Evaluating Electrode Kinetics in Nitrogen Reduction Catalyst Systems
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrogen Reduction Catalyst Evolution and Objectives
The evolution of nitrogen reduction catalysts represents a critical frontier in sustainable chemistry, with roots dating back to the early 20th century when Fritz Haber and Carl Bosch developed the industrial ammonia synthesis process. This breakthrough, while revolutionary, relied on harsh conditions including high temperatures (400-500°C) and pressures (150-300 bar), demanding substantial energy inputs and generating significant carbon emissions.
Over the past century, catalyst development has progressed through several distinct phases. The initial iron-based catalysts dominated industrial applications for decades, followed by the introduction of ruthenium-based systems in the 1970s that offered improved activity but at prohibitive costs. The 2000s witnessed a paradigm shift toward electrochemical nitrogen reduction approaches, seeking ambient-condition alternatives to the energy-intensive Haber-Bosch process.
Recent advances have focused on electrode kinetics as a fundamental limitation in nitrogen reduction reaction (NRR) systems. The sluggish electron transfer processes at the electrode-electrolyte interface represent a significant bottleneck in achieving commercially viable conversion rates. Current research indicates that understanding and optimizing these kinetic parameters is essential for developing next-generation catalysts capable of efficient nitrogen fixation under ambient conditions.
The primary technical objective in this field is to develop catalysts that can facilitate the electrochemical reduction of N₂ to NH₃ with high Faradaic efficiency (>60%) and production rates exceeding 10⁻⁹ mol cm⁻² s⁻¹ at ambient temperature and pressure. This requires overcoming several fundamental challenges, particularly the competing hydrogen evolution reaction that typically dominates aqueous systems.
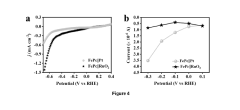
Electrode kinetics evaluation has emerged as a critical analytical approach, employing techniques such as electrochemical impedance spectroscopy (EIS), rotating disk electrode (RDE) measurements, and transient electrochemical methods to quantify exchange current densities, transfer coefficients, and activation energies. These parameters provide essential insights into reaction mechanisms and rate-limiting steps.
The technological trajectory suggests that future catalyst systems will likely incorporate advanced materials including single-atom catalysts, 2D materials, and metal-organic frameworks, all designed with precise control over active site structures to optimize electron transfer kinetics. Computational approaches using density functional theory are increasingly guiding rational catalyst design by predicting kinetic parameters before experimental validation.
Achieving the ambitious objectives in this field would revolutionize fertilizer production, potentially reducing global energy consumption by 1-2% and associated carbon emissions by a similar magnitude, representing a transformative advance in sustainable chemical manufacturing.
Over the past century, catalyst development has progressed through several distinct phases. The initial iron-based catalysts dominated industrial applications for decades, followed by the introduction of ruthenium-based systems in the 1970s that offered improved activity but at prohibitive costs. The 2000s witnessed a paradigm shift toward electrochemical nitrogen reduction approaches, seeking ambient-condition alternatives to the energy-intensive Haber-Bosch process.
Recent advances have focused on electrode kinetics as a fundamental limitation in nitrogen reduction reaction (NRR) systems. The sluggish electron transfer processes at the electrode-electrolyte interface represent a significant bottleneck in achieving commercially viable conversion rates. Current research indicates that understanding and optimizing these kinetic parameters is essential for developing next-generation catalysts capable of efficient nitrogen fixation under ambient conditions.
The primary technical objective in this field is to develop catalysts that can facilitate the electrochemical reduction of N₂ to NH₃ with high Faradaic efficiency (>60%) and production rates exceeding 10⁻⁹ mol cm⁻² s⁻¹ at ambient temperature and pressure. This requires overcoming several fundamental challenges, particularly the competing hydrogen evolution reaction that typically dominates aqueous systems.
