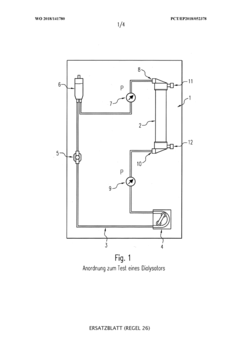
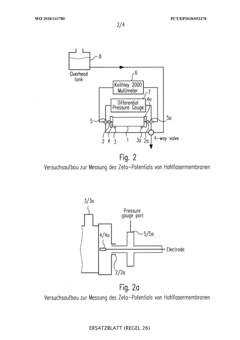
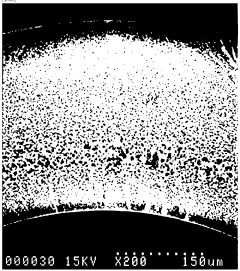
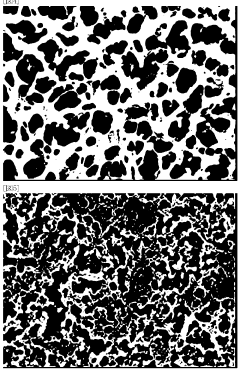
Hollow Fiber Membranes: Pharmaceutical/Food-Grade Compliance, Extractables And Sanitization
SEP 16, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hollow Fiber Membrane Technology Background and Objectives
Hollow fiber membranes represent a significant advancement in separation technology, evolving from early filtration methods to sophisticated membrane systems capable of precise molecular separation. These tubular, semi-permeable structures with diameters typically ranging from 0.5 to 3 mm have revolutionized various industries since their commercial introduction in the 1970s. The technology has progressed from cellulose-based materials to advanced polymeric compositions including polysulfone, polyethersulfone, and polyvinylidene fluoride, each offering specific advantages in different applications.
The pharmaceutical and food industries have increasingly adopted hollow fiber membrane technology due to its exceptional efficiency in separation processes, including ultrafiltration, microfiltration, and nanofiltration. These membranes enable critical operations such as protein purification, cell harvesting, and sterile filtration while maintaining product integrity. The historical trajectory shows a consistent trend toward membranes with higher selectivity, improved flow rates, and enhanced chemical resistance.
Current technological objectives focus on addressing several key challenges in pharmaceutical and food-grade applications. Primary among these is ensuring compliance with stringent regulatory standards, particularly FDA, USP, and EU regulations governing materials in contact with pharmaceuticals and food products. This necessitates the development of membranes with minimal extractables and leachables that could potentially contaminate products or interfere with bioprocessing.
Another critical objective is the advancement of sanitization protocols compatible with membrane integrity. Traditional sterilization methods often compromise membrane performance through chemical degradation or structural alteration. Research aims to develop membranes capable of withstanding repeated sanitization cycles using steam, chemical agents, or radiation without significant performance deterioration.
The industry is also pursuing innovations in membrane chemistry and structure to reduce protein adsorption and fouling, which remain significant operational challenges. This includes the development of novel surface modifications and composite materials that maintain separation efficiency while minimizing undesired interactions with process streams.
Looking forward, the technological trajectory points toward "smart" hollow fiber membranes with tailored surface properties, controlled pore architectures, and potentially responsive characteristics that adapt to processing conditions. The ultimate goal is to establish membrane technologies that combine pharmaceutical/food-grade compliance with operational excellence, providing high-throughput separation solutions while maintaining product purity and process economics.
The pharmaceutical and food industries have increasingly adopted hollow fiber membrane technology due to its exceptional efficiency in separation processes, including ultrafiltration, microfiltration, and nanofiltration. These membranes enable critical operations such as protein purification, cell harvesting, and sterile filtration while maintaining product integrity. The historical trajectory shows a consistent trend toward membranes with higher selectivity, improved flow rates, and enhanced chemical resistance.
