Enhancing the Catalytic Dehydrogenation of Isobutane for Propylene Production
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalytic Dehydrogenation Background and Objectives
Catalytic dehydrogenation of isobutane for propylene production has emerged as a critical process in the petrochemical industry. This technology has evolved significantly over the past few decades, driven by the increasing global demand for propylene, a key building block for various plastics and chemicals. The journey of this technology began in the mid-20th century when the need for on-purpose propylene production became apparent due to the limitations of traditional cracking processes.
The development of catalytic dehydrogenation has been marked by continuous improvements in catalyst design, reactor technology, and process optimization. Early catalysts were based on chromium oxide, which, while effective, posed environmental concerns. This led to the exploration of more sustainable alternatives, including platinum-based catalysts and, more recently, novel materials such as gallium-based systems.
As the technology progressed, the focus shifted towards enhancing selectivity, improving catalyst stability, and reducing energy consumption. These advancements have been crucial in addressing the inherent challenges of the dehydrogenation process, such as thermodynamic limitations and rapid catalyst deactivation due to coke formation.
The current technological landscape is characterized by a push towards more efficient and environmentally friendly processes. This includes the development of advanced reactor designs, such as fluidized bed reactors and membrane reactors, which offer improved heat management and product separation capabilities. Additionally, there is a growing interest in integrating renewable energy sources to power the endothermic dehydrogenation reaction, aligning with global sustainability goals.
Looking ahead, the primary objectives for enhancing catalytic dehydrogenation of isobutane include:
1. Increasing propylene yield and selectivity to maximize resource utilization.
2. Developing more robust catalysts with longer lifetimes and improved resistance to deactivation.
3. Reducing energy consumption and carbon footprint through process intensification and novel heat integration strategies.
4. Exploring new catalyst materials and structures, including nanocatalysts and single-atom catalysts, to push the boundaries of catalytic performance.
5. Integrating advanced process control and artificial intelligence to optimize real-time operation and predictive maintenance.
These objectives are driven by the need to meet the growing demand for propylene while addressing economic and environmental concerns. As the industry moves forward, the focus will be on developing more sustainable and efficient processes that can adapt to changing feedstock availability and market dynamics.
The development of catalytic dehydrogenation has been marked by continuous improvements in catalyst design, reactor technology, and process optimization. Early catalysts were based on chromium oxide, which, while effective, posed environmental concerns. This led to the exploration of more sustainable alternatives, including platinum-based catalysts and, more recently, novel materials such as gallium-based systems.
As the technology progressed, the focus shifted towards enhancing selectivity, improving catalyst stability, and reducing energy consumption. These advancements have been crucial in addressing the inherent challenges of the dehydrogenation process, such as thermodynamic limitations and rapid catalyst deactivation due to coke formation.
The current technological landscape is characterized by a push towards more efficient and environmentally friendly processes. This includes the development of advanced reactor designs, such as fluidized bed reactors and membrane reactors, which offer improved heat management and product separation capabilities. Additionally, there is a growing interest in integrating renewable energy sources to power the endothermic dehydrogenation reaction, aligning with global sustainability goals.
Looking ahead, the primary objectives for enhancing catalytic dehydrogenation of isobutane include:
1. Increasing propylene yield and selectivity to maximize resource utilization.
2. Developing more robust catalysts with longer lifetimes and improved resistance to deactivation.
3. Reducing energy consumption and carbon footprint through process intensification and novel heat integration strategies.
4. Exploring new catalyst materials and structures, including nanocatalysts and single-atom catalysts, to push the boundaries of catalytic performance.
5. Integrating advanced process control and artificial intelligence to optimize real-time operation and predictive maintenance.
These objectives are driven by the need to meet the growing demand for propylene while addressing economic and environmental concerns. As the industry moves forward, the focus will be on developing more sustainable and efficient processes that can adapt to changing feedstock availability and market dynamics.