Electrode kinetics evaluation has emerged as a critical analytical approach, employing techniques such as electrochemical impedance spectroscopy (EIS), rotating disk electrode (RDE) measurements, and transient electrochemical methods to quantify exchange current densities, transfer coefficients, and activation energies. These parameters provide essential insights into reaction mechanisms and rate-limiting steps.
The technological trajectory suggests that future catalyst systems will likely incorporate advanced materials including single-atom catalysts, 2D materials, and metal-organic frameworks, all designed with precise control over active site structures to optimize electron transfer kinetics. Computational approaches using density functional theory are increasingly guiding rational catalyst design by predicting kinetic parameters before experimental validation.
Achieving the ambitious objectives in this field would revolutionize fertilizer production, potentially reducing global energy consumption by 1-2% and associated carbon emissions by a similar magnitude, representing a transformative advance in sustainable chemical manufacturing.
Market Analysis for Nitrogen Fixation Technologies
The global nitrogen fixation market is experiencing significant growth, driven by increasing demand for fertilizers in agriculture and various industrial applications. Currently valued at approximately $25 billion, this market is projected to reach $32 billion by 2027, with a compound annual growth rate of 5.2%. The agricultural sector remains the dominant consumer, accounting for over 80% of fixed nitrogen usage worldwide, primarily in the form of ammonia-based fertilizers.
The conventional Haber-Bosch process has dominated industrial nitrogen fixation for over a century, but its high energy consumption and carbon footprint are creating market opportunities for alternative technologies. Electrochemical nitrogen reduction reaction (NRR) systems represent a promising segment with potential for substantial growth, especially as environmental regulations tighten globally and carbon pricing mechanisms become more widespread.
Regional analysis shows Asia-Pacific leading the nitrogen fixation market, with China being the largest producer and consumer of fixed nitrogen products. North America and Europe follow, with significant investments in research and development of sustainable nitrogen fixation technologies. Developing economies in South America and Africa represent emerging markets with high growth potential due to expanding agricultural sectors.
Market segmentation reveals increasing demand for decentralized nitrogen fixation solutions, particularly in remote agricultural regions where transportation of ammonia-based fertilizers is costly and logistically challenging. This trend is creating new opportunities for electrochemical NRR systems that can operate at ambient conditions using renewable electricity sources.
Consumer preferences are shifting toward environmentally sustainable products, creating premium market segments for "green ammonia" and other sustainably produced nitrogen compounds. Major agricultural corporations and chemical manufacturers are responding by investing in alternative nitrogen fixation technologies, including electrode-based systems with improved kinetics for nitrogen reduction.
The competitive landscape is evolving rapidly, with traditional chemical companies facing competition from technology startups and renewable energy providers entering the nitrogen fixation space. Strategic partnerships between established manufacturers and technology innovators are becoming increasingly common, accelerating the commercialization of novel electrode materials and catalyst systems.
Market barriers include high initial capital costs for new technologies, regulatory uncertainties, and technical challenges related to electrode stability and selectivity in NRR systems. However, favorable government policies supporting green technologies and increasing corporate sustainability commitments are expected to drive continued market expansion for advanced nitrogen fixation technologies.
The conventional Haber-Bosch process has dominated industrial nitrogen fixation for over a century, but its high energy consumption and carbon footprint are creating market opportunities for alternative technologies. Electrochemical nitrogen reduction reaction (NRR) systems represent a promising segment with potential for substantial growth, especially as environmental regulations tighten globally and carbon pricing mechanisms become more widespread.
Regional analysis shows Asia-Pacific leading the nitrogen fixation market, with China being the largest producer and consumer of fixed nitrogen products. North America and Europe follow, with significant investments in research and development of sustainable nitrogen fixation technologies. Developing economies in South America and Africa represent emerging markets with high growth potential due to expanding agricultural sectors.