Current technological objectives focus on addressing several key challenges in pharmaceutical and food-grade applications. Primary among these is ensuring compliance with stringent regulatory standards, particularly FDA, USP, and EU regulations governing materials in contact with pharmaceuticals and food products. This necessitates the development of membranes with minimal extractables and leachables that could potentially contaminate products or interfere with bioprocessing.
Another critical objective is the advancement of sanitization protocols compatible with membrane integrity. Traditional sterilization methods often compromise membrane performance through chemical degradation or structural alteration. Research aims to develop membranes capable of withstanding repeated sanitization cycles using steam, chemical agents, or radiation without significant performance deterioration.
The industry is also pursuing innovations in membrane chemistry and structure to reduce protein adsorption and fouling, which remain significant operational challenges. This includes the development of novel surface modifications and composite materials that maintain separation efficiency while minimizing undesired interactions with process streams.
Looking forward, the technological trajectory points toward "smart" hollow fiber membranes with tailored surface properties, controlled pore architectures, and potentially responsive characteristics that adapt to processing conditions. The ultimate goal is to establish membrane technologies that combine pharmaceutical/food-grade compliance with operational excellence, providing high-throughput separation solutions while maintaining product purity and process economics.
Market Demand Analysis for Pharmaceutical and Food-Grade Membranes
The global market for hollow fiber membranes in pharmaceutical and food industries has experienced significant growth over the past decade, driven by increasing demand for advanced filtration and separation technologies. The pharmaceutical sector, in particular, has shown robust demand due to stringent regulatory requirements for product purity and process efficiency. Current market estimates value the pharmaceutical-grade membrane market at several billion dollars, with a compound annual growth rate exceeding the general filtration market average.
In the pharmaceutical industry, hollow fiber membranes are primarily utilized in biopharmaceutical manufacturing processes, including protein purification, virus filtration, and cell harvesting. The rise of biologics and personalized medicine has substantially increased the need for high-performance, compliant membrane technologies that can ensure product safety while maximizing yield. Manufacturers are increasingly seeking membrane solutions that minimize extractables and leachables while withstanding aggressive sanitization protocols.
The food and beverage industry represents another significant market segment, where hollow fiber membranes are employed in clarification, concentration, and purification processes. Consumer demand for clean-label products with minimal processing and preservatives has accelerated the adoption of membrane filtration as an alternative to traditional thermal treatments. Dairy processing, fruit juice production, and brewing industries have emerged as key application areas within this sector.
Regulatory compliance has become a primary market driver, with manufacturers requiring membranes that meet FDA, USP Class VI, and other international standards. This has created a premium segment for membranes with comprehensive extractables profiles and validated sanitization protocols. Market research indicates that end-users are willing to pay significantly higher prices for membranes with thorough documentation and regulatory support.
Geographical analysis reveals that North America and Europe currently dominate the pharmaceutical-grade membrane market, while Asia-Pacific regions show the fastest growth rates. This growth is attributed to expanding pharmaceutical manufacturing capabilities in countries like China, India, and Singapore, coupled with increasing regulatory harmonization with Western standards.
The market is also witnessing a shift toward single-use membrane systems, particularly in pharmaceutical applications, driven by the need to reduce cross-contamination risks and cleaning validation requirements. This trend has created demand for pre-validated, ready-to-use membrane modules with comprehensive extractables data packages.
Customer feedback indicates growing interest in membranes with enhanced chemical compatibility that can withstand aggressive cleaning agents while maintaining performance characteristics. Additionally, there is increasing demand for membranes with improved fouling resistance to extend operational cycles between cleanings, thereby reducing production downtime and increasing overall process economics.
In the pharmaceutical industry, hollow fiber membranes are primarily utilized in biopharmaceutical manufacturing processes, including protein purification, virus filtration, and cell harvesting. The rise of biologics and personalized medicine has substantially increased the need for high-performance, compliant membrane technologies that can ensure product safety while maximizing yield. Manufacturers are increasingly seeking membrane solutions that minimize extractables and leachables while withstanding aggressive sanitization protocols.