Propylene Market Demand Analysis
The global propylene market has been experiencing significant growth, driven by increasing demand across various industries. Propylene, a key building block in the petrochemical industry, finds extensive applications in the production of plastics, fibers, and other chemical products. The market demand for propylene has been steadily rising, with a compound annual growth rate (CAGR) projected to remain strong in the coming years.
One of the primary factors contributing to the growing demand for propylene is the expanding automotive industry. As vehicle production continues to increase worldwide, the demand for polypropylene, a versatile plastic derived from propylene, has surged. Polypropylene is widely used in automotive components due to its lightweight properties, durability, and cost-effectiveness, making it an essential material for improving fuel efficiency and reducing vehicle weight.
The packaging industry has also played a crucial role in driving propylene demand. With the rise of e-commerce and changing consumer preferences, there has been a significant increase in the use of flexible packaging materials. Propylene-based polymers, such as polypropylene, are extensively used in food packaging, consumer goods packaging, and industrial packaging applications due to their excellent barrier properties and recyclability.
The construction sector has emerged as another major consumer of propylene-based products. The growing urbanization and infrastructure development in emerging economies have led to increased demand for propylene derivatives used in construction materials, such as pipes, fittings, and insulation. Additionally, the textile industry's demand for propylene-based fibers, particularly in the production of non-woven fabrics, has contributed to the overall market growth.
Geographically, Asia-Pacific has been the largest consumer of propylene, with China leading the demand. The region's rapid industrialization, expanding manufacturing sector, and growing population have been key drivers for propylene consumption. North America and Europe have also maintained steady demand, primarily driven by the automotive and packaging industries.
Despite the positive market outlook, the propylene industry faces challenges related to raw material availability and price volatility. The shift towards lighter feedstocks in the petrochemical industry has led to a reduction in propylene production from traditional steam cracking processes. This has created a propylene supply gap, driving the need for alternative production methods, such as on-purpose propylene technologies like propane dehydrogenation (PDH) and methanol-to-olefins (MTO).
In conclusion, the propylene market demand analysis indicates a robust growth trajectory, supported by diverse end-use applications across multiple industries. The increasing focus on sustainable and recyclable materials is expected to further boost propylene demand, particularly in developing economies. As the industry continues to evolve, innovative production technologies and strategies to address supply challenges will play a crucial role in meeting the growing market demand for propylene.
One of the primary factors contributing to the growing demand for propylene is the expanding automotive industry. As vehicle production continues to increase worldwide, the demand for polypropylene, a versatile plastic derived from propylene, has surged. Polypropylene is widely used in automotive components due to its lightweight properties, durability, and cost-effectiveness, making it an essential material for improving fuel efficiency and reducing vehicle weight.
The packaging industry has also played a crucial role in driving propylene demand. With the rise of e-commerce and changing consumer preferences, there has been a significant increase in the use of flexible packaging materials. Propylene-based polymers, such as polypropylene, are extensively used in food packaging, consumer goods packaging, and industrial packaging applications due to their excellent barrier properties and recyclability.
The construction sector has emerged as another major consumer of propylene-based products. The growing urbanization and infrastructure development in emerging economies have led to increased demand for propylene derivatives used in construction materials, such as pipes, fittings, and insulation. Additionally, the textile industry's demand for propylene-based fibers, particularly in the production of non-woven fabrics, has contributed to the overall market growth.
Geographically, Asia-Pacific has been the largest consumer of propylene, with China leading the demand. The region's rapid industrialization, expanding manufacturing sector, and growing population have been key drivers for propylene consumption. North America and Europe have also maintained steady demand, primarily driven by the automotive and packaging industries.
Despite the positive market outlook, the propylene industry faces challenges related to raw material availability and price volatility. The shift towards lighter feedstocks in the petrochemical industry has led to a reduction in propylene production from traditional steam cracking processes. This has created a propylene supply gap, driving the need for alternative production methods, such as on-purpose propylene technologies like propane dehydrogenation (PDH) and methanol-to-olefins (MTO).
