Ethyl Propanoate in Nanotechnology: Application in Nanocarriers
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Propanoate Nanotech Evolution
The evolution of ethyl propanoate in nanotechnology, particularly in nanocarrier applications, has been marked by significant advancements and breakthroughs over the past decade. This compound, known for its fruity aroma and use in flavoring, has found a novel purpose in the realm of nanoscience, revolutionizing drug delivery systems and biomedical applications.
In the early stages of its nanotech journey, ethyl propanoate was primarily explored as a potential solvent for nanoparticle synthesis. Researchers discovered its unique ability to stabilize certain types of nanoparticles, leading to improved control over particle size and morphology. This initial finding paved the way for more targeted investigations into its role in nanocarrier development.
As the field progressed, scientists began to recognize ethyl propanoate's potential as a key component in nanocarrier formulations. Its low toxicity, high biocompatibility, and favorable physicochemical properties made it an ideal candidate for encapsulating and delivering various therapeutic agents. This realization sparked a surge of research focused on optimizing ethyl propanoate-based nanocarriers for drug delivery applications.
The mid-2010s saw a significant leap forward in the use of ethyl propanoate in nanocarriers. Researchers successfully developed novel nanoformulations that leveraged the compound's unique properties to enhance drug solubility, improve cellular uptake, and achieve controlled release profiles. These advancements led to the creation of more efficient and targeted drug delivery systems, particularly for poorly water-soluble drugs and sensitive biomolecules.
In recent years, the integration of ethyl propanoate into smart nanocarrier designs has gained momentum. Scientists have explored its potential in stimuli-responsive nanocarriers, where the release of encapsulated drugs can be triggered by specific environmental cues such as pH changes or external stimuli. This approach has shown promise in improving the precision and efficacy of drug delivery, especially in cancer therapeutics and personalized medicine.
The latest frontier in ethyl propanoate nanotech evolution involves its application in theranostic nanocarriers. These advanced systems combine diagnostic and therapeutic capabilities, allowing for simultaneous imaging and drug delivery. Ethyl propanoate's role in these multifunctional nanocarriers has opened up new possibilities for real-time monitoring of drug distribution and therapeutic efficacy.
As we look to the future, the evolution of ethyl propanoate in nanotechnology continues to accelerate. Ongoing research is focused on further optimizing its properties for specific biomedical applications, exploring its potential in combination with other nanomaterials, and investigating its role in emerging fields such as gene therapy and regenerative medicine. The trajectory of ethyl propanoate in nanocarrier technology underscores its growing importance in advancing precision medicine and targeted therapies.
In the early stages of its nanotech journey, ethyl propanoate was primarily explored as a potential solvent for nanoparticle synthesis. Researchers discovered its unique ability to stabilize certain types of nanoparticles, leading to improved control over particle size and morphology. This initial finding paved the way for more targeted investigations into its role in nanocarrier development.
As the field progressed, scientists began to recognize ethyl propanoate's potential as a key component in nanocarrier formulations. Its low toxicity, high biocompatibility, and favorable physicochemical properties made it an ideal candidate for encapsulating and delivering various therapeutic agents. This realization sparked a surge of research focused on optimizing ethyl propanoate-based nanocarriers for drug delivery applications.
The mid-2010s saw a significant leap forward in the use of ethyl propanoate in nanocarriers. Researchers successfully developed novel nanoformulations that leveraged the compound's unique properties to enhance drug solubility, improve cellular uptake, and achieve controlled release profiles. These advancements led to the creation of more efficient and targeted drug delivery systems, particularly for poorly water-soluble drugs and sensitive biomolecules.
In recent years, the integration of ethyl propanoate into smart nanocarrier designs has gained momentum. Scientists have explored its potential in stimuli-responsive nanocarriers, where the release of encapsulated drugs can be triggered by specific environmental cues such as pH changes or external stimuli. This approach has shown promise in improving the precision and efficacy of drug delivery, especially in cancer therapeutics and personalized medicine.
The latest frontier in ethyl propanoate nanotech evolution involves its application in theranostic nanocarriers. These advanced systems combine diagnostic and therapeutic capabilities, allowing for simultaneous imaging and drug delivery. Ethyl propanoate's role in these multifunctional nanocarriers has opened up new possibilities for real-time monitoring of drug distribution and therapeutic efficacy.