The food and beverage industry represents another significant market segment, where hollow fiber membranes are employed in clarification, concentration, and purification processes. Consumer demand for clean-label products with minimal processing and preservatives has accelerated the adoption of membrane filtration as an alternative to traditional thermal treatments. Dairy processing, fruit juice production, and brewing industries have emerged as key application areas within this sector.
Regulatory compliance has become a primary market driver, with manufacturers requiring membranes that meet FDA, USP Class VI, and other international standards. This has created a premium segment for membranes with comprehensive extractables profiles and validated sanitization protocols. Market research indicates that end-users are willing to pay significantly higher prices for membranes with thorough documentation and regulatory support.
Geographical analysis reveals that North America and Europe currently dominate the pharmaceutical-grade membrane market, while Asia-Pacific regions show the fastest growth rates. This growth is attributed to expanding pharmaceutical manufacturing capabilities in countries like China, India, and Singapore, coupled with increasing regulatory harmonization with Western standards.
The market is also witnessing a shift toward single-use membrane systems, particularly in pharmaceutical applications, driven by the need to reduce cross-contamination risks and cleaning validation requirements. This trend has created demand for pre-validated, ready-to-use membrane modules with comprehensive extractables data packages.
Customer feedback indicates growing interest in membranes with enhanced chemical compatibility that can withstand aggressive cleaning agents while maintaining performance characteristics. Additionally, there is increasing demand for membranes with improved fouling resistance to extend operational cycles between cleanings, thereby reducing production downtime and increasing overall process economics.
Current Challenges in Compliance and Sanitization Technologies
The hollow fiber membrane industry faces significant challenges in meeting the stringent compliance requirements for pharmaceutical and food-grade applications. Current regulatory frameworks, including FDA, EMA, and ISO standards, demand extensive documentation and validation of membrane materials, manufacturing processes, and quality control measures. These regulations are continuously evolving, creating a moving target for manufacturers attempting to maintain compliance across global markets.
Extractables and leachables present a particularly complex challenge for hollow fiber membrane technologies. The potential migration of chemical compounds from membrane materials into process streams raises serious safety concerns, especially in pharmaceutical and food applications. Current analytical methods for identifying and quantifying these compounds often lack standardization across the industry, leading to inconsistent testing protocols and results interpretation.
Material compatibility issues further complicate compliance efforts. The interaction between membrane materials and various process fluids, cleaning agents, and sanitization chemicals can compromise membrane integrity over time. This degradation may not only affect separation performance but also increase the risk of extractables leaching into the final product. The industry currently lacks comprehensive compatibility databases that account for the wide range of chemicals used in different applications.
Sanitization technologies present another significant hurdle. Traditional methods such as steam sterilization, chemical sanitization, and hot water sanitization each come with limitations when applied to hollow fiber membranes. Steam sterilization can cause thermal damage to certain membrane polymers, while chemical sanitization may leave residues or create new extractable compounds through chemical reactions with the membrane material. Hot water sanitization, while gentler, may not achieve the microbial reduction levels required for pharmaceutical applications.
The validation of cleaning and sanitization protocols represents a substantial technical challenge. Current methodologies often struggle to demonstrate complete removal of process residues, cleaning agents, and microbial contaminants from the complex internal structures of hollow fiber membrane modules. The small diameter of hollow fibers creates flow distribution challenges that can result in inadequate sanitization of certain regions within the membrane module.
Biofilm formation on membrane surfaces remains a persistent problem despite current sanitization approaches. Microorganisms can develop protective biofilms that resist standard cleaning procedures, leading to contamination risks and reduced membrane performance. The industry lacks effective strategies for preventing biofilm formation specifically tailored to hollow fiber geometries and materials.
Cross-contamination prevention between production batches presents additional challenges, particularly in multi-product manufacturing facilities. Current changeover protocols may not adequately address the risk of product carryover in hollow fiber systems, where residual material can be trapped in dead spaces or adsorbed onto membrane surfaces.
