Hollow Fiber Membranes: Bubble-Point/Pressure-Hold Methods, Sensitivity And Defect Mapping
SEP 16, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hollow Fiber Membrane Technology Evolution and Objectives
Hollow fiber membrane technology has evolved significantly since its inception in the late 1960s, transforming from experimental laboratory concepts to sophisticated industrial applications. Initially developed for gas separation and water purification, these membranes have progressively expanded into diverse fields including hemodialysis, pharmaceutical processing, and food industry applications. The cylindrical, hollow structure provides exceptional surface area-to-volume ratios, making these membranes particularly efficient for separation processes compared to flat sheet configurations.
The evolution of hollow fiber membrane technology has been marked by several key advancements. Early membranes suffered from limited selectivity and mechanical integrity, but material science breakthroughs in the 1980s introduced more robust polymers such as polysulfone, polyethersulfone, and PVDF. The 1990s witnessed significant improvements in spinning techniques, allowing for precise control over wall thickness and pore structure. More recently, composite and asymmetric membrane structures have emerged, offering enhanced performance characteristics.
Quality control methodologies have evolved in parallel with membrane technology itself. The bubble-point method, introduced in the early 1980s, represented the first standardized approach to integrity testing. This technique has since been refined into more sophisticated pressure-hold methods that offer greater sensitivity and reproducibility. Modern defect mapping capabilities now allow manufacturers to identify and characterize membrane imperfections with unprecedented precision.
The primary objective in hollow fiber membrane technology development is achieving optimal balance between permeability and selectivity while maintaining mechanical integrity. Current research focuses on enhancing detection sensitivity for increasingly smaller defects, as even microscopic imperfections can significantly impact membrane performance in high-precision applications. The industry aims to develop standardized testing protocols that can reliably detect defects at the sub-micron level.
Another critical objective is the development of non-destructive testing methodologies that can be implemented in-line during manufacturing processes. This would enable real-time quality control and reduce production costs. Additionally, there is growing interest in correlating defect characteristics with specific performance parameters, allowing for more targeted membrane design and application-specific optimization.
Looking forward, the field is moving toward automated defect mapping systems that integrate machine learning algorithms to predict membrane performance based on detected imperfection patterns. This predictive capability would revolutionize quality control processes and enable more efficient membrane production with consistently higher performance standards.
The evolution of hollow fiber membrane technology has been marked by several key advancements. Early membranes suffered from limited selectivity and mechanical integrity, but material science breakthroughs in the 1980s introduced more robust polymers such as polysulfone, polyethersulfone, and PVDF. The 1990s witnessed significant improvements in spinning techniques, allowing for precise control over wall thickness and pore structure. More recently, composite and asymmetric membrane structures have emerged, offering enhanced performance characteristics.
Quality control methodologies have evolved in parallel with membrane technology itself. The bubble-point method, introduced in the early 1980s, represented the first standardized approach to integrity testing. This technique has since been refined into more sophisticated pressure-hold methods that offer greater sensitivity and reproducibility. Modern defect mapping capabilities now allow manufacturers to identify and characterize membrane imperfections with unprecedented precision.
The primary objective in hollow fiber membrane technology development is achieving optimal balance between permeability and selectivity while maintaining mechanical integrity. Current research focuses on enhancing detection sensitivity for increasingly smaller defects, as even microscopic imperfections can significantly impact membrane performance in high-precision applications. The industry aims to develop standardized testing protocols that can reliably detect defects at the sub-micron level.
Another critical objective is the development of non-destructive testing methodologies that can be implemented in-line during manufacturing processes. This would enable real-time quality control and reduce production costs. Additionally, there is growing interest in correlating defect characteristics with specific performance parameters, allowing for more targeted membrane design and application-specific optimization.
Looking forward, the field is moving toward automated defect mapping systems that integrate machine learning algorithms to predict membrane performance based on detected imperfection patterns. This predictive capability would revolutionize quality control processes and enable more efficient membrane production with consistently higher performance standards.
Market Applications and Demand Analysis for Integrity Testing
The integrity testing market for hollow fiber membranes has experienced significant growth in recent years, driven by increasing applications in pharmaceutical manufacturing, water treatment, and biotechnology sectors. The global market for membrane integrity testing was valued at approximately $4.3 billion in 2022 and is projected to grow at a compound annual growth rate of 7.8% through 2030, according to industry analyses.