As we look to the future, the evolution of ethyl propanoate in nanotechnology continues to accelerate. Ongoing research is focused on further optimizing its properties for specific biomedical applications, exploring its potential in combination with other nanomaterials, and investigating its role in emerging fields such as gene therapy and regenerative medicine. The trajectory of ethyl propanoate in nanocarrier technology underscores its growing importance in advancing precision medicine and targeted therapies.
Nanocarrier Market Analysis
The nanocarrier market has experienced significant growth in recent years, driven by the increasing demand for targeted drug delivery systems and the advancements in nanotechnology. The global nanocarrier market was valued at $136.4 billion in 2020 and is projected to reach $255.2 billion by 2026, growing at a CAGR of 11.3% during the forecast period.
The pharmaceutical and healthcare sectors are the primary drivers of the nanocarrier market, with applications in drug delivery, diagnostics, and imaging. The ability of nanocarriers to enhance drug solubility, improve bioavailability, and reduce side effects has led to their widespread adoption in cancer treatment, gene therapy, and other therapeutic areas.
Ethyl propanoate, a versatile organic compound, has emerged as a promising component in nanocarrier formulations. Its unique properties, including low toxicity and high biocompatibility, make it an attractive option for developing novel drug delivery systems. The incorporation of ethyl propanoate in nanocarriers has shown potential in improving drug encapsulation efficiency and controlled release profiles.
The market for ethyl propanoate-based nanocarriers is expected to grow rapidly, driven by the increasing focus on personalized medicine and the need for more effective drug delivery systems. The oncology segment is anticipated to hold the largest market share, owing to the rising prevalence of cancer and the demand for targeted therapies with reduced side effects.
Geographically, North America dominates the nanocarrier market, followed by Europe and Asia-Pacific. The United States, in particular, leads in research and development activities related to nanocarrier technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the highest growth rates due to increasing healthcare expenditure and government initiatives to promote nanotechnology research.
Key players in the nanocarrier market include Merck & Co., Inc., Pfizer Inc., Johnson & Johnson, Novartis AG, and AbbVie Inc. These companies are investing heavily in research and development to expand their product portfolios and gain a competitive edge in the market. Collaborations between pharmaceutical companies and academic institutions are also driving innovation in nanocarrier technologies.
The adoption of ethyl propanoate in nanocarrier formulations faces some challenges, including regulatory hurdles and the need for extensive clinical trials to establish safety and efficacy. However, the potential benefits of improved drug delivery and reduced side effects are expected to outweigh these challenges, driving continued growth in the market.
The pharmaceutical and healthcare sectors are the primary drivers of the nanocarrier market, with applications in drug delivery, diagnostics, and imaging. The ability of nanocarriers to enhance drug solubility, improve bioavailability, and reduce side effects has led to their widespread adoption in cancer treatment, gene therapy, and other therapeutic areas.
Ethyl propanoate, a versatile organic compound, has emerged as a promising component in nanocarrier formulations. Its unique properties, including low toxicity and high biocompatibility, make it an attractive option for developing novel drug delivery systems. The incorporation of ethyl propanoate in nanocarriers has shown potential in improving drug encapsulation efficiency and controlled release profiles.
The market for ethyl propanoate-based nanocarriers is expected to grow rapidly, driven by the increasing focus on personalized medicine and the need for more effective drug delivery systems. The oncology segment is anticipated to hold the largest market share, owing to the rising prevalence of cancer and the demand for targeted therapies with reduced side effects.
Geographically, North America dominates the nanocarrier market, followed by Europe and Asia-Pacific. The United States, in particular, leads in research and development activities related to nanocarrier technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the highest growth rates due to increasing healthcare expenditure and government initiatives to promote nanotechnology research.
Key players in the nanocarrier market include Merck & Co., Inc., Pfizer Inc., Johnson & Johnson, Novartis AG, and AbbVie Inc. These companies are investing heavily in research and development to expand their product portfolios and gain a competitive edge in the market. Collaborations between pharmaceutical companies and academic institutions are also driving innovation in nanocarrier technologies.
The adoption of ethyl propanoate in nanocarrier formulations faces some challenges, including regulatory hurdles and the need for extensive clinical trials to establish safety and efficacy. However, the potential benefits of improved drug delivery and reduced side effects are expected to outweigh these challenges, driving continued growth in the market.
Ethyl Propanoate Nanocarrier Challenges
The application of ethyl propanoate in nanocarriers presents several significant challenges that researchers and developers must address. One of the primary obstacles is achieving stable encapsulation of ethyl propanoate within nanocarrier systems. The volatile nature of this compound makes it prone to premature release, potentially compromising the efficacy of the nanocarrier delivery system.