In conclusion, the propylene market demand analysis indicates a robust growth trajectory, supported by diverse end-use applications across multiple industries. The increasing focus on sustainable and recyclable materials is expected to further boost propylene demand, particularly in developing economies. As the industry continues to evolve, innovative production technologies and strategies to address supply challenges will play a crucial role in meeting the growing market demand for propylene.
Current Challenges in Isobutane Dehydrogenation
The catalytic dehydrogenation of isobutane for propylene production faces several significant challenges that hinder its widespread industrial adoption and efficiency. One of the primary issues is the thermodynamic limitations of the reaction. The dehydrogenation process is highly endothermic, requiring substantial energy input to drive the reaction forward. This not only increases operational costs but also poses challenges in maintaining optimal reaction conditions.
Catalyst deactivation remains a persistent problem in isobutane dehydrogenation. The formation of coke on the catalyst surface leads to a gradual loss of catalytic activity over time. This necessitates frequent regeneration cycles or catalyst replacement, impacting process continuity and overall economics. The development of more coke-resistant catalysts or innovative regeneration techniques is crucial to address this challenge.
Selectivity is another critical concern in the dehydrogenation process. Side reactions, such as cracking and isomerization, can lead to the formation of unwanted by-products, reducing the yield of the desired propylene. Improving catalyst selectivity while maintaining high conversion rates is a delicate balance that researchers are continuously striving to optimize.
The high operating temperatures required for the dehydrogenation reaction present materials and safety challenges. Reactor design and construction materials must withstand these extreme conditions while ensuring process safety and longevity. Additionally, the high temperatures contribute to increased energy consumption and potential thermal degradation of the catalyst.
Hydrogen management is a significant operational challenge in isobutane dehydrogenation. The production of hydrogen as a by-product can lead to equilibrium limitations and reduced conversion rates. Effective strategies for hydrogen removal or utilization are necessary to enhance process efficiency and economics.
Scale-up and process integration pose challenges when transitioning from laboratory-scale experiments to industrial-scale production. Maintaining consistent performance, heat management, and process stability at larger scales requires careful engineering and optimization.
Lastly, the volatility of propylene market prices and feedstock costs introduces economic uncertainties. Developing more cost-effective and flexible production processes that can adapt to market fluctuations is essential for the long-term viability of isobutane dehydrogenation technology.
Catalyst deactivation remains a persistent problem in isobutane dehydrogenation. The formation of coke on the catalyst surface leads to a gradual loss of catalytic activity over time. This necessitates frequent regeneration cycles or catalyst replacement, impacting process continuity and overall economics. The development of more coke-resistant catalysts or innovative regeneration techniques is crucial to address this challenge.
Selectivity is another critical concern in the dehydrogenation process. Side reactions, such as cracking and isomerization, can lead to the formation of unwanted by-products, reducing the yield of the desired propylene. Improving catalyst selectivity while maintaining high conversion rates is a delicate balance that researchers are continuously striving to optimize.
The high operating temperatures required for the dehydrogenation reaction present materials and safety challenges. Reactor design and construction materials must withstand these extreme conditions while ensuring process safety and longevity. Additionally, the high temperatures contribute to increased energy consumption and potential thermal degradation of the catalyst.
Hydrogen management is a significant operational challenge in isobutane dehydrogenation. The production of hydrogen as a by-product can lead to equilibrium limitations and reduced conversion rates. Effective strategies for hydrogen removal or utilization are necessary to enhance process efficiency and economics.
Scale-up and process integration pose challenges when transitioning from laboratory-scale experiments to industrial-scale production. Maintaining consistent performance, heat management, and process stability at larger scales requires careful engineering and optimization.
Lastly, the volatility of propylene market prices and feedstock costs introduces economic uncertainties. Developing more cost-effective and flexible production processes that can adapt to market fluctuations is essential for the long-term viability of isobutane dehydrogenation technology.
