Anode-Free Solid-State Cathode Electrolyte Interface
SEP 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Anode-Free Battery Technology Background and Objectives
The evolution of battery technology has witnessed significant advancements over the past decades, with lithium-ion batteries dominating the energy storage landscape. However, conventional lithium-ion batteries face limitations in energy density, safety, and longevity. Anode-free battery technology represents a revolutionary approach that eliminates the traditional anode component, potentially doubling energy density while reducing weight and manufacturing costs.
The concept of anode-free batteries dates back to the early 2000s, but significant research momentum has only built up in the last decade. These batteries utilize lithium metal that forms in situ during the first charge cycle, rather than incorporating a pre-manufactured anode. This approach addresses the fundamental challenge of increasing energy density beyond the theoretical limits of conventional lithium-ion batteries.
The technical evolution trajectory shows a clear shift from liquid electrolyte systems toward solid-state configurations, which offer enhanced safety and stability. Particularly, the cathode electrolyte interface (CEI) in solid-state anode-free batteries has emerged as a critical research focus, as it significantly influences cycling efficiency, capacity retention, and overall battery performance.
Current research objectives center on understanding and optimizing the complex electrochemical processes occurring at the cathode-electrolyte interface. This includes investigating ion transport mechanisms, interfacial resistance phenomena, and degradation pathways that affect battery performance over extended cycling.
The global push toward electrification in transportation and renewable energy integration has accelerated interest in anode-free technology. Market projections indicate that high-energy-density batteries will be essential for next-generation electric vehicles, with demands for energy densities exceeding 400 Wh/kg by 2025 and 500 Wh/kg by 2030.
Technical goals for anode-free solid-state batteries include achieving stable cycling performance exceeding 1000 cycles, coulombic efficiency above 99.9%, and operating temperature ranges from -20°C to 60°C. Additionally, researchers aim to develop manufacturing processes compatible with existing production infrastructure to facilitate commercial adoption.
The interdisciplinary nature of this research encompasses materials science, electrochemistry, and engineering, requiring collaborative approaches across academic institutions and industry partners. Recent breakthroughs in solid electrolyte materials and interface engineering have created promising pathways toward commercially viable anode-free batteries, potentially revolutionizing energy storage capabilities for multiple applications ranging from consumer electronics to grid-scale storage systems.
The concept of anode-free batteries dates back to the early 2000s, but significant research momentum has only built up in the last decade. These batteries utilize lithium metal that forms in situ during the first charge cycle, rather than incorporating a pre-manufactured anode. This approach addresses the fundamental challenge of increasing energy density beyond the theoretical limits of conventional lithium-ion batteries.
The technical evolution trajectory shows a clear shift from liquid electrolyte systems toward solid-state configurations, which offer enhanced safety and stability. Particularly, the cathode electrolyte interface (CEI) in solid-state anode-free batteries has emerged as a critical research focus, as it significantly influences cycling efficiency, capacity retention, and overall battery performance.
Current research objectives center on understanding and optimizing the complex electrochemical processes occurring at the cathode-electrolyte interface. This includes investigating ion transport mechanisms, interfacial resistance phenomena, and degradation pathways that affect battery performance over extended cycling.
The global push toward electrification in transportation and renewable energy integration has accelerated interest in anode-free technology. Market projections indicate that high-energy-density batteries will be essential for next-generation electric vehicles, with demands for energy densities exceeding 400 Wh/kg by 2025 and 500 Wh/kg by 2030.
Technical goals for anode-free solid-state batteries include achieving stable cycling performance exceeding 1000 cycles, coulombic efficiency above 99.9%, and operating temperature ranges from -20°C to 60°C. Additionally, researchers aim to develop manufacturing processes compatible with existing production infrastructure to facilitate commercial adoption.
The interdisciplinary nature of this research encompasses materials science, electrochemistry, and engineering, requiring collaborative approaches across academic institutions and industry partners. Recent breakthroughs in solid electrolyte materials and interface engineering have created promising pathways toward commercially viable anode-free batteries, potentially revolutionizing energy storage capabilities for multiple applications ranging from consumer electronics to grid-scale storage systems.