Pharmaceutical and biopharmaceutical industries represent the largest market segment for hollow fiber membrane integrity testing, accounting for nearly 45% of the total market share. This dominance stems from stringent regulatory requirements for product quality and safety, particularly in sterile filtration processes where membrane integrity is critical for preventing contamination.
Water treatment applications constitute the second-largest market segment, with municipal water treatment facilities increasingly adopting hollow fiber membrane technologies for their superior filtration capabilities. The growing global water scarcity concerns and stricter regulations regarding water quality have accelerated the adoption of membrane filtration systems, consequently driving demand for reliable integrity testing methods.
Healthcare facilities represent another significant market segment, where hollow fiber membranes are extensively used in hemodialysis and other medical applications. The critical nature of these applications necessitates regular and reliable integrity testing to ensure patient safety.
Geographically, North America leads the market for hollow fiber membrane integrity testing, followed by Europe and Asia-Pacific. The Asia-Pacific region is expected to witness the highest growth rate due to rapid industrialization, increasing healthcare expenditure, and growing awareness about water quality issues.
The demand for automated and non-destructive integrity testing methods, particularly bubble-point and pressure-hold techniques, has been increasing due to their reliability and efficiency. End-users are increasingly seeking testing solutions that offer higher sensitivity for defect detection while maintaining operational efficiency.
Market trends indicate a growing preference for integrated testing systems that can perform multiple integrity tests and provide comprehensive data analysis. This trend is particularly evident in pharmaceutical manufacturing, where data integrity and compliance with regulatory standards are paramount.
The COVID-19 pandemic has further accelerated market growth, as it highlighted the importance of reliable filtration systems in healthcare settings and increased focus on water quality. This has led to increased investments in advanced membrane technologies and corresponding integrity testing solutions.
Pharmaceutical and biopharmaceutical industries represent the largest market segment for hollow fiber membrane integrity testing, accounting for nearly 45% of the total market share. This dominance stems from stringent regulatory requirements for product quality and safety, particularly in sterile filtration processes where membrane integrity is critical for preventing contamination.
Water treatment applications constitute the second-largest market segment, with municipal water treatment facilities increasingly adopting hollow fiber membrane technologies for their superior filtration capabilities. The growing global water scarcity concerns and stricter regulations regarding water quality have accelerated the adoption of membrane filtration systems, consequently driving demand for reliable integrity testing methods.
Healthcare facilities represent another significant market segment, where hollow fiber membranes are extensively used in hemodialysis and other medical applications. The critical nature of these applications necessitates regular and reliable integrity testing to ensure patient safety.
Geographically, North America leads the market for hollow fiber membrane integrity testing, followed by Europe and Asia-Pacific. The Asia-Pacific region is expected to witness the highest growth rate due to rapid industrialization, increasing healthcare expenditure, and growing awareness about water quality issues.
The demand for automated and non-destructive integrity testing methods, particularly bubble-point and pressure-hold techniques, has been increasing due to their reliability and efficiency. End-users are increasingly seeking testing solutions that offer higher sensitivity for defect detection while maintaining operational efficiency.
Market trends indicate a growing preference for integrated testing systems that can perform multiple integrity tests and provide comprehensive data analysis. This trend is particularly evident in pharmaceutical manufacturing, where data integrity and compliance with regulatory standards are paramount.
The COVID-19 pandemic has further accelerated market growth, as it highlighted the importance of reliable filtration systems in healthcare settings and increased focus on water quality. This has led to increased investments in advanced membrane technologies and corresponding integrity testing solutions.
Current Challenges in Membrane Defect Detection
Despite significant advancements in hollow fiber membrane technology, defect detection remains a critical challenge in quality control processes. Current membrane defect detection methods, particularly bubble-point and pressure-hold techniques, face several limitations that impact their effectiveness and reliability in industrial applications.