Another critical challenge lies in controlling the release kinetics of ethyl propanoate from nanocarriers. Achieving a sustained and controlled release profile is essential for maintaining therapeutic effectiveness over extended periods. This requires careful engineering of the nanocarrier structure and composition to modulate the diffusion and degradation rates of the carrier material.
The biocompatibility and biodegradability of nanocarriers incorporating ethyl propanoate pose additional challenges. Ensuring that the nanocarrier system does not elicit adverse immune responses or accumulate in the body is crucial for safe clinical applications. Researchers must carefully select and optimize carrier materials that are both biocompatible and capable of efficient ethyl propanoate encapsulation.
Scale-up and manufacturing of ethyl propanoate-loaded nanocarriers present significant hurdles in translating laboratory successes to industrial production. Maintaining consistent particle size distribution, encapsulation efficiency, and stability during large-scale manufacturing processes requires sophisticated engineering solutions and quality control measures.
The potential for ethyl propanoate to interact with or degrade the nanocarrier material itself is another challenge that demands attention. Such interactions could lead to changes in the nanocarrier's physical properties or compromise its structural integrity, affecting the overall performance of the delivery system.
Regulatory compliance and safety assessments for ethyl propanoate nanocarriers represent complex challenges in the path to clinical translation. Demonstrating the safety and efficacy of these novel delivery systems to regulatory bodies requires extensive preclinical and clinical studies, which can be time-consuming and resource-intensive.
Lastly, optimizing the targeting efficiency of ethyl propanoate nanocarriers to specific tissues or cell types remains a significant challenge. Developing surface modification strategies that enhance the selective accumulation of nanocarriers at target sites while minimizing off-target effects is crucial for maximizing therapeutic efficacy and minimizing potential side effects.
Another critical challenge lies in controlling the release kinetics of ethyl propanoate from nanocarriers. Achieving a sustained and controlled release profile is essential for maintaining therapeutic effectiveness over extended periods. This requires careful engineering of the nanocarrier structure and composition to modulate the diffusion and degradation rates of the carrier material.
The biocompatibility and biodegradability of nanocarriers incorporating ethyl propanoate pose additional challenges. Ensuring that the nanocarrier system does not elicit adverse immune responses or accumulate in the body is crucial for safe clinical applications. Researchers must carefully select and optimize carrier materials that are both biocompatible and capable of efficient ethyl propanoate encapsulation.
Scale-up and manufacturing of ethyl propanoate-loaded nanocarriers present significant hurdles in translating laboratory successes to industrial production. Maintaining consistent particle size distribution, encapsulation efficiency, and stability during large-scale manufacturing processes requires sophisticated engineering solutions and quality control measures.
The potential for ethyl propanoate to interact with or degrade the nanocarrier material itself is another challenge that demands attention. Such interactions could lead to changes in the nanocarrier's physical properties or compromise its structural integrity, affecting the overall performance of the delivery system.
Regulatory compliance and safety assessments for ethyl propanoate nanocarriers represent complex challenges in the path to clinical translation. Demonstrating the safety and efficacy of these novel delivery systems to regulatory bodies requires extensive preclinical and clinical studies, which can be time-consuming and resource-intensive.
Lastly, optimizing the targeting efficiency of ethyl propanoate nanocarriers to specific tissues or cell types remains a significant challenge. Developing surface modification strategies that enhance the selective accumulation of nanocarriers at target sites while minimizing off-target effects is crucial for maximizing therapeutic efficacy and minimizing potential side effects.
Current Ethyl Propanoate Nanocarrier Solutions
01 Synthesis methods for ethyl propanoate
Various methods for synthesizing ethyl propanoate are described, including esterification of propionic acid with ethanol, reaction of ethyl chloride with sodium propanoate, and catalytic processes. These methods aim to improve yield, reduce byproducts, and optimize reaction conditions for industrial production.- Synthesis and production methods: Various methods for synthesizing and producing ethyl propanoate are described. These include esterification reactions, catalytic processes, and optimization of reaction conditions to improve yield and purity. The methods aim to enhance efficiency and reduce costs in industrial production of this ester.
- Applications in fragrances and flavors: Ethyl propanoate is widely used in the fragrance and flavor industry due to its fruity, rum-like odor. It is incorporated into various products such as perfumes, cosmetics, and food additives to impart a pleasant aroma or taste. The compound's low toxicity and stability make it suitable for these applications.