Existing Catalytic Solutions for Isobutane Dehydrogenation
01 Catalyst composition for isobutane dehydrogenation
Various catalyst compositions are used to improve the efficiency of isobutane dehydrogenation. These catalysts often include noble metals, such as platinum or chromium, supported on metal oxides like alumina or silica. The composition and preparation method of these catalysts significantly influence their activity, selectivity, and stability in the dehydrogenation process.- Catalyst composition for isobutane dehydrogenation: Various catalyst compositions are used to improve the efficiency of isobutane dehydrogenation. These catalysts often include noble metals, such as platinum or chromium, supported on alumina or other metal oxides. The composition and preparation method of the catalyst significantly influence its activity, selectivity, and stability in the dehydrogenation process.
- Reactor design and process conditions: The design of the reactor and the optimization of process conditions play crucial roles in enhancing catalytic efficiency. Factors such as temperature, pressure, residence time, and feed composition are carefully controlled to maximize isobutylene yield while minimizing side reactions and coke formation. Novel reactor designs, including fluidized bed and membrane reactors, are explored to improve heat transfer and product separation.
- Catalyst regeneration and deactivation prevention: Strategies to maintain catalyst activity over extended periods are essential for improving overall process efficiency. This includes developing regeneration techniques to remove coke deposits and restore catalyst performance, as well as implementing methods to prevent or slow down catalyst deactivation. Continuous regeneration processes and the use of promoters or stabilizers in the catalyst formulation are common approaches.
- Process integration and heat management: Efficient heat management and process integration are crucial for improving the overall efficiency of isobutane dehydrogenation. This includes the use of heat recovery systems, integration with other processes, and the development of novel heat transfer methods. Advanced process configurations, such as oxidative dehydrogenation or the use of membrane reactors, are explored to overcome thermodynamic limitations and reduce energy consumption.
- Feed purification and product separation: The purity of the isobutane feed and efficient separation of the products significantly impact the overall catalytic efficiency. Techniques for removing impurities from the feed stream and advanced separation methods for isolating high-purity isobutylene are developed. This includes the use of adsorption processes, membrane separations, and reactive distillation to enhance product yield and quality.
02 Process conditions optimization
Optimizing process conditions is crucial for enhancing catalytic efficiency in isobutane dehydrogenation. Key parameters include temperature, pressure, residence time, and feed composition. Careful control of these conditions can improve conversion rates, product selectivity, and catalyst longevity while minimizing side reactions and coke formation.Expand Specific Solutions03 Reactor design and configuration
The design and configuration of reactors play a significant role in the catalytic efficiency of isobutane dehydrogenation. Various reactor types, such as fixed-bed, fluidized-bed, or moving-bed reactors, are employed. Innovations in reactor design focus on improving heat transfer, minimizing pressure drop, and optimizing catalyst distribution to enhance overall process efficiency.Expand Specific Solutions04 Catalyst regeneration and deactivation prevention
Strategies for catalyst regeneration and prevention of deactivation are essential for maintaining high catalytic efficiency in isobutane dehydrogenation. These include in-situ regeneration techniques, the use of promoters to enhance catalyst stability, and the development of coke-resistant catalyst formulations. Effective regeneration methods can significantly extend catalyst lifetime and improve overall process economics.Expand Specific Solutions05 Novel catalytic systems and process intensification