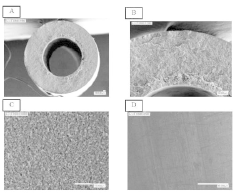
The sensitivity threshold of conventional detection methods presents a primary challenge. While bubble-point testing can identify larger defects, it struggles to consistently detect micro-defects below 1-10 μm in size. These microscopic imperfections, though small, can significantly compromise membrane performance in critical applications such as hemodialysis, water purification, and gas separation processes.
Spatial resolution limitations further complicate defect mapping across the entire membrane surface. Current methods often provide only localized information or averaged results across membrane sections, making it difficult to create comprehensive defect distribution maps. This incomplete spatial characterization leads to uncertainty in quality assessment and potentially allows defective membrane sections to pass quality control.
Test standardization represents another significant hurdle. The industry lacks universally accepted protocols for bubble-point and pressure-hold testing, resulting in variability between manufacturers and testing facilities. This inconsistency makes it challenging to compare results across different membrane types or production batches, hindering quality benchmarking efforts.
Real-time monitoring capabilities remain underdeveloped, with most detection methods requiring membrane samples to be removed from production lines for testing. This destructive testing approach not only increases waste but also creates delays in the manufacturing process, preventing immediate corrective actions when defects are identified.
Environmental factors such as temperature fluctuations, humidity variations, and testing fluid properties significantly influence test results, yet current methods often fail to adequately account for these variables. This leads to inconsistent measurements and reduced reliability in defect detection outcomes.
The correlation between detected defects and actual membrane performance presents perhaps the most fundamental challenge. Current methods struggle to establish clear relationships between identified defects and their impact on critical performance parameters such as flux, rejection rates, and mechanical integrity under operating conditions. This disconnect complicates decision-making regarding membrane acceptance or rejection criteria.
Automation and integration of defect detection into manufacturing workflows remain limited, with many facilities still relying on labor-intensive manual testing procedures that introduce human error and reduce throughput. The industry needs more sophisticated, integrated detection systems that can operate continuously within production environments.
The sensitivity threshold of conventional detection methods presents a primary challenge. While bubble-point testing can identify larger defects, it struggles to consistently detect micro-defects below 1-10 μm in size. These microscopic imperfections, though small, can significantly compromise membrane performance in critical applications such as hemodialysis, water purification, and gas separation processes.
Spatial resolution limitations further complicate defect mapping across the entire membrane surface. Current methods often provide only localized information or averaged results across membrane sections, making it difficult to create comprehensive defect distribution maps. This incomplete spatial characterization leads to uncertainty in quality assessment and potentially allows defective membrane sections to pass quality control.
Test standardization represents another significant hurdle. The industry lacks universally accepted protocols for bubble-point and pressure-hold testing, resulting in variability between manufacturers and testing facilities. This inconsistency makes it challenging to compare results across different membrane types or production batches, hindering quality benchmarking efforts.
Real-time monitoring capabilities remain underdeveloped, with most detection methods requiring membrane samples to be removed from production lines for testing. This destructive testing approach not only increases waste but also creates delays in the manufacturing process, preventing immediate corrective actions when defects are identified.
Environmental factors such as temperature fluctuations, humidity variations, and testing fluid properties significantly influence test results, yet current methods often fail to adequately account for these variables. This leads to inconsistent measurements and reduced reliability in defect detection outcomes.
The correlation between detected defects and actual membrane performance presents perhaps the most fundamental challenge. Current methods struggle to establish clear relationships between identified defects and their impact on critical performance parameters such as flux, rejection rates, and mechanical integrity under operating conditions. This disconnect complicates decision-making regarding membrane acceptance or rejection criteria.
Automation and integration of defect detection into manufacturing workflows remain limited, with many facilities still relying on labor-intensive manual testing procedures that introduce human error and reduce throughput. The industry needs more sophisticated, integrated detection systems that can operate continuously within production environments.