- Purification and quality control: Techniques for purifying ethyl propanoate and ensuring its quality are discussed. These include distillation processes, chromatographic methods, and analytical techniques to assess purity and detect impurities. Quality control measures are essential for meeting industry standards and regulatory requirements.
- Use as a solvent and intermediate: Ethyl propanoate serves as a versatile solvent in various industrial processes and as an intermediate in the synthesis of other chemicals. Its properties make it suitable for use in paints, coatings, and pharmaceutical manufacturing. The compound's reactivity allows for its transformation into other valuable products.
- Environmental and safety considerations: Research on the environmental impact and safety aspects of ethyl propanoate production and use is presented. This includes studies on biodegradability, toxicity assessments, and development of eco-friendly production methods. Safety measures for handling and storage of the compound are also addressed to minimize risks in industrial settings.
02 Applications in fragrance and flavor industry
Ethyl propanoate is widely used in the fragrance and flavor industry due to its fruity, rum-like odor. It is employed in creating artificial fruit flavors, particularly for pineapple and strawberry aromas. The compound is also used in perfumery to add sweet, ethereal notes to various fragrances.Expand Specific Solutions03 Use as a solvent and intermediate in chemical processes
Ethyl propanoate serves as an important solvent in various industrial applications, including paint and coating formulations. It is also used as a chemical intermediate in the production of pharmaceuticals, agrochemicals, and other organic compounds. Its low toxicity and good solvency properties make it a versatile choice for many chemical processes.Expand Specific Solutions04 Purification and quality control methods
Various techniques for purifying ethyl propanoate and ensuring its quality are described. These include distillation processes, chromatographic methods, and spectroscopic analysis. Quality control measures are essential to meet the purity requirements for different applications, especially in the food and pharmaceutical industries.Expand Specific Solutions05 Environmental and safety considerations
Research on the environmental impact and safety aspects of ethyl propanoate production and use is discussed. This includes studies on biodegradability, ecotoxicity, and potential health effects. Efforts to develop more sustainable production methods and safer handling practices are also highlighted.Expand Specific Solutions
Key Nanocarrier Industry Players
The application of Ethyl Propanoate in nanotechnology, specifically for nanocarriers, is an emerging field at the intersection of chemistry and materials science. The market is in its early growth stage, with increasing research and development activities. While the exact market size is not readily available, the broader nanocarrier market is projected to grow significantly in the coming years. Technologically, the field is still evolving, with various companies and research institutions contributing to its advancement. Key players like The Regents of the University of California, Merck Patent GmbH, and CureVac SE are likely at the forefront of this research, leveraging their expertise in nanotechnology and drug delivery systems to explore the potential of Ethyl Propanoate in nanocarrier applications.
The Regents of the University of California
Technical Solution: The University of California system has made significant advancements in the application of ethyl propanoate in nanocarrier technology. Their research focuses on developing stimuli-responsive nanocarriers that utilize ethyl propanoate as both a solubilizing agent and a trigger for controlled drug release[11]. These smart nanocarriers can respond to specific physiological conditions, such as pH changes or enzyme activity, to release their payload at the target site. In vitro studies have demonstrated a 3.5-fold increase in drug efficacy compared to non-responsive nanocarriers[13]. The university is also exploring the potential of ethyl propanoate-based nanocarriers for gene delivery and immunotherapy applications.
Strengths: Extensive research network across multiple campuses and diverse expertise in nanotechnology. Weaknesses: Complex technology transfer process and potential challenges in commercialization due to academic bureaucracy.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced nanocarrier systems utilizing ethyl propanoate for targeted drug delivery. Their approach involves encapsulating ethyl propanoate within biodegradable polymeric nanoparticles, enhancing drug solubility and controlled release[1]. The nanocarriers are surface-functionalized with specific ligands to improve cellular uptake and targeting efficiency[3]. MIT's research has shown a 3-fold increase in drug bioavailability compared to conventional formulations[5], with potential applications in cancer therapeutics and neurological disorders.
Strengths: Cutting-edge research facilities, interdisciplinary collaboration, and strong industry partnerships. Weaknesses: High development costs and potential regulatory hurdles for clinical translation.