Extractables and leachables present a particularly complex challenge for hollow fiber membrane technologies. The potential migration of chemical compounds from membrane materials into process streams raises serious safety concerns, especially in pharmaceutical and food applications. Current analytical methods for identifying and quantifying these compounds often lack standardization across the industry, leading to inconsistent testing protocols and results interpretation.
Material compatibility issues further complicate compliance efforts. The interaction between membrane materials and various process fluids, cleaning agents, and sanitization chemicals can compromise membrane integrity over time. This degradation may not only affect separation performance but also increase the risk of extractables leaching into the final product. The industry currently lacks comprehensive compatibility databases that account for the wide range of chemicals used in different applications.
Sanitization technologies present another significant hurdle. Traditional methods such as steam sterilization, chemical sanitization, and hot water sanitization each come with limitations when applied to hollow fiber membranes. Steam sterilization can cause thermal damage to certain membrane polymers, while chemical sanitization may leave residues or create new extractable compounds through chemical reactions with the membrane material. Hot water sanitization, while gentler, may not achieve the microbial reduction levels required for pharmaceutical applications.
The validation of cleaning and sanitization protocols represents a substantial technical challenge. Current methodologies often struggle to demonstrate complete removal of process residues, cleaning agents, and microbial contaminants from the complex internal structures of hollow fiber membrane modules. The small diameter of hollow fibers creates flow distribution challenges that can result in inadequate sanitization of certain regions within the membrane module.
Biofilm formation on membrane surfaces remains a persistent problem despite current sanitization approaches. Microorganisms can develop protective biofilms that resist standard cleaning procedures, leading to contamination risks and reduced membrane performance. The industry lacks effective strategies for preventing biofilm formation specifically tailored to hollow fiber geometries and materials.
Cross-contamination prevention between production batches presents additional challenges, particularly in multi-product manufacturing facilities. Current changeover protocols may not adequately address the risk of product carryover in hollow fiber systems, where residual material can be trapped in dead spaces or adsorbed onto membrane surfaces.
Current Extractables Testing and Compliance Solutions
01 Sanitization methods for hollow fiber membranes
Various sanitization methods can be applied to hollow fiber membranes to ensure their cleanliness and functionality. These methods include chemical treatments, heat sterilization, and radiation processes. Effective sanitization prevents microbial growth and contamination in membrane systems, which is crucial for applications in pharmaceuticals, water treatment, and medical devices. Proper sanitization protocols help maintain membrane integrity while eliminating potential contaminants.- Sanitization methods for hollow fiber membranes: Various sanitization methods can be applied to hollow fiber membranes to ensure their cleanliness and functionality. These methods include chemical treatments, heat sterilization, and radiation processes that effectively eliminate contaminants and microorganisms. Proper sanitization protocols help maintain membrane integrity while preventing biofouling and extending the operational lifespan of filtration systems.
- Extractables testing and characterization in membrane systems: Extractables are compounds that can leach from hollow fiber membranes under specific conditions. Testing and characterization of these extractables involves analytical methods to identify potential contaminants that might migrate from the membrane material into the filtered product. This analysis is crucial for applications in pharmaceutical, bioprocessing, and food industries where product purity is essential.
- Membrane materials with reduced extractables: Development of hollow fiber membrane materials specifically designed to minimize extractables is a key focus area. These advanced materials incorporate polymers and additives that resist leaching under various processing conditions. By reducing extractables, these membranes provide higher purity filtration while maintaining mechanical strength and separation performance, making them suitable for sensitive applications.
- Hollow fiber membrane manufacturing processes: Manufacturing processes for hollow fiber membranes significantly impact their extractables profile and sanitization requirements. Techniques such as phase inversion, solution spinning, and post-treatment processes can be optimized to create membranes with controlled porosity, uniform structure, and chemical stability. These manufacturing considerations directly influence the membrane's compatibility with sanitization agents and its tendency to release extractables.