Research into novel catalytic systems and process intensification techniques aims to further improve the efficiency of isobutane dehydrogenation. This includes the development of membrane reactors, catalytic membrane reactors, and structured catalysts. These innovations seek to overcome thermodynamic limitations, enhance product yield, and reduce energy consumption in the dehydrogenation process.Expand Specific Solutions
Key Players in Propylene Production Industry
The catalytic dehydrogenation of isobutane for propylene production is in a mature stage of development, with a growing market driven by increasing demand for propylene in various industries. The global market size for this technology is substantial, estimated to be in the billions of dollars annually. Technologically, the process is well-established, with major players like China Petroleum & Chemical Corp. (Sinopec), ExxonMobil Chemical Patents, Inc., and SABIC Global Technologies BV leading the field. These companies, along with others such as Clariant International AG and Haldor Topsøe A/S, are continuously improving catalyst efficiency and process optimization to enhance yield and reduce energy consumption, indicating ongoing technological advancements in this mature market.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an advanced catalytic dehydrogenation process for isobutane to enhance propylene production. Their approach utilizes a novel Pt-Sn/Al2O3 catalyst with improved stability and selectivity[1]. The catalyst is modified with alkaline earth metals to reduce coke formation and extend catalyst lifetime[2]. Sinopec's process operates at lower temperatures (520-560°C) compared to conventional methods, reducing energy consumption by up to 15%[3]. They have also implemented a proprietary reactor design that enhances heat transfer and reaction efficiency, resulting in a propylene yield increase of 5-7%[4]. Additionally, Sinopec has integrated an advanced coke removal system that uses periodic oxygen pulsing to regenerate the catalyst in-situ, minimizing downtime and improving overall process economics[5].
Strengths: High propylene yield, energy-efficient, extended catalyst lifetime. Weaknesses: Potential higher initial investment costs, may require specialized operational expertise.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed a proprietary catalytic dehydrogenation technology called MTDH-P (Mobil Thermal Dehydrogenation Process) for isobutane conversion to propylene. Their process utilizes a highly selective Pt-Sn catalyst supported on a modified alumina carrier[6]. The catalyst formulation includes promoters that enhance resistance to coking and sintering, extending the catalyst life to over 2 years[7]. ExxonMobil's reactor design incorporates a multi-stage fluidized bed system, allowing for continuous catalyst regeneration and optimal heat management[8]. This design achieves conversion rates of up to 55% per pass and propylene selectivity exceeding 90%[9]. The process also features an innovative product recovery system that employs pressure swing adsorption, reducing energy consumption in the separation stage by approximately 20% compared to conventional distillation methods[10].
Strengths: High conversion rates, excellent selectivity, continuous catalyst regeneration. Weaknesses: Complex reactor design may lead to higher capital costs, potential scalability challenges for smaller operations.
Innovative Catalyst Designs for Enhanced Performance
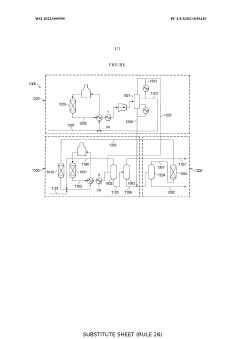
Isobutylene to propylene process flow improvement
PatentWO2022005995A1
Innovation
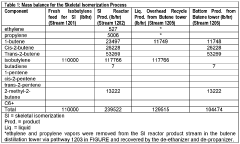
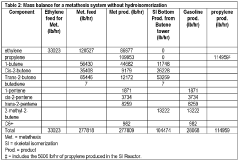
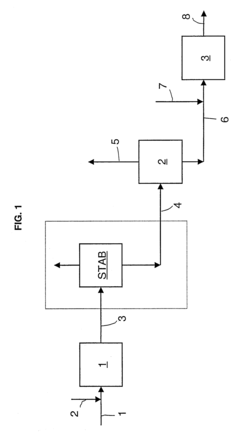
- An improved system and method that converts isobutylene into a usable metathesis reactant by using a skeletal isomerization reactor and a metathesis reactor, with a hydroisomerization hydrotreater to convert 1-butene to 2-butene, forming a 2-butene-rich stream for recycling, which increases propylene production and extends the lifetime of the metathesis catalyst.