Market segmentation reveals increasing demand for decentralized nitrogen fixation solutions, particularly in remote agricultural regions where transportation of ammonia-based fertilizers is costly and logistically challenging. This trend is creating new opportunities for electrochemical NRR systems that can operate at ambient conditions using renewable electricity sources.
Consumer preferences are shifting toward environmentally sustainable products, creating premium market segments for "green ammonia" and other sustainably produced nitrogen compounds. Major agricultural corporations and chemical manufacturers are responding by investing in alternative nitrogen fixation technologies, including electrode-based systems with improved kinetics for nitrogen reduction.
The competitive landscape is evolving rapidly, with traditional chemical companies facing competition from technology startups and renewable energy providers entering the nitrogen fixation space. Strategic partnerships between established manufacturers and technology innovators are becoming increasingly common, accelerating the commercialization of novel electrode materials and catalyst systems.
Market barriers include high initial capital costs for new technologies, regulatory uncertainties, and technical challenges related to electrode stability and selectivity in NRR systems. However, favorable government policies supporting green technologies and increasing corporate sustainability commitments are expected to drive continued market expansion for advanced nitrogen fixation technologies.
Current Challenges in Electrode Kinetics Assessment
The assessment of electrode kinetics in nitrogen reduction catalyst systems presents several significant challenges that impede accurate evaluation and comparison of catalytic performance. Current methodologies suffer from inconsistencies in experimental protocols, leading to difficulties in reproducing results across different research groups and laboratories.
One primary challenge lies in the complex multi-step electron transfer processes involved in nitrogen reduction reactions (NRR). Unlike simpler electrochemical reactions, NRR involves multiple intermediates and parallel reaction pathways, making the isolation and quantification of individual kinetic parameters extremely difficult. Researchers struggle to decouple the effects of mass transport, electron transfer rates, and chemical reaction steps.
The presence of competing hydrogen evolution reactions (HER) further complicates kinetic measurements. Since most NRR catalysts operate in aqueous environments, proton reduction to hydrogen often dominates the current response, obscuring the true kinetic parameters associated with nitrogen reduction. This parasitic reaction creates significant background signals that must be carefully accounted for in kinetic analyses.
Surface heterogeneity of catalyst materials presents another substantial challenge. Most practical electrocatalysts feature multiple types of active sites with varying activities, making it difficult to correlate macroscopic measurements with microscopic kinetic parameters. The dynamic nature of catalyst surfaces under reaction conditions further complicates matters, as restructuring and compositional changes can occur during operation.
Limitations in analytical techniques also hinder accurate kinetic assessments. Current methods for product detection, particularly for ammonia quantification, lack the sensitivity required for real-time kinetic measurements. The low Faradaic efficiencies typical of NRR catalysts (often below 10%) mean that product formation rates are at the detection limits of many analytical methods.
Temperature and pressure dependencies add another layer of complexity. Most laboratory studies are conducted at ambient conditions, while industrial processes operate at elevated temperatures and pressures. Extrapolating kinetic parameters across these different regimes introduces significant uncertainties in performance predictions.
The lack of standardized benchmarking protocols represents perhaps the most pressing challenge in the field. Different research groups employ varying electrode preparations, electrolyte compositions, cell configurations, and data analysis methods, making direct comparisons between catalysts nearly impossible. This inconsistency has led to contradictory reports in the literature and hindered systematic progress in catalyst development.
One primary challenge lies in the complex multi-step electron transfer processes involved in nitrogen reduction reactions (NRR). Unlike simpler electrochemical reactions, NRR involves multiple intermediates and parallel reaction pathways, making the isolation and quantification of individual kinetic parameters extremely difficult. Researchers struggle to decouple the effects of mass transport, electron transfer rates, and chemical reaction steps.
The presence of competing hydrogen evolution reactions (HER) further complicates kinetic measurements. Since most NRR catalysts operate in aqueous environments, proton reduction to hydrogen often dominates the current response, obscuring the true kinetic parameters associated with nitrogen reduction. This parasitic reaction creates significant background signals that must be carefully accounted for in kinetic analyses.