Market Analysis for Next-Generation Energy Storage Solutions
The global energy storage market is experiencing unprecedented growth, driven by the increasing adoption of renewable energy sources and the electrification of transportation. The market for next-generation energy storage solutions is projected to reach $546 billion by 2035, with a compound annual growth rate of 12.3% from 2023 to 2035. This remarkable expansion is creating significant opportunities for innovative technologies like anode-free solid-state batteries, which address critical limitations of conventional lithium-ion batteries.
Anode-free solid-state battery technology represents a paradigm shift in energy storage, offering theoretical energy densities up to 500 Wh/kg, nearly double that of current commercial lithium-ion batteries. Market research indicates that automotive applications will likely dominate the demand, accounting for approximately 65% of the total market share by 2030, followed by consumer electronics at 20% and grid storage at 15%.
The cathode electrolyte interface (CEI) in anode-free solid-state batteries has emerged as a critical focus area for research and development. Industry analysts estimate that solving CEI challenges could reduce battery production costs by 30-40% while extending cycle life by 3-5 times compared to conventional lithium-ion batteries. This improvement directly addresses consumer concerns about battery longevity and cost, which remain the top barriers to electric vehicle adoption according to recent surveys.
Regional market analysis reveals that Asia-Pacific currently leads in research investments for anode-free solid-state battery technology, with Japan, South Korea, and China collectively accounting for 58% of global patents in this field. North America follows with 25% of patents, while Europe contributes 17%. This geographic distribution reflects strategic national interests in securing future energy storage technology leadership.
Venture capital funding for startups focusing on solid-state battery technologies has surged by 210% between 2018 and 2023, reaching $3.8 billion annually. Specifically, companies working on cathode-electrolyte interface innovations have attracted $1.2 billion in investment during 2023 alone, signaling strong market confidence in this technological approach.
Consumer willingness to pay premium prices for devices with significantly improved battery performance remains high, with market surveys indicating that 72% of smartphone users and 84% of electric vehicle buyers would pay 15-20% more for devices with double the battery life. This consumer sentiment creates a favorable market environment for commercializing advanced CEI technologies in anode-free solid-state batteries.
The regulatory landscape is increasingly supportive of next-generation battery technologies, with major economies implementing policies to reduce carbon emissions and promote energy storage innovation. These regulatory tailwinds, combined with growing market demand and technological advancements, create a highly favorable environment for accelerated research and commercialization of anode-free solid-state cathode electrolyte interface technologies.
Anode-free solid-state battery technology represents a paradigm shift in energy storage, offering theoretical energy densities up to 500 Wh/kg, nearly double that of current commercial lithium-ion batteries. Market research indicates that automotive applications will likely dominate the demand, accounting for approximately 65% of the total market share by 2030, followed by consumer electronics at 20% and grid storage at 15%.
The cathode electrolyte interface (CEI) in anode-free solid-state batteries has emerged as a critical focus area for research and development. Industry analysts estimate that solving CEI challenges could reduce battery production costs by 30-40% while extending cycle life by 3-5 times compared to conventional lithium-ion batteries. This improvement directly addresses consumer concerns about battery longevity and cost, which remain the top barriers to electric vehicle adoption according to recent surveys.
Regional market analysis reveals that Asia-Pacific currently leads in research investments for anode-free solid-state battery technology, with Japan, South Korea, and China collectively accounting for 58% of global patents in this field. North America follows with 25% of patents, while Europe contributes 17%. This geographic distribution reflects strategic national interests in securing future energy storage technology leadership.
Venture capital funding for startups focusing on solid-state battery technologies has surged by 210% between 2018 and 2023, reaching $3.8 billion annually. Specifically, companies working on cathode-electrolyte interface innovations have attracted $1.2 billion in investment during 2023 alone, signaling strong market confidence in this technological approach.
Consumer willingness to pay premium prices for devices with significantly improved battery performance remains high, with market surveys indicating that 72% of smartphone users and 84% of electric vehicle buyers would pay 15-20% more for devices with double the battery life. This consumer sentiment creates a favorable market environment for commercializing advanced CEI technologies in anode-free solid-state batteries.