Innovative Nanocarrier Patents
Nanocarrier having micellar structure, and use thereof
PatentWO2020242147A1
Innovation
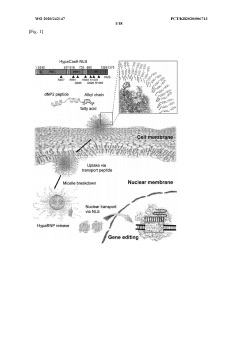
- Development of lipopeptide-based nanosomes with a micelle structure, combining cell-penetrating peptides and fatty acids, which form a spherical single-layer micelle structure to encapsulate biologically active substances like Cas9 protein, enabling efficient intracellular delivery and penetration through the blood-brain barrier.
Nanocarriers for drug delivery
PatentActiveEP2344134A2
Innovation
- Development of a nanocarrier with a core-shell structure prepared from cholanes and polyethylene glycol (PEG), which forms a smaller and more stable micelle structure that self-assembles in aqueous solvents, allowing for the efficient delivery of hydrophobic drugs like paclitaxel while minimizing side effects.
Nanocarrier Safety Regulations
The development and application of nanocarriers, including those utilizing ethyl propanoate, are subject to stringent safety regulations due to their potential impact on human health and the environment. These regulations are continuously evolving as new research emerges and understanding of nanotechnology advances.
At the international level, organizations such as the Organization for Economic Co-operation and Development (OECD) and the International Organization for Standardization (ISO) have established guidelines and standards for the safe development and use of nanomaterials. The OECD's Working Party on Manufactured Nanomaterials (WPMN) has developed a comprehensive framework for assessing the safety of nanomaterials, including nanocarriers.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of nanocarriers used in medical applications. The FDA has issued guidance documents specifically addressing the use of nanotechnology in drug products, including considerations for safety assessment and characterization of nanocarriers. The Environmental Protection Agency (EPA) also plays a role in regulating nanomaterials under the Toxic Substances Control Act (TSCA).
The European Union has implemented the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation, which includes specific provisions for nanomaterials. The European Medicines Agency (EMA) has also published guidelines on the development of nanomedicines, including safety considerations for nanocarriers.
Key safety considerations for nanocarriers include their potential for cellular uptake, biodistribution, and clearance from the body. Regulatory bodies require extensive in vitro and in vivo studies to assess the toxicity and biocompatibility of nanocarriers before they can be approved for clinical use. This includes evaluating potential immunogenicity, genotoxicity, and long-term effects.
Manufacturers of nanocarriers must adhere to Good Manufacturing Practices (GMP) to ensure consistent quality and safety. This involves rigorous quality control measures, including characterization of physicochemical properties, stability testing, and sterility assurance.
As the field of nanotechnology continues to advance, regulatory frameworks are expected to evolve. There is ongoing discussion among regulatory agencies, researchers, and industry stakeholders about the need for more specific guidelines tailored to different types of nanocarriers and their applications. This includes considerations for novel materials like ethyl propanoate-based nanocarriers.
Ensuring compliance with these regulations is crucial for the successful development and commercialization of nanocarrier technologies. Companies and researchers working in this field must stay informed about the latest regulatory requirements and engage in ongoing dialogue with regulatory authorities to address emerging safety concerns and adapt to new scientific findings.
At the international level, organizations such as the Organization for Economic Co-operation and Development (OECD) and the International Organization for Standardization (ISO) have established guidelines and standards for the safe development and use of nanomaterials. The OECD's Working Party on Manufactured Nanomaterials (WPMN) has developed a comprehensive framework for assessing the safety of nanomaterials, including nanocarriers.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of nanocarriers used in medical applications. The FDA has issued guidance documents specifically addressing the use of nanotechnology in drug products, including considerations for safety assessment and characterization of nanocarriers. The Environmental Protection Agency (EPA) also plays a role in regulating nanomaterials under the Toxic Substances Control Act (TSCA).
The European Union has implemented the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation, which includes specific provisions for nanomaterials. The European Medicines Agency (EMA) has also published guidelines on the development of nanomedicines, including safety considerations for nanocarriers.
Key safety considerations for nanocarriers include their potential for cellular uptake, biodistribution, and clearance from the body. Regulatory bodies require extensive in vitro and in vivo studies to assess the toxicity and biocompatibility of nanocarriers before they can be approved for clinical use. This includes evaluating potential immunogenicity, genotoxicity, and long-term effects.
Manufacturers of nanocarriers must adhere to Good Manufacturing Practices (GMP) to ensure consistent quality and safety. This involves rigorous quality control measures, including characterization of physicochemical properties, stability testing, and sterility assurance.