Surface heterogeneity of catalyst materials presents another substantial challenge. Most practical electrocatalysts feature multiple types of active sites with varying activities, making it difficult to correlate macroscopic measurements with microscopic kinetic parameters. The dynamic nature of catalyst surfaces under reaction conditions further complicates matters, as restructuring and compositional changes can occur during operation.
Limitations in analytical techniques also hinder accurate kinetic assessments. Current methods for product detection, particularly for ammonia quantification, lack the sensitivity required for real-time kinetic measurements. The low Faradaic efficiencies typical of NRR catalysts (often below 10%) mean that product formation rates are at the detection limits of many analytical methods.
Temperature and pressure dependencies add another layer of complexity. Most laboratory studies are conducted at ambient conditions, while industrial processes operate at elevated temperatures and pressures. Extrapolating kinetic parameters across these different regimes introduces significant uncertainties in performance predictions.
The lack of standardized benchmarking protocols represents perhaps the most pressing challenge in the field. Different research groups employ varying electrode preparations, electrolyte compositions, cell configurations, and data analysis methods, making direct comparisons between catalysts nearly impossible. This inconsistency has led to contradictory reports in the literature and hindered systematic progress in catalyst development.
Methodologies for Electrode Kinetics Evaluation
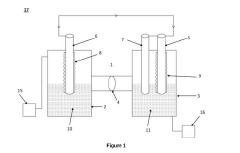
01 Metal-based catalysts for nitrogen reduction
Various metal-based catalysts have been developed for efficient nitrogen reduction reactions. These catalysts typically include transition metals such as iron, nickel, cobalt, and molybdenum, which can facilitate the breaking of the strong N≡N triple bond. The catalysts are designed with specific structures and compositions to enhance electron transfer kinetics and improve the overall efficiency of nitrogen reduction. These metal-based systems often demonstrate superior performance in terms of conversion rates and selectivity.- Metal-based catalysts for nitrogen reduction: Various metal-based catalysts have been developed for efficient nitrogen reduction reactions. These catalysts typically include transition metals such as iron, nickel, cobalt, and their alloys or compounds that facilitate the breaking of the strong N≡N triple bond. The catalysts are designed with specific surface structures and electronic properties to lower the activation energy barrier for nitrogen reduction, thereby improving reaction kinetics and efficiency at ambient conditions.
- Electrode design and materials for enhanced nitrogen reduction: Advanced electrode designs and materials play a crucial role in nitrogen reduction systems. Electrodes with high surface area, controlled porosity, and optimized morphology can significantly improve reaction kinetics by providing more active sites for nitrogen adsorption and reduction. Novel electrode materials including carbon-based supports, metal oxides, and composite structures are engineered to enhance electron transfer rates and catalytic activity while maintaining stability during the electrochemical nitrogen reduction process.
- Electrolyte composition effects on nitrogen reduction kinetics: The composition and properties of electrolytes significantly influence the kinetics of electrochemical nitrogen reduction reactions. Factors such as pH, ionic strength, and the presence of specific ions can alter the reaction pathway, affect the stability of intermediates, and impact the overall efficiency of the process. Optimized electrolyte formulations can enhance proton availability, improve mass transport, and minimize competing reactions like hydrogen evolution, thereby increasing the selectivity and rate of nitrogen reduction.
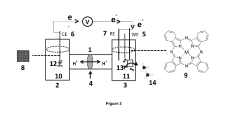
- Reaction mechanisms and kinetic modeling of nitrogen reduction: Understanding the reaction mechanisms and developing accurate kinetic models are essential for advancing nitrogen reduction catalyst systems. Research focuses on elucidating the elementary steps involved in nitrogen activation, protonation sequences, and the formation of intermediates like N2H2, N2H4, and NH3. Computational methods and experimental techniques are combined to identify rate-determining steps, quantify activation energies, and predict catalyst performance under various operating conditions, guiding the rational design of more efficient catalyst systems.