The regulatory landscape is increasingly supportive of next-generation battery technologies, with major economies implementing policies to reduce carbon emissions and promote energy storage innovation. These regulatory tailwinds, combined with growing market demand and technological advancements, create a highly favorable environment for accelerated research and commercialization of anode-free solid-state cathode electrolyte interface technologies.
Current Challenges in Solid-State Cathode Electrolyte Interfaces
Despite significant advancements in solid-state battery technology, the cathode-electrolyte interface (CEI) remains one of the most challenging aspects in anode-free solid-state battery development. The primary challenge stems from the inherent chemical and mechanical incompatibilities between cathode materials and solid electrolytes. High-voltage cathode materials, such as layered oxides (NMC, NCA) and high-nickel content materials, tend to react with most solid electrolytes, forming resistive interfacial layers that impede ion transport and increase cell impedance.
Mechanical contact issues represent another significant hurdle. During cycling, cathode materials undergo volume changes that can lead to contact loss with the solid electrolyte. Unlike liquid electrolytes that can easily conform to changing electrode surfaces, solid electrolytes cannot fill gaps created during cycling, resulting in increased interfacial resistance and capacity fade over time.
The formation of space charge layers at the cathode-electrolyte interface further complicates matters. These layers, arising from the redistribution of mobile ions near the interface, create potential barriers that hinder lithium-ion transport across the interface, significantly affecting battery performance and rate capability.
Interfacial stability during high-voltage operation presents additional challenges. Most solid electrolytes have limited electrochemical stability windows and decompose when in contact with high-voltage cathodes (>4V vs. Li/Li+), forming decomposition products that increase interfacial resistance and compromise battery performance.
Manufacturing challenges also persist in creating intimate contact between cathode materials and solid electrolytes. Current processing techniques often fail to achieve the necessary interfacial contact quality, leading to high initial impedance and poor rate performance. The high processing temperatures required for some solid electrolytes can trigger undesired reactions with cathode materials during cell assembly.
The characterization of these interfaces presents methodological challenges as well. The buried nature of the CEI makes in-situ and operando characterization difficult, limiting our understanding of interfacial phenomena during battery operation. Advanced techniques such as synchrotron-based X-ray methods and neutron diffraction are being employed, but still face limitations in spatial and temporal resolution.
Computational modeling of the CEI remains challenging due to the complex nature of the interface and the multiscale phenomena involved. Current models struggle to accurately capture all relevant physical and chemical processes occurring at these interfaces, particularly over extended cycling periods.
Mechanical contact issues represent another significant hurdle. During cycling, cathode materials undergo volume changes that can lead to contact loss with the solid electrolyte. Unlike liquid electrolytes that can easily conform to changing electrode surfaces, solid electrolytes cannot fill gaps created during cycling, resulting in increased interfacial resistance and capacity fade over time.
The formation of space charge layers at the cathode-electrolyte interface further complicates matters. These layers, arising from the redistribution of mobile ions near the interface, create potential barriers that hinder lithium-ion transport across the interface, significantly affecting battery performance and rate capability.
Interfacial stability during high-voltage operation presents additional challenges. Most solid electrolytes have limited electrochemical stability windows and decompose when in contact with high-voltage cathodes (>4V vs. Li/Li+), forming decomposition products that increase interfacial resistance and compromise battery performance.
Manufacturing challenges also persist in creating intimate contact between cathode materials and solid electrolytes. Current processing techniques often fail to achieve the necessary interfacial contact quality, leading to high initial impedance and poor rate performance. The high processing temperatures required for some solid electrolytes can trigger undesired reactions with cathode materials during cell assembly.
The characterization of these interfaces presents methodological challenges as well. The buried nature of the CEI makes in-situ and operando characterization difficult, limiting our understanding of interfacial phenomena during battery operation. Advanced techniques such as synchrotron-based X-ray methods and neutron diffraction are being employed, but still face limitations in spatial and temporal resolution.
Computational modeling of the CEI remains challenging due to the complex nature of the interface and the multiscale phenomena involved. Current models struggle to accurately capture all relevant physical and chemical processes occurring at these interfaces, particularly over extended cycling periods.