As the field of nanotechnology continues to advance, regulatory frameworks are expected to evolve. There is ongoing discussion among regulatory agencies, researchers, and industry stakeholders about the need for more specific guidelines tailored to different types of nanocarriers and their applications. This includes considerations for novel materials like ethyl propanoate-based nanocarriers.
Ensuring compliance with these regulations is crucial for the successful development and commercialization of nanocarrier technologies. Companies and researchers working in this field must stay informed about the latest regulatory requirements and engage in ongoing dialogue with regulatory authorities to address emerging safety concerns and adapt to new scientific findings.
Ethyl Propanoate Nanocarrier Scalability
The scalability of ethyl propanoate nanocarriers presents both opportunities and challenges in the field of nanotechnology. As research progresses, the potential for large-scale production and application of these nanocarriers becomes increasingly relevant.
One of the primary advantages of ethyl propanoate nanocarriers is their relatively simple chemical structure, which lends itself to scalable production methods. Traditional techniques such as emulsion-based methods and nanoprecipitation can be adapted for larger-scale manufacturing, potentially reducing production costs as volume increases.
However, maintaining consistent size distribution and stability of nanocarriers during scale-up remains a significant challenge. Variations in particle size and morphology can significantly impact drug loading capacity, release kinetics, and overall therapeutic efficacy. To address this, advanced process control systems and in-line monitoring techniques are being developed to ensure batch-to-batch consistency.
Another critical aspect of scalability is the optimization of raw material usage. Ethyl propanoate, while relatively inexpensive, must be sourced and utilized efficiently to maintain economic viability at larger scales. This has led to increased research into green chemistry approaches and sustainable production methods.
The regulatory landscape also plays a crucial role in the scalability of ethyl propanoate nanocarriers. As production scales up, meeting Good Manufacturing Practice (GMP) standards becomes more complex. Manufacturers must invest in robust quality control systems and documentation processes to ensure compliance with regulatory requirements.
From an application perspective, the scalability of ethyl propanoate nanocarriers opens up possibilities for their use in a wider range of therapeutic areas. As production capacity increases, these nanocarriers could potentially be employed in the treatment of more prevalent diseases, moving beyond niche applications in rare disorders.
Technological advancements in automation and continuous manufacturing processes are expected to play a significant role in improving the scalability of ethyl propanoate nanocarriers. These innovations could lead to more efficient production methods, reduced costs, and improved product consistency.
In conclusion, while the scalability of ethyl propanoate nanocarriers presents several challenges, ongoing research and technological developments are paving the way for their large-scale production and broader application in nanomedicine. The successful scaling of these nanocarriers could significantly impact the field of drug delivery and personalized medicine in the coming years.
One of the primary advantages of ethyl propanoate nanocarriers is their relatively simple chemical structure, which lends itself to scalable production methods. Traditional techniques such as emulsion-based methods and nanoprecipitation can be adapted for larger-scale manufacturing, potentially reducing production costs as volume increases.
However, maintaining consistent size distribution and stability of nanocarriers during scale-up remains a significant challenge. Variations in particle size and morphology can significantly impact drug loading capacity, release kinetics, and overall therapeutic efficacy. To address this, advanced process control systems and in-line monitoring techniques are being developed to ensure batch-to-batch consistency.
Another critical aspect of scalability is the optimization of raw material usage. Ethyl propanoate, while relatively inexpensive, must be sourced and utilized efficiently to maintain economic viability at larger scales. This has led to increased research into green chemistry approaches and sustainable production methods.
The regulatory landscape also plays a crucial role in the scalability of ethyl propanoate nanocarriers. As production scales up, meeting Good Manufacturing Practice (GMP) standards becomes more complex. Manufacturers must invest in robust quality control systems and documentation processes to ensure compliance with regulatory requirements.
From an application perspective, the scalability of ethyl propanoate nanocarriers opens up possibilities for their use in a wider range of therapeutic areas. As production capacity increases, these nanocarriers could potentially be employed in the treatment of more prevalent diseases, moving beyond niche applications in rare disorders.
Technological advancements in automation and continuous manufacturing processes are expected to play a significant role in improving the scalability of ethyl propanoate nanocarriers. These innovations could lead to more efficient production methods, reduced costs, and improved product consistency.
In conclusion, while the scalability of ethyl propanoate nanocarriers presents several challenges, ongoing research and technological developments are paving the way for their large-scale production and broader application in nanomedicine. The successful scaling of these nanocarriers could significantly impact the field of drug delivery and personalized medicine in the coming years.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!