- Sustainable and scalable nitrogen reduction technologies: Development of environmentally sustainable and industrially scalable nitrogen reduction technologies is a growing focus area. These technologies aim to replace the energy-intensive Haber-Bosch process with electrochemical or photocatalytic approaches that operate under mild conditions. Research efforts concentrate on improving energy efficiency, increasing ammonia yield rates, enhancing catalyst stability and longevity, and developing integrated systems that can utilize renewable energy sources for nitrogen fixation, contributing to more sustainable fertilizer production and energy storage solutions.
02 Electrode materials and structures for nitrogen reduction
The design and composition of electrode materials play a crucial role in nitrogen reduction reactions. Advanced electrode structures with high surface area, controlled porosity, and optimized morphology can significantly enhance the kinetics of nitrogen reduction. These electrodes are often modified with specific functional groups or nanostructures to improve the adsorption of nitrogen molecules and facilitate electron transfer. The electrode architecture is tailored to minimize mass transport limitations and maximize catalytic activity.Expand Specific Solutions03 Electrolyte composition effects on nitrogen reduction kinetics
The composition of the electrolyte significantly influences the kinetics of nitrogen reduction reactions. Factors such as pH, ionic strength, and the presence of specific ions can affect the stability of reaction intermediates and the overall reaction pathway. Optimized electrolyte formulations can enhance catalyst performance by facilitating proton transfer, stabilizing reaction intermediates, and preventing catalyst poisoning. The electrolyte composition is carefully designed to work synergistically with the catalyst system to achieve high efficiency and selectivity.Expand Specific Solutions04 Reaction mechanisms and kinetic studies of nitrogen reduction
Understanding the reaction mechanisms and kinetics of nitrogen reduction is essential for catalyst design and optimization. Research in this area focuses on identifying rate-determining steps, characterizing reaction intermediates, and elucidating electron transfer processes. Advanced spectroscopic and electrochemical techniques are employed to study the reaction pathways and kinetics in real-time. These mechanistic insights guide the rational design of more efficient catalyst systems with improved kinetic parameters.Expand Specific Solutions05 Novel catalyst supports and composite materials
The development of novel catalyst supports and composite materials has significantly advanced nitrogen reduction catalyst systems. These supports, including carbon-based materials, metal oxides, and conductive polymers, provide high surface area, improved conductivity, and enhanced stability. Composite materials that combine different functional components can create synergistic effects that improve catalytic performance. The interaction between the catalyst and support material plays a crucial role in determining the overall electrode kinetics and efficiency of nitrogen reduction.Expand Specific Solutions
Leading Research Groups and Industrial Players
The nitrogen reduction catalyst market is currently in an early growth phase, characterized by intensive R&D efforts across academic institutions and industrial players. The global market size remains relatively modest but is projected to expand significantly as sustainable ammonia production becomes increasingly critical for decarbonization efforts. From a technological maturity perspective, the field is still evolving, with key players demonstrating varying levels of advancement. Academic institutions like California Institute of Technology, Osaka University, and Tokyo Institute of Technology are pioneering fundamental research, while industrial leaders including Toyota, BASF, Topsoe, and Siemens Energy are developing practical applications. Companies like Hitachi and SK Innovation are leveraging their materials expertise to enhance electrode performance. The competitive landscape reveals a balanced ecosystem of automotive companies, chemical manufacturers, and energy corporations collaborating with research institutions to overcome the significant kinetic barriers in nitrogen reduction reactions.
Toyota Motor Corp.