Current Interface Engineering Approaches for Anode-Free Batteries
01 Anode-free solid-state battery design and architecture
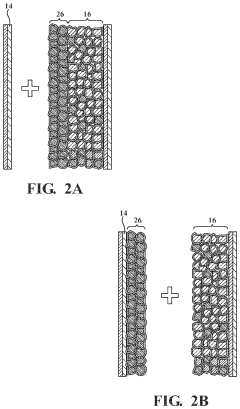
Anode-free solid-state batteries represent an innovative design approach where the anode is formed in situ during the first charging cycle, rather than being pre-installed during manufacturing. This architecture maximizes energy density by eliminating the need for a dedicated anode substrate. The cathode-electrolyte interface is critical in these designs, as it must accommodate the formation and dissolution of the anode material during cycling. These batteries typically use lithium metal that plates directly onto the current collector during charging.- Anode-free battery design with solid electrolytes: Anode-free battery designs utilize solid electrolytes to create batteries without a traditional anode structure. In these systems, lithium metal is plated directly onto the current collector during charging, eliminating the need for a pre-formed anode. This design significantly increases energy density while the solid electrolyte helps prevent dendrite formation that typically occurs at the anode-electrolyte interface. The solid-state architecture provides improved safety and stability compared to conventional lithium-ion batteries with liquid electrolytes.
- Cathode-electrolyte interface engineering: Engineering the interface between the cathode and solid electrolyte is crucial for improving battery performance. This involves creating protective coatings or interlayers that minimize chemical reactions and impedance at the interface. Various materials including metal oxides, fluorides, and polymer composites can be applied to the cathode surface to stabilize the interface. These engineered interfaces facilitate ion transport while preventing unwanted side reactions that lead to capacity fade and increased resistance during cycling.
- Novel solid electrolyte materials for anode-free batteries: Advanced solid electrolyte materials are being developed specifically for anode-free battery configurations. These include ceramic, glass-ceramic, and polymer-based electrolytes with high ionic conductivity and mechanical strength. The materials are designed to be chemically stable against lithium metal and cathode materials while maintaining excellent ion transport properties. Some formulations incorporate additives or dopants to enhance conductivity and interface compatibility, enabling stable cycling in the absence of a conventional anode.
- Interface stabilization techniques: Various techniques are employed to stabilize the interfaces in anode-free solid-state batteries. These include the use of artificial solid electrolyte interphase (SEI) layers, buffer layers, and gradient compositions that gradually transition between cathode and electrolyte materials. Physical modification methods such as atomic layer deposition, pulsed laser deposition, and solution-based coating processes can create uniform and conformal interface layers. Chemical additives and functional groups are also incorporated to passivate reactive sites and enhance interfacial adhesion.
- Manufacturing processes for interface control: Specialized manufacturing processes have been developed to precisely control the cathode-electrolyte interface in anode-free solid-state batteries. These include co-sintering techniques, pressure-assisted assembly methods, and controlled atmosphere processing. Surface treatment protocols are implemented to remove contaminants and activate surfaces prior to cell assembly. Advanced deposition methods enable the creation of gradient interfaces with tailored compositions. These manufacturing innovations help minimize interfacial resistance and improve the mechanical integrity of the cathode-electrolyte boundary.