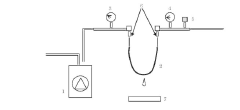
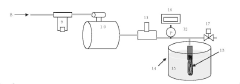
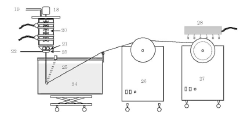
Bubble-Point and Pressure-Hold Testing Methodologies
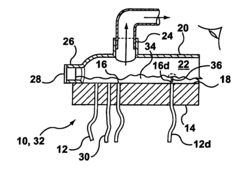
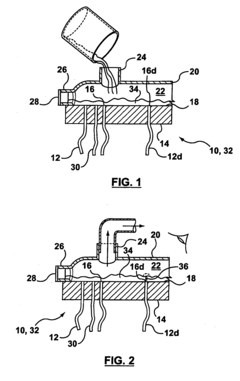
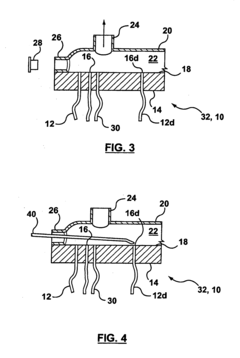
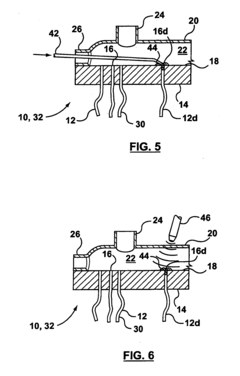
01 Defect detection methods for hollow fiber membranes
Various methods are employed to detect defects in hollow fiber membranes, including optical inspection systems, imaging techniques, and automated detection algorithms. These methods can identify pinholes, cracks, and other structural imperfections that may compromise membrane performance. Advanced imaging technologies enable high-resolution scanning of membrane surfaces to map defects with precision, allowing for quality control during manufacturing processes.- Defect detection methods for hollow fiber membranes: Various methods can be employed to detect defects in hollow fiber membranes, including optical inspection systems, pressure-based testing, and imaging techniques. These methods allow for the identification of structural flaws, pinholes, or irregularities that might compromise membrane performance. Advanced detection systems can identify defects at microscopic levels, enabling quality control during manufacturing and maintenance processes.
- Sensitivity analysis and performance evaluation techniques: Techniques for evaluating the sensitivity and performance of hollow fiber membranes include integrity testing, permeability measurements, and rejection rate analysis. These methods help determine the membrane's effectiveness in filtration applications and its response to various operating conditions. Sensitivity analysis can identify critical parameters affecting membrane performance and establish optimal operating ranges for specific applications.
- Manufacturing processes affecting membrane integrity: The manufacturing process significantly impacts hollow fiber membrane integrity and defect formation. Factors such as spinning conditions, polymer composition, coagulation parameters, and post-treatment methods can influence membrane structure and defect prevalence. Optimized manufacturing techniques can reduce defect formation and enhance membrane performance, leading to more reliable filtration systems with improved longevity.
- Automated mapping and visualization of membrane defects: Advanced systems for automated mapping and visualization of hollow fiber membrane defects utilize computer vision, machine learning algorithms, and specialized imaging techniques. These systems can create comprehensive defect maps that highlight the location, size, and type of defects across membrane modules. Automated mapping enables more efficient quality control processes and facilitates targeted repairs or replacements of defective membrane sections.
- Integrity testing and leak detection systems: Specialized integrity testing and leak detection systems for hollow fiber membranes include pressure decay tests, bubble point measurements, and diffusive flow techniques. These systems can identify breaches in membrane integrity that might allow contaminants to pass through. Real-time monitoring capabilities enable continuous assessment of membrane performance and early detection of developing defects, preventing system failures in critical applications.