Technical Solution: Toyota has developed an innovative approach to nitrogen reduction catalyst evaluation focused on automotive applications and distributed ammonia production. Their system employs a combination of computational screening and high-throughput experimental validation to identify promising catalyst candidates. Toyota's electrode design incorporates nanostructured transition metal nitrides supported on carbon nanotubes, with particular emphasis on molybdenum-based materials that demonstrate enhanced N₂ activation. Their evaluation protocol utilizes a custom-built electrochemical cell that enables precise control of reaction conditions while simultaneously monitoring product formation through colorimetric detection methods[4]. Toyota has pioneered the use of ionic liquid electrolytes to suppress competing hydrogen evolution reactions, achieving Faradaic efficiencies for ammonia production approaching 10% under ambient conditions. Their kinetic analysis framework incorporates Tafel slope measurements, exchange current density determination, and activation energy calculations to provide comprehensive performance metrics. Recent developments include integration with renewable energy sources to create decentralized ammonia synthesis systems suitable for on-site fertilizer production or hydrogen storage applications.
Strengths: Strong systems integration capabilities; extensive experience with electrochemical systems in transportation applications; well-established manufacturing infrastructure for potential commercialization. Weaknesses: Relatively new entrant to chemical catalysis compared to specialized chemical companies; technology still requires further efficiency improvements for commercial viability; catalyst durability under variable operating conditions remains challenging.
Technische Universität München
Technical Solution: Technische Universität München (TUM) has established a comprehensive approach to evaluating electrode kinetics in nitrogen reduction catalysts, combining advanced spectroelectrochemical methods with theoretical calculations. Their methodology employs operando Raman spectroscopy and differential electrochemical mass spectrometry to monitor reaction intermediates and products in real-time. TUM researchers have developed novel catalyst systems based on metal-organic frameworks with atomically dispersed active sites, demonstrating enhanced selectivity for nitrogen reduction over competing hydrogen evolution. Their electrode design incorporates hierarchical porosity to optimize mass transport while maintaining high active site density[6]. TUM's kinetic evaluation protocol includes detailed analysis of charge transfer coefficients, reaction orders, and activation energies across different potential regions to identify rate-determining steps. The university has pioneered the use of isotopically labeled reactants combined with nuclear magnetic resonance spectroscopy to track nitrogen incorporation into ammonia, providing unambiguous evidence of electrochemical N2 reduction. Recent innovations include the development of tandem catalyst systems that spatially separate N2 activation from protonation steps, significantly enhancing reaction efficiency and suppressing unwanted side reactions.
Strengths: Exceptional fundamental research capabilities; strong interdisciplinary approach combining electrochemistry, materials science, and theoretical modeling; access to advanced characterization facilities. Weaknesses: Limited focus on industrial scalability and manufacturing considerations; catalyst systems often utilize precious metals or complex structures challenging for mass production; technology readiness level remains relatively low compared to industrial requirements.
Key Innovations in Catalyst Interface Characterization
Method for evaluation of electrode catalyst
PatentInactiveJP2016131099A
Innovation
- A method involving cyclic voltammetry with specific potential sweeps and holding times on both electrodes of a fuel cell, allowing estimation of the active surface area decline by calculating 'metal stress' from charge differences in cyclic voltammograms.
Electrochemical cell for generating ammonia
PatentPendingIN202211074333A
Innovation
- An electrochemical cell system with a cathode electrode coated with a transition metal-based catalyst layer, such as Iron (Fe), Cobalt (Co), or Copper (Cu) phthalocyanine, and an anode electrode coated with Ruthenium (IV) oxide, using sodium tetrafluoroborate as the catholyte and potassium hydroxide as the anolyte, which improves nitrogen reduction reaction efficiency and oxygen evolution reaction kinetics.
Environmental Impact Assessment
The environmental implications of nitrogen reduction catalyst systems extend far beyond their immediate technological applications. These systems, designed to convert atmospheric nitrogen into ammonia, represent a potential paradigm shift in fertilizer production, which currently relies heavily on the energy-intensive Haber-Bosch process. This conventional process consumes approximately 1-2% of global energy production and generates significant greenhouse gas emissions, contributing substantially to climate change.