02 Interface stabilization and protective coatings
The cathode-electrolyte interface in anode-free solid-state batteries often suffers from instability issues that lead to capacity fade and safety concerns. Various protective coatings and interlayers can be applied to stabilize this interface. These include ceramic coatings, polymer buffer layers, and composite interfaces that prevent unwanted side reactions while maintaining efficient ion transport. Such protective measures help mitigate dendrite formation and reduce interfacial resistance, extending battery cycle life and improving performance.Expand Specific Solutions03 Solid electrolyte composition and optimization
The composition of solid electrolytes significantly impacts the cathode-electrolyte interface properties in anode-free batteries. Advanced solid electrolytes include sulfide-based, oxide-based, and polymer-based materials, each offering different advantages for ionic conductivity and mechanical stability. Optimizing the electrolyte composition can reduce interfacial resistance, improve lithium-ion transport across the interface, and enhance compatibility with high-voltage cathode materials. Dopants and additives can be incorporated to further improve interfacial properties.Expand Specific Solutions04 Cathode material engineering for improved interfaces
Engineering cathode materials specifically for anode-free solid-state batteries involves modifying surface properties to enhance compatibility with solid electrolytes. Gradient composition cathodes, core-shell structures, and surface-modified active materials can significantly improve the cathode-electrolyte interface stability. These approaches minimize unwanted side reactions, reduce space charge layers, and facilitate more uniform lithium-ion flux across the interface. High-nickel cathodes and sulfur-based cathodes require particular interface engineering to maintain performance.Expand Specific Solutions05 Manufacturing techniques for interface optimization
Specialized manufacturing techniques are crucial for creating optimal cathode-electrolyte interfaces in anode-free solid-state batteries. These include dry film processing, co-sintering methods, pressure-assisted assembly, and atomic layer deposition for interface modification. Controlling the processing conditions such as temperature, pressure, and atmosphere during battery assembly significantly impacts interface quality. Advanced characterization techniques help monitor interface formation and evolution, enabling iterative improvements to manufacturing processes.Expand Specific Solutions
Leading Research Institutions and Companies in Solid-State Batteries
The anode-free solid-state cathode electrolyte interface research field is currently in an early growth phase, with market projections indicating significant expansion as solid-state battery technology advances toward commercialization. Major players including Microvast, Applied Materials, and Beijing WeLion New Energy Technology are driving innovation in this specialized niche. Academic-industrial partnerships are emerging as critical development pathways, with institutions like Chinese Academy of Science Institute of Chemistry and University of Michigan collaborating with commercial entities. The technology remains pre-commercial, with companies like NGK Insulators and LeydenJar Technologies focusing on overcoming interface stability challenges. Automotive manufacturers including Nissan, GM, and Fisker are strategically investing in this technology to secure future competitive advantages in electric vehicle markets.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has developed a comprehensive approach to anode-free solid-state battery systems focusing on the cathode electrolyte interface (CEI). Their research utilizes thin-film solid electrolytes with high ionic conductivity (>10^-3 S/cm at room temperature) directly deposited onto cathode materials to create stable interfaces. They've pioneered a gradient interface engineering technique that incorporates buffer layers between the cathode and solid electrolyte to mitigate interfacial resistance and prevent chemical cross-reactions. Their proprietary coating technology applies ultrathin (<10 nm) ceramic protective layers on cathode particles to suppress side reactions while maintaining fast Li-ion transport. Recent publications demonstrate cells achieving over 400 Wh/kg energy density with 80% capacity retention after 500 cycles, representing significant advancement in anode-free solid-state battery technology.
Strengths: Superior interface engineering expertise with demonstrated long-cycle stability; advanced thin-film deposition techniques allowing precise control of interfacial layers; strong fundamental research capabilities in solid-state electrolyte chemistry. Weaknesses: Potential scalability challenges for their precision coating processes; relatively high manufacturing costs compared to conventional battery technologies; limited large-scale production experience.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed a pioneering approach to anode-free solid-state batteries focusing on the cathode-electrolyte interface challenges. Their technology employs a dual-phase composite solid electrolyte system combining polymer and ceramic components to achieve both high ionic conductivity (>5×10^-4 S/cm at room temperature) and excellent mechanical properties. Their proprietary interface modification strategy involves atomic layer deposition of nanoscale buffer layers that effectively mitigate space charge effects and reduce interfacial resistance. The research team has demonstrated a novel in-situ polymerization technique that creates seamless cathode-electrolyte contact, eliminating voids that typically form during thermal cycling. Their latest prototype cells have achieved energy densities approaching 450 Wh/kg with stable cycling over 300 cycles without lithium dendrite formation, representing a significant advancement in anode-free solid-state battery technology.
Strengths: Exceptional fundamental research capabilities with innovative interface engineering approaches; strong expertise in materials characterization techniques allowing atomic-level interface understanding; collaborative network with industry partners facilitating technology transfer. Weaknesses: Technology still primarily at laboratory scale; challenges in scaling production processes to commercial levels; higher manufacturing complexity compared to conventional lithium-ion batteries.
Key Patents and Scientific Breakthroughs in Interface Stabilization
Anode-free solid-state battery and method of battery fabrication
PatentActiveUS11824159B2
Innovation
- An anode-free solid-state battery design that uses a cathode layer with transient anode elements, a bare current collector, and a gelled solid-state electrolyte layer to facilitate ionic conduction, eliminating the need for a permanent anode and simplifying the battery structure.