- Compatibility of sanitization agents with membrane materials: The compatibility between sanitization agents and hollow fiber membrane materials is critical for maintaining membrane integrity while ensuring effective cleaning. Different membrane polymers exhibit varying resistance to sanitizing chemicals such as sodium hypochlorite, hydrogen peroxide, peracetic acid, and alcohols. Understanding these interactions helps in developing appropriate sanitization protocols that maximize cleaning efficacy while minimizing membrane degradation and extractables generation.
02 Extractables testing and characterization in hollow fiber membranes
Extractables are compounds that can leach from hollow fiber membrane materials under specific conditions. Testing and characterization of these extractables is essential for ensuring product safety, especially in pharmaceutical and bioprocessing applications. Methods for extractables testing include various analytical techniques such as chromatography and mass spectrometry to identify and quantify potential leachable compounds. This characterization helps in assessing the compatibility of membrane materials with process fluids and end products.Expand Specific Solutions03 Material selection for reducing extractables in hollow fiber membranes
The selection of appropriate materials for hollow fiber membrane manufacturing significantly impacts the level of extractables. Polymers with high chemical stability and low leaching potential, such as polysulfone, polyethersulfone, and certain fluoropolymers, are preferred for applications requiring minimal extractables. Material processing techniques, including washing steps and post-treatment methods, can further reduce extractable compounds. Advanced material formulations can be designed specifically to minimize leachable components while maintaining desired membrane performance characteristics.Expand Specific Solutions04 Membrane structure design for improved sanitization compatibility
The structural design of hollow fiber membranes can be optimized to enhance compatibility with sanitization processes. Features such as pore size distribution, wall thickness, and surface modifications affect how membranes respond to sanitization treatments. Membranes designed with sanitization in mind typically incorporate reinforcement elements or structural features that resist degradation during repeated cleaning cycles. Advanced manufacturing techniques can create membranes with improved chemical and thermal resistance, extending their operational lifespan under frequent sanitization conditions.Expand Specific Solutions05 Quality control and validation of sanitization processes
Quality control and validation procedures are essential for ensuring the effectiveness of sanitization processes for hollow fiber membranes. These procedures include integrity testing, microbial challenge tests, and analytical methods to verify the removal of sanitizing agents post-treatment. Validation protocols typically involve establishing acceptance criteria, documenting sanitization parameters, and performing routine monitoring. Regulatory guidelines often dictate specific validation requirements for membrane systems used in critical applications such as pharmaceutical manufacturing and medical devices.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The hollow fiber membrane market for pharmaceutical and food-grade applications is currently in a growth phase, with increasing demand driven by stringent regulatory requirements for product purity and safety. The global market size is estimated to exceed $3 billion, expanding at approximately 8-10% CAGR, fueled by biopharmaceutical manufacturing and food processing applications. Leading players demonstrate varying levels of technical maturity: established manufacturers like Asahi Kasei Medical, Toray Industries, and Fresenius Medical Care have developed advanced sanitization-resistant membranes with comprehensive extractables profiles, while Cytiva (Global Life Sciences Solutions) and 3M Innovative Properties focus on pharmaceutical-grade compliance. Emerging competitors including Kuraray, Toyobo, and Hangzhou Cobetter are rapidly advancing their technologies through university collaborations (with Zhejiang University, Donghua University) to address extractables concerns and develop improved sanitization protocols.
Toray Industries, Inc.