Electrode-based nitrogen reduction reaction (NRR) catalysts offer a promising alternative with substantially reduced environmental footprints. When powered by renewable energy sources, these electrochemical systems can operate at ambient temperature and pressure, eliminating the need for the extreme conditions required by traditional methods. Quantitative assessments indicate potential reductions in carbon emissions by 60-80% compared to conventional ammonia synthesis, representing a significant contribution to global decarbonization efforts.
Water consumption represents another critical environmental consideration. Conventional ammonia production requires substantial water inputs for cooling and steam generation. Electrochemical nitrogen reduction systems typically demonstrate 30-50% lower water requirements, though this advantage may be partially offset by water needs for electrode maintenance and electrolyte preparation. In water-stressed regions, this reduction could prove particularly valuable for sustainable agricultural practices.
The materials used in electrode fabrication present both challenges and opportunities for environmental sustainability. Many high-performance catalysts incorporate precious metals or rare earth elements, raising concerns about resource depletion and mining impacts. Life cycle assessments of various catalyst compositions reveal significant variations in environmental burdens, with noble metal-based catalysts generally showing higher extraction-phase impacts but potentially longer operational lifespans than transition metal alternatives.
Waste generation and management constitute additional environmental considerations. Catalyst degradation products and spent electrolytes require proper handling and disposal. However, emerging research in catalyst regeneration techniques demonstrates potential for circular economy approaches, with some systems achieving 70-85% materials recovery rates, significantly reducing waste streams compared to single-use catalyst systems.
Land use impacts also differ substantially between conventional and electrochemical nitrogen fixation. Distributed, smaller-scale electrochemical systems could reduce the need for large industrial facilities and extensive transportation networks for ammonia distribution, potentially decreasing habitat disruption and allowing for more localized agricultural nutrient cycles that better mimic natural systems.
Electrode-based nitrogen reduction reaction (NRR) catalysts offer a promising alternative with substantially reduced environmental footprints. When powered by renewable energy sources, these electrochemical systems can operate at ambient temperature and pressure, eliminating the need for the extreme conditions required by traditional methods. Quantitative assessments indicate potential reductions in carbon emissions by 60-80% compared to conventional ammonia synthesis, representing a significant contribution to global decarbonization efforts.
Water consumption represents another critical environmental consideration. Conventional ammonia production requires substantial water inputs for cooling and steam generation. Electrochemical nitrogen reduction systems typically demonstrate 30-50% lower water requirements, though this advantage may be partially offset by water needs for electrode maintenance and electrolyte preparation. In water-stressed regions, this reduction could prove particularly valuable for sustainable agricultural practices.
The materials used in electrode fabrication present both challenges and opportunities for environmental sustainability. Many high-performance catalysts incorporate precious metals or rare earth elements, raising concerns about resource depletion and mining impacts. Life cycle assessments of various catalyst compositions reveal significant variations in environmental burdens, with noble metal-based catalysts generally showing higher extraction-phase impacts but potentially longer operational lifespans than transition metal alternatives.
Waste generation and management constitute additional environmental considerations. Catalyst degradation products and spent electrolytes require proper handling and disposal. However, emerging research in catalyst regeneration techniques demonstrates potential for circular economy approaches, with some systems achieving 70-85% materials recovery rates, significantly reducing waste streams compared to single-use catalyst systems.
Land use impacts also differ substantially between conventional and electrochemical nitrogen fixation. Distributed, smaller-scale electrochemical systems could reduce the need for large industrial facilities and extensive transportation networks for ammonia distribution, potentially decreasing habitat disruption and allowing for more localized agricultural nutrient cycles that better mimic natural systems.
Scalability and Economic Viability
The scalability of nitrogen reduction catalyst systems represents a critical challenge in transitioning laboratory-scale electrode kinetics research to industrial applications. Current electrode systems demonstrating promising nitrogen reduction reaction (NRR) kinetics typically operate at small scales under controlled laboratory conditions, with electrode surface areas rarely exceeding a few square centimeters. Scaling these systems to industrial dimensions introduces significant engineering challenges, particularly in maintaining uniform reaction conditions across larger electrode surfaces.