Production of high-purity isobutene and propylene from hydrocarbon fractions with four carbon atoms
PatentInactiveUS6686510B2
Innovation
- A process involving selective hydrogenation and isomerization of diolefins and acetylenic impurities, followed by metathesis of butenes-2 with ethylene, to produce high-purity isobutene and propylene, using catalysts like palladium on alumina for hydrogenation and rhenium oxide on alumina for metathesis, integrated with catalytic distillation to separate and optimize product yields.
Environmental Impact and Sustainability Considerations
The catalytic dehydrogenation of isobutane for propylene production is a process that requires careful consideration of its environmental impact and sustainability. This process, while essential for meeting the growing demand for propylene, has significant implications for energy consumption, greenhouse gas emissions, and resource utilization.
One of the primary environmental concerns associated with this process is its high energy intensity. The endothermic nature of the dehydrogenation reaction necessitates substantial heat input, typically supplied through the combustion of fossil fuels. This reliance on non-renewable energy sources contributes to carbon dioxide emissions and exacerbates climate change concerns. To address this issue, researchers are exploring alternative energy sources, such as renewable electricity or hydrogen, to power the process and reduce its carbon footprint.
Water consumption is another critical environmental factor to consider. The process requires significant amounts of water for cooling and steam generation, potentially straining local water resources in water-scarce regions. Implementing water recycling systems and exploring air-cooled alternatives can help mitigate this impact and improve the overall sustainability of the process.
The catalysts used in the dehydrogenation process also present environmental challenges. Many conventional catalysts contain precious metals or rare earth elements, which are often mined using environmentally damaging practices. Developing more sustainable catalyst formulations, utilizing abundant and less environmentally impactful materials, is crucial for improving the long-term viability of the process.
Waste management is an additional concern, as the process generates spent catalysts and by-products that require proper disposal or recycling. Implementing effective catalyst regeneration techniques and exploring ways to valorize by-products can significantly reduce waste generation and improve resource efficiency.
From a sustainability perspective, enhancing the selectivity and conversion efficiency of the catalytic dehydrogenation process is paramount. Higher yields of propylene per unit of isobutane feed not only improve economic viability but also reduce resource consumption and waste generation. This can be achieved through catalyst optimization, process intensification, and the development of novel reactor designs.
Lastly, the integration of circular economy principles into the propylene production process is gaining attention. This involves exploring opportunities for utilizing waste streams from other industrial processes as feedstock, as well as finding innovative applications for the by-products of the dehydrogenation reaction. Such approaches can help close material loops and reduce the overall environmental footprint of the propylene value chain.
One of the primary environmental concerns associated with this process is its high energy intensity. The endothermic nature of the dehydrogenation reaction necessitates substantial heat input, typically supplied through the combustion of fossil fuels. This reliance on non-renewable energy sources contributes to carbon dioxide emissions and exacerbates climate change concerns. To address this issue, researchers are exploring alternative energy sources, such as renewable electricity or hydrogen, to power the process and reduce its carbon footprint.
Water consumption is another critical environmental factor to consider. The process requires significant amounts of water for cooling and steam generation, potentially straining local water resources in water-scarce regions. Implementing water recycling systems and exploring air-cooled alternatives can help mitigate this impact and improve the overall sustainability of the process.
The catalysts used in the dehydrogenation process also present environmental challenges. Many conventional catalysts contain precious metals or rare earth elements, which are often mined using environmentally damaging practices. Developing more sustainable catalyst formulations, utilizing abundant and less environmentally impactful materials, is crucial for improving the long-term viability of the process.
Waste management is an additional concern, as the process generates spent catalysts and by-products that require proper disposal or recycling. Implementing effective catalyst regeneration techniques and exploring ways to valorize by-products can significantly reduce waste generation and improve resource efficiency.
From a sustainability perspective, enhancing the selectivity and conversion efficiency of the catalytic dehydrogenation process is paramount. Higher yields of propylene per unit of isobutane feed not only improve economic viability but also reduce resource consumption and waste generation. This can be achieved through catalyst optimization, process intensification, and the development of novel reactor designs.