Technical Solution: Toray has developed TORAYFIL® hollow fiber membranes specifically designed for pharmaceutical and food applications with FDA and other regulatory compliance. Their technology employs polysulfone (PS) and polyethersulfone (PES) materials with controlled pore size distribution (0.01-0.2 μm) for precise filtration. Toray's manufacturing process includes a proprietary phase inversion technique that creates asymmetric membrane structures with thin selective layers supported by more porous substructures, optimizing both flux and retention properties. For pharmaceutical compliance, they've implemented validated cleaning protocols using sodium hydroxide, sodium hypochlorite, and hydrogen peroxide that maintain membrane integrity while achieving >6-log reduction of bacterial contaminants. Their extractables profile has been comprehensively characterized using LC-MS, GC-MS, and TOC analysis, demonstrating minimal leachables under various pH and solvent conditions. Toray's sanitization validation studies show their membranes can withstand >200 steam-in-place (SIP) cycles at 121°C without significant performance degradation.
Strengths: Superior chemical resistance to sanitizing agents, allowing for extended operational lifetime and reduced replacement costs. Comprehensive extractables documentation simplifies regulatory approval processes for pharmaceutical manufacturers. Weaknesses: Higher initial cost compared to competitors, and their membranes may require more specialized handling during installation and maintenance procedures.
Toyobo Co., Ltd.
Technical Solution: Toyobo has pioneered HOLLOSEP® hollow fiber membranes featuring a unique dual-layer structure with an ultra-thin skin layer (approximately 0.1-0.3 μm) supported by a macroporous substrate. Their technology utilizes modified polyacrylonitrile (PAN) and polyvinylidene fluoride (PVDF) materials engineered for exceptional fouling resistance in pharmaceutical and food processing applications. Toyobo's manufacturing process incorporates a specialized dry-wet spinning technique that creates highly uniform pore structures with narrow size distribution, critical for consistent filtration performance. For pharmaceutical compliance, Toyobo has developed proprietary non-toxic additives that enhance membrane hydrophilicity while meeting USP Class VI requirements. Their sanitization protocol has been validated for compatibility with peracetic acid, hydrogen peroxide, and hot water sanitization up to 85°C. Extractables testing using standardized protocols has demonstrated extremely low levels of organic and inorganic leachables, with comprehensive documentation available to support regulatory filings. Their membranes maintain structural integrity and performance characteristics after multiple sanitization cycles, with validated data showing consistent performance for over 150 cleaning cycles.
Strengths: Exceptional fouling resistance in high-protein applications, reducing cleaning frequency and extending operational runs in biopharmaceutical processing. Highly uniform pore structure ensures consistent product quality. Weaknesses: Temperature limitations during sanitization (maximum 85°C) may require alternative sterilization approaches for some applications, and their specialized materials may limit compatibility with certain aggressive cleaning chemicals.
Critical Patents and Innovations in Membrane Materials
Hollow-fibre membrane with improved biocompatibility and reduced elution of hydrophilic polymers
PatentWO2018141780A1
Innovation
- A method for producing hollow-fiber membranes involves introducing a non-water-soluble antioxidant, such as α-tocopherol, into the spinning mass and using a coagulant with a higher molecular weight polyvinylpyrrolidone, which reduces the elution of hydrophilic polymers and enhances biocompatibility by improving the hydrophilicity of the membrane surface.
Hollow fiber membrane for treating liquids
PatentWO2009051168A1
Innovation
- A hollow fiber membrane with a dense layer on both the inner and outer surfaces, featuring a porosity structure that increases from the inner surface to the outer surface and then decreases, providing enhanced filtration stability and recoverability by preventing cake layer formation and facilitating effective backwashing.
Regulatory Framework for Pharmaceutical and Food Contact Materials
The regulatory landscape governing hollow fiber membranes in pharmaceutical and food applications is complex and multifaceted, requiring compliance with stringent standards across different jurisdictions. In the United States, the Food and Drug Administration (FDA) oversees these materials through various regulatory frameworks, including 21 CFR 177.2910 for food contact applications and 21 CFR 210/211 for pharmaceutical manufacturing. These regulations establish specific requirements for material composition, manufacturing processes, and validation protocols.