02 Integrity testing and sensitivity analysis of hollow fiber membranes
Integrity testing procedures are used to evaluate the functional performance and sensitivity of hollow fiber membranes. These tests measure parameters such as bubble point, pressure decay, and diffusion rates to determine membrane integrity. Sensitivity analysis helps identify critical operational parameters that affect membrane performance and longevity. These testing methods are essential for ensuring that membranes meet required specifications and can reliably perform filtration tasks.Expand Specific Solutions03 Manufacturing techniques to minimize defects in hollow fiber membranes
Specialized manufacturing techniques have been developed to minimize defects in hollow fiber membranes. These include controlled spinning processes, precise polymer formulation, and optimized solidification conditions. Post-production treatments such as annealing and coating can further reduce defect formation. Quality control measures implemented during manufacturing help identify and address potential defect sources, resulting in more reliable and consistent membrane products.Expand Specific Solutions04 Non-destructive testing for hollow fiber membrane defect mapping
Non-destructive testing methods allow for comprehensive defect mapping of hollow fiber membranes without compromising their structure. These techniques include ultrasonic inspection, infrared thermography, and electrical conductivity measurements. Advanced imaging systems can create detailed maps of membrane surfaces and internal structures, identifying defects that might be missed by conventional testing. These non-destructive approaches are particularly valuable for quality assurance and failure analysis.Expand Specific Solutions05 Sensitivity enhancement and defect repair in hollow fiber membranes
Methods for enhancing the sensitivity of hollow fiber membranes and repairing defects have been developed to improve membrane performance. These include surface modification techniques, selective coating applications, and targeted repair procedures for identified defects. Sensitivity enhancement can involve the incorporation of functional additives or post-treatment processes that improve selectivity and permeability. Defect repair technologies can extend membrane lifespan and maintain filtration efficiency even after defects are detected.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The hollow fiber membrane market is in a growth phase, with increasing applications in water treatment, medical devices, and industrial processes. The global market size is projected to expand significantly due to rising demand for clean water technologies and advanced filtration systems. Technologically, the field is maturing with established players like Toray Industries, Asahi Kasei, and Sumitomo Electric leading innovation in bubble-point/pressure-hold testing methods. Companies such as Fresenius Medical Care and Gambro focus on medical applications, while Toyobo and Mitsubishi Rayon specialize in industrial membranes. Chinese manufacturers like Hangzhou Cobetter are rapidly advancing, particularly in defect mapping technologies. Academic-industry collaborations with institutions like National University of Singapore are accelerating sensitivity improvements and standardization of quality control methodologies.
Toray Industries, Inc.
Technical Solution: Toray has developed advanced bubble-point/pressure-hold testing systems for hollow fiber membranes with high sensitivity detection capabilities. Their technology utilizes automated pressure ramping with precise digital pressure transducers capable of detecting defects as small as 1-3 microns. The system incorporates multi-point testing along membrane modules to create comprehensive defect maps. Toray's approach includes proprietary algorithms that analyze pressure decay curves to differentiate between true defects and normal membrane porosity characteristics. Their testing protocol integrates both bubble-point (visual detection of first bubble) and pressure-hold methods (measuring pressure decay over time) to provide complementary data on membrane integrity. For critical applications like hemodialysis membranes, Toray implements 100% in-line testing with automated image recognition to identify bubble formation at precise pressure thresholds.
Strengths: Superior detection sensitivity for submicron defects; comprehensive defect mapping capabilities; high throughput automated testing suitable for production environments. Weaknesses: System requires significant calibration for different membrane materials; higher implementation cost compared to basic testing methods; may produce false positives in certain membrane geometries.
Toyobo Co., Ltd.
Technical Solution: Toyobo has developed a sophisticated hollow fiber membrane testing platform that integrates both bubble-point and extended pressure-hold methodologies. Their system employs high-precision digital pressure sensors (accuracy of ±0.1% full scale) coupled with computerized data acquisition systems capable of detecting pressure changes as small as 0.5 mbar over extended periods. Toyobo's technology incorporates specialized membrane wetting protocols using carefully formulated test fluids with controlled surface tension properties to ensure consistent results across different membrane materials. Their defect mapping approach utilizes segmented testing along membrane modules with automated pressure ramping and decay analysis to precisely locate integrity issues. For high-value applications like pharmaceutical filtration, Toyobo implements a dual-testing methodology where membranes undergo both bubble-point visual inspection and quantitative pressure-hold analysis. The system also features automated temperature compensation and environmental control to minimize testing variations due to ambient conditions.
Strengths: High detection sensitivity suitable for critical separation applications; excellent reproducibility across different production batches; comprehensive defect characterization capabilities. Weaknesses: Testing process requires specialized operator training; system optimization is material-specific; relatively slower throughput compared to basic integrity testing methods.
Critical Parameters Affecting Test Sensitivity
Polyamide hollow fiber membrane
PatentActiveJP2016068005A
Innovation
- A polyamide hollow fiber membrane produced via a thermally induced phase separation method, incorporating a polyamide resin with a fatty acid amide in the polymer solution, achieves high bubble point and water permeability by controlling pore size and enhancing structural strength.