Economic viability analysis reveals that electrode materials constitute a substantial portion of system costs. Noble metal catalysts such as ruthenium and platinum demonstrate superior kinetics but remain prohibitively expensive for large-scale deployment. Recent advances in non-precious metal catalysts and metal-nitrogen-carbon (M-N-C) composites offer more cost-effective alternatives, though often with reduced reaction rates and selectivity.
Energy efficiency metrics indicate that most current NRR systems operate at electrical-to-chemical energy conversion efficiencies below 10%, significantly impacting operational economics. Calculations based on current technology suggest production costs of $2,000-4,000 per ton of ammonia via electrochemical routes, compared to $400-600 per ton through conventional Haber-Bosch processes. This substantial cost differential highlights the necessity for dramatic improvements in electrode kinetics and system design.
Infrastructure requirements present additional scaling challenges. Most high-performance electrode systems require specialized electrolytes, precise pH control, and carefully regulated temperatures. The capital expenditure for such control systems increases non-linearly with scale, creating economic barriers to industrial implementation. Furthermore, electrode degradation rates observed in laboratory studies suggest replacement cycles that would be economically unsustainable at industrial scales.
Recent techno-economic analyses suggest that electrochemical nitrogen reduction could become competitive with conventional processes if three key metrics are achieved simultaneously: catalyst current densities exceeding 300 mA/cm², Faradaic efficiencies above 60%, and operational stability beyond 5,000 hours. Current state-of-the-art systems typically achieve only one or two of these metrics, indicating substantial room for improvement.
Renewable energy integration offers a potential pathway to economic viability. Coupling NRR systems with intermittent renewable energy sources could provide value beyond simple ammonia production costs, particularly in grid-balancing applications. Models suggest that such integrated systems could achieve economic viability at performance metrics 30-40% lower than those required for standalone operations.
Economic viability analysis reveals that electrode materials constitute a substantial portion of system costs. Noble metal catalysts such as ruthenium and platinum demonstrate superior kinetics but remain prohibitively expensive for large-scale deployment. Recent advances in non-precious metal catalysts and metal-nitrogen-carbon (M-N-C) composites offer more cost-effective alternatives, though often with reduced reaction rates and selectivity.
Energy efficiency metrics indicate that most current NRR systems operate at electrical-to-chemical energy conversion efficiencies below 10%, significantly impacting operational economics. Calculations based on current technology suggest production costs of $2,000-4,000 per ton of ammonia via electrochemical routes, compared to $400-600 per ton through conventional Haber-Bosch processes. This substantial cost differential highlights the necessity for dramatic improvements in electrode kinetics and system design.
Infrastructure requirements present additional scaling challenges. Most high-performance electrode systems require specialized electrolytes, precise pH control, and carefully regulated temperatures. The capital expenditure for such control systems increases non-linearly with scale, creating economic barriers to industrial implementation. Furthermore, electrode degradation rates observed in laboratory studies suggest replacement cycles that would be economically unsustainable at industrial scales.
Recent techno-economic analyses suggest that electrochemical nitrogen reduction could become competitive with conventional processes if three key metrics are achieved simultaneously: catalyst current densities exceeding 300 mA/cm², Faradaic efficiencies above 60%, and operational stability beyond 5,000 hours. Current state-of-the-art systems typically achieve only one or two of these metrics, indicating substantial room for improvement.
Renewable energy integration offers a potential pathway to economic viability. Coupling NRR systems with intermittent renewable energy sources could provide value beyond simple ammonia production costs, particularly in grid-balancing applications. Models suggest that such integrated systems could achieve economic viability at performance metrics 30-40% lower than those required for standalone operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!