The European Union implements a more comprehensive approach through the European Medicines Agency (EMA) guidelines and the EU Food Contact Materials Regulation (EC) No 1935/2004. These frameworks emphasize the need for thorough risk assessment and documentation of extractables and leachables profiles, particularly for materials used in critical applications such as pharmaceutical filtration or food processing.
International harmonization efforts are led by organizations such as the International Conference on Harmonisation (ICH) and ISO, which have developed standards like ISO 10993 for biocompatibility assessment and ISO 13408 for aseptic processing. These standards provide globally recognized methodologies for evaluating material safety and performance in regulated applications.
Specific to hollow fiber membranes, regulatory bodies require comprehensive extractables studies following protocols such as those outlined in USP <665> and <1665>. These studies must identify potential leachable compounds under various processing conditions, including exposure to different solvents, pH levels, and temperatures that simulate actual use conditions.
Sanitization validation represents another critical regulatory requirement, with authorities mandating documented evidence that cleaning and sanitization procedures effectively remove process residues, potential contaminants, and microbial contamination without compromising membrane integrity or introducing harmful substances.
Material qualification procedures typically involve a hierarchical approach beginning with raw material certification (often requiring compliance with pharmacopeial standards such as USP, EP, or JP), followed by process validation and final product testing. For pharmaceutical applications, this often includes bacterial endotoxin testing per USP <85> and particulate matter analysis according to USP <788>.
Recent regulatory trends indicate increasing scrutiny of previously overlooked extractables, particularly per- and polyfluoroalkyl substances (PFAS) and nanoplastics. Regulatory agencies worldwide are implementing more stringent requirements for analytical characterization and risk assessment of these compounds, necessitating more sophisticated testing methodologies and comprehensive documentation.
Compliance strategies for membrane manufacturers must therefore incorporate robust quality systems, detailed material characterization, comprehensive validation protocols, and thorough documentation practices to navigate this complex regulatory environment successfully.
The European Union implements a more comprehensive approach through the European Medicines Agency (EMA) guidelines and the EU Food Contact Materials Regulation (EC) No 1935/2004. These frameworks emphasize the need for thorough risk assessment and documentation of extractables and leachables profiles, particularly for materials used in critical applications such as pharmaceutical filtration or food processing.
International harmonization efforts are led by organizations such as the International Conference on Harmonisation (ICH) and ISO, which have developed standards like ISO 10993 for biocompatibility assessment and ISO 13408 for aseptic processing. These standards provide globally recognized methodologies for evaluating material safety and performance in regulated applications.
Specific to hollow fiber membranes, regulatory bodies require comprehensive extractables studies following protocols such as those outlined in USP <665> and <1665>. These studies must identify potential leachable compounds under various processing conditions, including exposure to different solvents, pH levels, and temperatures that simulate actual use conditions.
Sanitization validation represents another critical regulatory requirement, with authorities mandating documented evidence that cleaning and sanitization procedures effectively remove process residues, potential contaminants, and microbial contamination without compromising membrane integrity or introducing harmful substances.
Material qualification procedures typically involve a hierarchical approach beginning with raw material certification (often requiring compliance with pharmacopeial standards such as USP, EP, or JP), followed by process validation and final product testing. For pharmaceutical applications, this often includes bacterial endotoxin testing per USP <85> and particulate matter analysis according to USP <788>.
Recent regulatory trends indicate increasing scrutiny of previously overlooked extractables, particularly per- and polyfluoroalkyl substances (PFAS) and nanoplastics. Regulatory agencies worldwide are implementing more stringent requirements for analytical characterization and risk assessment of these compounds, necessitating more sophisticated testing methodologies and comprehensive documentation.
Compliance strategies for membrane manufacturers must therefore incorporate robust quality systems, detailed material characterization, comprehensive validation protocols, and thorough documentation practices to navigate this complex regulatory environment successfully.