Method of locating or repairing damaged hollow fiber membranes or header or module assembly
PatentInactiveUS20060113226A1
Innovation
- A header assembly with a translucent cover and port system allows for the application of a pressure differential to create bubbles at damaged fibers, enabling visual identification through a transparent cover, and a method for sealing damaged fibers using sealing materials and energy sources to repair them, with optional access enhancements for better access to all fibers.
Quality Control Standards and Compliance Requirements
Quality control standards for hollow fiber membrane manufacturing are governed by multiple regulatory frameworks that ensure product safety and performance. The International Organization for Standardization (ISO) provides several standards specifically applicable to membrane testing, including ISO 15747 for pharmaceutical applications and ISO 10993 series for biocompatibility assessment. These standards establish minimum requirements for bubble-point and pressure-hold testing methodologies, including acceptable pressure ranges, testing frequencies, and documentation protocols.
In the United States, the Food and Drug Administration (FDA) enforces compliance through 21 CFR Part 210/211 for pharmaceutical applications and 21 CFR Part 820 for medical devices incorporating hollow fiber membranes. These regulations mandate validation of testing methods, calibration of testing equipment, and implementation of statistical process control for defect detection. The FDA guidance document "Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing" specifically addresses integrity testing requirements for filtration systems.
European regulatory frameworks include the European Medicines Agency (EMA) guidelines and the Medical Device Regulation (MDR 2017/745), which establish more stringent requirements for defect mapping and sensitivity analysis. These regulations require manufacturers to implement risk-based approaches to quality control, with particular emphasis on critical process parameters affecting membrane integrity.
Industry-specific standards from organizations such as ASTM International provide detailed testing protocols. ASTM F838-15a establishes standardized procedures for bacterial retention testing of membrane filters, while ASTM D6908 addresses pressure-hold test methodologies. These standards specify acceptance criteria based on application requirements, with more stringent thresholds for pharmaceutical and medical applications compared to industrial or water treatment applications.
Compliance with these standards requires manufacturers to implement robust quality management systems that include detailed documentation of testing procedures, calibration records, and validation studies. Statistical process control methods must be employed to establish control limits for bubble-point and pressure-hold test results, with clear action and alert limits defined. Manufacturers must also maintain traceability records linking raw materials, production parameters, and final test results.
Recent regulatory trends indicate increasing emphasis on real-time monitoring and automated defect mapping technologies. Regulatory bodies are beginning to recognize advanced analytical methods that can provide more comprehensive membrane integrity assessment than traditional bubble-point testing alone. This shift reflects growing recognition of the limitations of conventional testing methods and the potential benefits of more sophisticated quality control approaches.
In the United States, the Food and Drug Administration (FDA) enforces compliance through 21 CFR Part 210/211 for pharmaceutical applications and 21 CFR Part 820 for medical devices incorporating hollow fiber membranes. These regulations mandate validation of testing methods, calibration of testing equipment, and implementation of statistical process control for defect detection. The FDA guidance document "Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing" specifically addresses integrity testing requirements for filtration systems.
European regulatory frameworks include the European Medicines Agency (EMA) guidelines and the Medical Device Regulation (MDR 2017/745), which establish more stringent requirements for defect mapping and sensitivity analysis. These regulations require manufacturers to implement risk-based approaches to quality control, with particular emphasis on critical process parameters affecting membrane integrity.
Industry-specific standards from organizations such as ASTM International provide detailed testing protocols. ASTM F838-15a establishes standardized procedures for bacterial retention testing of membrane filters, while ASTM D6908 addresses pressure-hold test methodologies. These standards specify acceptance criteria based on application requirements, with more stringent thresholds for pharmaceutical and medical applications compared to industrial or water treatment applications.
Compliance with these standards requires manufacturers to implement robust quality management systems that include detailed documentation of testing procedures, calibration records, and validation studies. Statistical process control methods must be employed to establish control limits for bubble-point and pressure-hold test results, with clear action and alert limits defined. Manufacturers must also maintain traceability records linking raw materials, production parameters, and final test results.