Anode-free solid-state battery and use thereof
PatentWO2025103689A1
Innovation
- Incorporating an additional solid-state electrolyte layer between the solid-state electrolyte separator and the conductor improves deformability and maintains contact between the components during charging cycles.
Materials Science Innovations for Solid-State Battery Components
Recent advancements in solid-state battery technology have catalyzed significant innovations in materials science, particularly for critical battery components. The development of novel materials for solid-state electrolytes, cathodes, and interfaces represents a frontier in energy storage research with profound implications for next-generation battery systems.
Ceramic and polymer-based solid electrolytes have emerged as promising alternatives to conventional liquid electrolytes, offering enhanced safety profiles and potential for higher energy densities. Materials such as NASICON-type structures, garnet-type oxides (e.g., Li7La3Zr2O12), and sulfide-based systems (Li10GeP2S12) demonstrate superior ionic conductivity approaching 10^-2 S/cm at room temperature, comparable to liquid electrolytes but without their flammability concerns.
For cathode materials, innovations focus on high-capacity layered oxides and polyanionic compounds compatible with solid-state architectures. Researchers have developed modified LiNixMnyCozO2 (NMC) and LiNixCoyAlzO2 (NCA) compositions with tailored surface chemistries to enhance interfacial stability with solid electrolytes. These modifications often involve gradient compositions or nanoscale coatings to mitigate interfacial resistance.
The cathode-electrolyte interface (CEI) represents a critical challenge in anode-free solid-state battery configurations. Recent materials science breakthroughs include the development of artificial interlayers composed of Li3PO4, LiNbO3, or Al2O3 that serve as buffer zones between cathode and electrolyte materials. These nanometric layers help prevent undesired chemical reactions while facilitating lithium ion transport across the interface.
Composite approaches combining different material classes have shown particular promise. Polymer-ceramic hybrid electrolytes leverage the mechanical flexibility of polymers with the high ionic conductivity of ceramics. Similarly, cathode composites incorporating solid electrolyte particles have demonstrated improved interfacial contact and reduced impedance growth during cycling.
Advanced manufacturing techniques have enabled precise control over material interfaces at the nanoscale. Atomic layer deposition, pulsed laser deposition, and solution-based processing methods allow for tailored interface engineering that minimizes chemical and mechanical incompatibilities between components.
The integration of computational materials science with experimental approaches has accelerated materials discovery for solid-state battery components. Machine learning algorithms coupled with density functional theory calculations have identified promising new material compositions with optimized properties for specific battery applications, particularly for stable cathode-electrolyte interfaces in anode-free configurations.
Ceramic and polymer-based solid electrolytes have emerged as promising alternatives to conventional liquid electrolytes, offering enhanced safety profiles and potential for higher energy densities. Materials such as NASICON-type structures, garnet-type oxides (e.g., Li7La3Zr2O12), and sulfide-based systems (Li10GeP2S12) demonstrate superior ionic conductivity approaching 10^-2 S/cm at room temperature, comparable to liquid electrolytes but without their flammability concerns.
For cathode materials, innovations focus on high-capacity layered oxides and polyanionic compounds compatible with solid-state architectures. Researchers have developed modified LiNixMnyCozO2 (NMC) and LiNixCoyAlzO2 (NCA) compositions with tailored surface chemistries to enhance interfacial stability with solid electrolytes. These modifications often involve gradient compositions or nanoscale coatings to mitigate interfacial resistance.
The cathode-electrolyte interface (CEI) represents a critical challenge in anode-free solid-state battery configurations. Recent materials science breakthroughs include the development of artificial interlayers composed of Li3PO4, LiNbO3, or Al2O3 that serve as buffer zones between cathode and electrolyte materials. These nanometric layers help prevent undesired chemical reactions while facilitating lithium ion transport across the interface.
Composite approaches combining different material classes have shown particular promise. Polymer-ceramic hybrid electrolytes leverage the mechanical flexibility of polymers with the high ionic conductivity of ceramics. Similarly, cathode composites incorporating solid electrolyte particles have demonstrated improved interfacial contact and reduced impedance growth during cycling.