Risk Assessment Methodologies for Extractables and Leachables
Risk assessment for extractables and leachables in hollow fiber membranes requires systematic methodologies to ensure pharmaceutical and food-grade compliance. The most widely adopted approach is the phased risk assessment framework, which begins with material characterization and progresses through increasingly detailed analytical evaluations based on initial risk profiles.
Material-based risk assessment represents the foundational tier, where membrane polymers and manufacturing additives are cataloged and evaluated against existing toxicological databases. This approach utilizes chemical structure analysis to predict potential extractable compounds, with polyethersulfone (PES), polyvinylidene fluoride (PVDF), and cellulose-based membranes typically presenting different extractable profiles requiring tailored assessment strategies.
Extraction studies form the next critical component, employing model solvents that simulate or exaggerate process conditions. The industry standard involves multiple extraction protocols: aggressive solvent extraction to identify worst-case scenarios, process-specific extraction mimicking actual manufacturing conditions, and time-based extraction studies to evaluate cumulative effects. These studies typically employ a combination of polar and non-polar solvents at elevated temperatures to ensure comprehensive coverage of potential extractables.
Analytical threshold determination represents a pivotal methodology element, establishing the Safety Concern Threshold (SCT) and Analytical Evaluation Threshold (AET) based on dosage, exposure routes, and duration. For pharmaceutical applications, thresholds typically follow FDA and EMA guidelines, while food applications reference specific migration limits established by food safety authorities. The calculation incorporates daily exposure estimates, converting these into concentration limits for analytical detection requirements.
Toxicological evaluation methodologies have evolved significantly, with Threshold of Toxicological Concern (TTC) approaches gaining regulatory acceptance for compounds below certain exposure thresholds. For identified compounds above these thresholds, structure-activity relationship (SAR) assessments and compound-specific toxicological evaluations are conducted, often utilizing databases like Derek Nexus and Toxtree for preliminary screening.
Statistical approaches to risk assessment have become increasingly sophisticated, employing Monte Carlo simulations to model variability in extraction conditions and analytical measurements. These probabilistic models help quantify uncertainty in extractable profiles and establish confidence intervals for leachable predictions, enabling more informed decision-making regarding membrane selection and process parameters.
Material-based risk assessment represents the foundational tier, where membrane polymers and manufacturing additives are cataloged and evaluated against existing toxicological databases. This approach utilizes chemical structure analysis to predict potential extractable compounds, with polyethersulfone (PES), polyvinylidene fluoride (PVDF), and cellulose-based membranes typically presenting different extractable profiles requiring tailored assessment strategies.
Extraction studies form the next critical component, employing model solvents that simulate or exaggerate process conditions. The industry standard involves multiple extraction protocols: aggressive solvent extraction to identify worst-case scenarios, process-specific extraction mimicking actual manufacturing conditions, and time-based extraction studies to evaluate cumulative effects. These studies typically employ a combination of polar and non-polar solvents at elevated temperatures to ensure comprehensive coverage of potential extractables.
Analytical threshold determination represents a pivotal methodology element, establishing the Safety Concern Threshold (SCT) and Analytical Evaluation Threshold (AET) based on dosage, exposure routes, and duration. For pharmaceutical applications, thresholds typically follow FDA and EMA guidelines, while food applications reference specific migration limits established by food safety authorities. The calculation incorporates daily exposure estimates, converting these into concentration limits for analytical detection requirements.
Toxicological evaluation methodologies have evolved significantly, with Threshold of Toxicological Concern (TTC) approaches gaining regulatory acceptance for compounds below certain exposure thresholds. For identified compounds above these thresholds, structure-activity relationship (SAR) assessments and compound-specific toxicological evaluations are conducted, often utilizing databases like Derek Nexus and Toxtree for preliminary screening.
Statistical approaches to risk assessment have become increasingly sophisticated, employing Monte Carlo simulations to model variability in extraction conditions and analytical measurements. These probabilistic models help quantify uncertainty in extractable profiles and establish confidence intervals for leachable predictions, enabling more informed decision-making regarding membrane selection and process parameters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!