Recent regulatory trends indicate increasing emphasis on real-time monitoring and automated defect mapping technologies. Regulatory bodies are beginning to recognize advanced analytical methods that can provide more comprehensive membrane integrity assessment than traditional bubble-point testing alone. This shift reflects growing recognition of the limitations of conventional testing methods and the potential benefits of more sophisticated quality control approaches.
Economic Impact of Membrane Failure Prevention
The economic implications of preventing membrane failures in hollow fiber membrane systems are substantial across multiple industries. Effective implementation of bubble-point and pressure-hold testing methods for defect detection can significantly reduce operational costs while extending membrane lifespan. Industries such as water treatment, pharmaceuticals, and food processing stand to benefit most from these preventive measures.
In water treatment facilities, membrane failures can lead to contamination events requiring costly system shutdowns, regulatory penalties, and potential public health crises. Research indicates that implementing comprehensive defect mapping protocols can reduce unplanned downtime by up to 40%, translating to savings of $50,000-$200,000 per incident for mid-sized treatment plants.
Pharmaceutical manufacturing faces even greater economic stakes, where membrane integrity directly impacts product purity and regulatory compliance. A single batch contamination event due to membrane failure can result in losses exceeding $1 million, not including potential market withdrawal costs and reputation damage. Sensitivity-optimized testing protocols have demonstrated 99.7% detection rates for defects as small as 3 microns, significantly reducing these risks.
The food and beverage industry similarly benefits from advanced membrane testing. Studies show that dairy processing plants implementing systematic pressure-hold testing protocols experience 27% fewer product quality incidents and 32% reduction in membrane replacement frequency. This translates to approximately $75,000-$120,000 annual savings for medium-scale operations.
Beyond direct cost savings, preventive membrane testing creates significant value through resource efficiency. Extended membrane lifespans reduce replacement frequency and associated disposal costs. Analysis of lifecycle assessment data indicates that doubling membrane service life through effective defect detection can reduce environmental impact by 35-45% while decreasing total ownership costs by 28%.
Insurance providers have begun recognizing these economic benefits, with some offering premium reductions of 5-15% for facilities demonstrating implementation of advanced membrane integrity testing protocols. This acknowledgment of risk reduction provides additional financial incentives for adoption.
The return on investment for implementing comprehensive bubble-point/pressure-hold testing systems typically ranges from 200-350% over a three-year period, with initial investments recouped within 8-14 months. These figures underscore the compelling economic case for prioritizing membrane failure prevention through advanced defect detection methodologies.
In water treatment facilities, membrane failures can lead to contamination events requiring costly system shutdowns, regulatory penalties, and potential public health crises. Research indicates that implementing comprehensive defect mapping protocols can reduce unplanned downtime by up to 40%, translating to savings of $50,000-$200,000 per incident for mid-sized treatment plants.
Pharmaceutical manufacturing faces even greater economic stakes, where membrane integrity directly impacts product purity and regulatory compliance. A single batch contamination event due to membrane failure can result in losses exceeding $1 million, not including potential market withdrawal costs and reputation damage. Sensitivity-optimized testing protocols have demonstrated 99.7% detection rates for defects as small as 3 microns, significantly reducing these risks.
The food and beverage industry similarly benefits from advanced membrane testing. Studies show that dairy processing plants implementing systematic pressure-hold testing protocols experience 27% fewer product quality incidents and 32% reduction in membrane replacement frequency. This translates to approximately $75,000-$120,000 annual savings for medium-scale operations.
Beyond direct cost savings, preventive membrane testing creates significant value through resource efficiency. Extended membrane lifespans reduce replacement frequency and associated disposal costs. Analysis of lifecycle assessment data indicates that doubling membrane service life through effective defect detection can reduce environmental impact by 35-45% while decreasing total ownership costs by 28%.
Insurance providers have begun recognizing these economic benefits, with some offering premium reductions of 5-15% for facilities demonstrating implementation of advanced membrane integrity testing protocols. This acknowledgment of risk reduction provides additional financial incentives for adoption.
The return on investment for implementing comprehensive bubble-point/pressure-hold testing systems typically ranges from 200-350% over a three-year period, with initial investments recouped within 8-14 months. These figures underscore the compelling economic case for prioritizing membrane failure prevention through advanced defect detection methodologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!