Lastly, the integration of circular economy principles into the propylene production process is gaining attention. This involves exploring opportunities for utilizing waste streams from other industrial processes as feedstock, as well as finding innovative applications for the by-products of the dehydrogenation reaction. Such approaches can help close material loops and reduce the overall environmental footprint of the propylene value chain.
Process Optimization Strategies
Process optimization strategies for enhancing the catalytic dehydrogenation of isobutane for propylene production focus on maximizing yield, selectivity, and energy efficiency while minimizing catalyst deactivation. One key approach involves optimizing reactor design and operating conditions. This includes fine-tuning temperature profiles, pressure levels, and residence times to achieve optimal conversion rates without compromising catalyst stability.
Advanced reactor configurations, such as fluidized bed reactors or membrane reactors, can significantly improve heat transfer and product separation. Fluidized bed reactors offer better temperature control and catalyst regeneration capabilities, while membrane reactors allow for continuous product removal, shifting the equilibrium towards higher conversion.
Catalyst design and formulation play a crucial role in process optimization. Developing catalysts with higher activity, selectivity, and stability is essential. This may involve incorporating promoters, optimizing support materials, or engineering novel nanostructured catalysts. Recent advancements in catalyst design have focused on enhancing coke resistance and improving regeneration characteristics.
Process integration strategies can further enhance overall efficiency. Heat integration techniques, such as using waste heat from the dehydrogenation reaction to preheat feed streams, can significantly reduce energy consumption. Additionally, integrating separation processes, like adsorption or membrane separation, directly with the reaction system can improve product recovery and purity.
Implementing advanced process control systems is another vital optimization strategy. Real-time monitoring and control of key process parameters, coupled with predictive modeling and machine learning algorithms, can help maintain optimal operating conditions and quickly respond to process disturbances.
Catalyst regeneration strategies are critical for maintaining long-term process efficiency. Developing effective in-situ or ex-situ regeneration protocols, potentially using novel oxidation techniques or plasma-assisted methods, can extend catalyst lifetime and reduce downtime.
Finally, exploring alternative feedstocks or co-feed strategies can open new avenues for process optimization. For instance, investigating the co-feeding of light alkanes or incorporating renewable feedstocks could lead to more sustainable and economically viable processes for propylene production.
Advanced reactor configurations, such as fluidized bed reactors or membrane reactors, can significantly improve heat transfer and product separation. Fluidized bed reactors offer better temperature control and catalyst regeneration capabilities, while membrane reactors allow for continuous product removal, shifting the equilibrium towards higher conversion.
Catalyst design and formulation play a crucial role in process optimization. Developing catalysts with higher activity, selectivity, and stability is essential. This may involve incorporating promoters, optimizing support materials, or engineering novel nanostructured catalysts. Recent advancements in catalyst design have focused on enhancing coke resistance and improving regeneration characteristics.
Process integration strategies can further enhance overall efficiency. Heat integration techniques, such as using waste heat from the dehydrogenation reaction to preheat feed streams, can significantly reduce energy consumption. Additionally, integrating separation processes, like adsorption or membrane separation, directly with the reaction system can improve product recovery and purity.
Implementing advanced process control systems is another vital optimization strategy. Real-time monitoring and control of key process parameters, coupled with predictive modeling and machine learning algorithms, can help maintain optimal operating conditions and quickly respond to process disturbances.
Catalyst regeneration strategies are critical for maintaining long-term process efficiency. Developing effective in-situ or ex-situ regeneration protocols, potentially using novel oxidation techniques or plasma-assisted methods, can extend catalyst lifetime and reduce downtime.
Finally, exploring alternative feedstocks or co-feed strategies can open new avenues for process optimization. For instance, investigating the co-feeding of light alkanes or incorporating renewable feedstocks could lead to more sustainable and economically viable processes for propylene production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!