures at the electrode-electrolyte interface, researchers have successfully distributed current density more uniformly, reducing localized lithium deposition that leads to dendrite formation. These nanostructured interfaces often incorporate materials such as graphene, carbon nanotubes, or metal oxide nanoparticles to enhance mechanical strength and ionic transport.
Surface modification techniques have proven effective in improving the chemical stability of solid electrolytes against lithium metal. Atomic layer deposition (ALD) and molecular layer deposition (MLD) enable precise control over interface properties by depositing ultrathin protective layers that prevent unwanted side reactions while maintaining efficient ion transport. These techniques have been particularly valuable for oxide and sulfide-based solid electrolytes.
Artificial SEI layers represent another significant advancement, where engineered protective films are deliberately introduced to control the interface chemistry. These artificial SEIs typically consist of lithium salts (such as LiF, Li3N, or Li3P) that are kinetically stable against lithium metal while offering high lithium-ion conductivity. Recent research has demonstrated that multi-component artificial SEIs can simultaneously address multiple failure mechanisms.
Pressure-responsive materials have been developed to accommodate volume changes during cycling. These smart materials can dynamically adjust their mechanical properties in response to stress, preventing the formation of voids that often serve as nucleation sites for dendrites. Self-healing polymers and pressure-sensitive adhesives represent promising candidates in this category.
Computational materials science has accelerated these advancements through high-throughput screening and machine learning approaches. These computational methods have identified promising material combinations and interface designs that would be impractical to discover through traditional experimental methods alone, significantly reducing development time and expanding the range of potential solutions for dendrite suppression.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!