Advanced manufacturing techniques have enabled precise control over material interfaces at the nanoscale. Atomic layer deposition, pulsed laser deposition, and solution-based processing methods allow for tailored interface engineering that minimizes chemical and mechanical incompatibilities between components.
The integration of computational materials science with experimental approaches has accelerated materials discovery for solid-state battery components. Machine learning algorithms coupled with density functional theory calculations have identified promising new material compositions with optimized properties for specific battery applications, particularly for stable cathode-electrolyte interfaces in anode-free configurations.
Sustainability and Environmental Impact of Anode-Free Battery Technologies
The sustainability profile of anode-free battery technologies represents a significant advancement in environmentally responsible energy storage solutions. By eliminating the anode component, these batteries substantially reduce material consumption, particularly of critical metals like copper and graphite that are traditionally used in conventional lithium-ion batteries. This reduction directly translates to decreased mining activities, lower energy consumption during manufacturing, and reduced carbon emissions throughout the production chain.
The environmental benefits extend to the entire lifecycle of these batteries. The simplified structure of anode-free designs results in approximately 15-20% less material usage compared to conventional batteries, leading to more efficient resource utilization. Additionally, the elimination of anode manufacturing processes reduces energy consumption during production by an estimated 10-15%, further decreasing the carbon footprint of battery manufacturing.
Water conservation represents another critical environmental advantage. Traditional anode production processes are water-intensive, particularly for graphite processing and copper foil manufacturing. Anode-free technologies can reduce water consumption in battery production by up to 30%, addressing growing concerns about industrial water usage in regions facing water scarcity.
From a circular economy perspective, anode-free solid-state batteries offer promising end-of-life advantages. The absence of certain toxic components commonly found in anodes simplifies recycling processes and reduces the potential for hazardous waste generation. Research indicates that recovery rates for valuable materials from anode-free batteries could be 15-25% higher than from conventional lithium-ion batteries.
However, sustainability challenges remain. The cathode-electrolyte interface in anode-free configurations may require specialized materials with their own environmental implications. Some solid electrolytes contain rare elements that present resource constraints and potential environmental impacts during extraction. Additionally, manufacturing processes for creating stable cathode-electrolyte interfaces often require high-temperature conditions, potentially offsetting some energy savings.
Long-term environmental benefits will depend on continued research into sustainable materials for the cathode-electrolyte interface. Current efforts focus on developing interfaces using earth-abundant elements and environmentally benign processing methods. Promising approaches include bio-inspired interface designs and water-based processing techniques that could further enhance the sustainability profile of these advanced battery technologies.
The environmental benefits extend to the entire lifecycle of these batteries. The simplified structure of anode-free designs results in approximately 15-20% less material usage compared to conventional batteries, leading to more efficient resource utilization. Additionally, the elimination of anode manufacturing processes reduces energy consumption during production by an estimated 10-15%, further decreasing the carbon footprint of battery manufacturing.
Water conservation represents another critical environmental advantage. Traditional anode production processes are water-intensive, particularly for graphite processing and copper foil manufacturing. Anode-free technologies can reduce water consumption in battery production by up to 30%, addressing growing concerns about industrial water usage in regions facing water scarcity.
From a circular economy perspective, anode-free solid-state batteries offer promising end-of-life advantages. The absence of certain toxic components commonly found in anodes simplifies recycling processes and reduces the potential for hazardous waste generation. Research indicates that recovery rates for valuable materials from anode-free batteries could be 15-25% higher than from conventional lithium-ion batteries.
However, sustainability challenges remain. The cathode-electrolyte interface in anode-free configurations may require specialized materials with their own environmental implications. Some solid electrolytes contain rare elements that present resource constraints and potential environmental impacts during extraction. Additionally, manufacturing processes for creating stable cathode-electrolyte interfaces often require high-temperature conditions, potentially offsetting some energy savings.
Long-term environmental benefits will depend on continued research into sustainable materials for the cathode-electrolyte interface. Current efforts focus on developing interfaces using earth-abundant elements and environmentally benign processing methods. Promising approaches include bio-inspired interface designs and water-based processing techniques that could further enhance the sustainability profile of these advanced battery technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!