How Hollow Fiber Membranes Are Qualified With Bubble-Point/Integrity Acceptance Across Lines?
SEP 16, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hollow Fiber Membrane Technology Background and Objectives
Hollow fiber membrane technology has evolved significantly over the past five decades since its initial development in the 1960s. These semi-permeable barriers, characterized by their tubular structure with diameters typically ranging from 0.5 to 3.0 mm, have revolutionized separation processes across multiple industries. The technology leverages the principle of selective permeation, where specific molecules can pass through while others are retained, based on size exclusion, diffusion rates, or charge interactions.
The historical trajectory shows a steady progression from rudimentary designs with limited selectivity to today's sophisticated membranes with precisely engineered pore sizes and surface properties. Early applications focused primarily on hemodialysis, but the technology quickly expanded into water purification, gas separation, and biopharmaceutical processing. This diversification has driven continuous innovation in membrane materials, from cellulose acetate to advanced polymers like polysulfone, PVDF, and PES, each offering specific advantages in mechanical strength, chemical resistance, and separation efficiency.
Current market trends indicate accelerating adoption of hollow fiber membrane technology, particularly in biopharmaceutical manufacturing where product purity requirements are increasingly stringent. The bubble-point/integrity testing methodology has emerged as the gold standard for quality assurance across production lines, ensuring membrane consistency and performance reliability. This non-destructive testing approach measures the pressure required to force air through water-filled pores, providing critical data on membrane integrity and filtration capabilities.
The primary objective of hollow fiber membrane qualification via bubble-point testing is to establish standardized protocols that ensure consistent performance across manufacturing lines while maintaining compliance with regulatory requirements. This includes developing precise acceptance criteria that can accurately predict membrane filtration efficiency and detect potential defects before deployment in critical applications.
Technical challenges persist in achieving uniform testing conditions across different production scales and environments. Variables such as temperature fluctuations, wetting agent concentrations, and pressure application rates can significantly impact test results, necessitating robust standardization efforts. Additionally, correlating bubble-point measurements with actual filtration performance remains an area requiring further research to enhance predictive capabilities.
The industry aims to advance toward automated, in-line integrity testing systems that can provide real-time quality assurance while minimizing human intervention and contamination risks. This evolution aligns with broader trends toward continuous manufacturing processes in regulated industries, where consistent quality verification is paramount for operational efficiency and regulatory compliance.
The historical trajectory shows a steady progression from rudimentary designs with limited selectivity to today's sophisticated membranes with precisely engineered pore sizes and surface properties. Early applications focused primarily on hemodialysis, but the technology quickly expanded into water purification, gas separation, and biopharmaceutical processing. This diversification has driven continuous innovation in membrane materials, from cellulose acetate to advanced polymers like polysulfone, PVDF, and PES, each offering specific advantages in mechanical strength, chemical resistance, and separation efficiency.
Current market trends indicate accelerating adoption of hollow fiber membrane technology, particularly in biopharmaceutical manufacturing where product purity requirements are increasingly stringent. The bubble-point/integrity testing methodology has emerged as the gold standard for quality assurance across production lines, ensuring membrane consistency and performance reliability. This non-destructive testing approach measures the pressure required to force air through water-filled pores, providing critical data on membrane integrity and filtration capabilities.
The primary objective of hollow fiber membrane qualification via bubble-point testing is to establish standardized protocols that ensure consistent performance across manufacturing lines while maintaining compliance with regulatory requirements. This includes developing precise acceptance criteria that can accurately predict membrane filtration efficiency and detect potential defects before deployment in critical applications.
Technical challenges persist in achieving uniform testing conditions across different production scales and environments. Variables such as temperature fluctuations, wetting agent concentrations, and pressure application rates can significantly impact test results, necessitating robust standardization efforts. Additionally, correlating bubble-point measurements with actual filtration performance remains an area requiring further research to enhance predictive capabilities.
The industry aims to advance toward automated, in-line integrity testing systems that can provide real-time quality assurance while minimizing human intervention and contamination risks. This evolution aligns with broader trends toward continuous manufacturing processes in regulated industries, where consistent quality verification is paramount for operational efficiency and regulatory compliance.
Market Analysis of Hollow Fiber Membrane Applications
The hollow fiber membrane market has experienced significant growth over the past decade, driven primarily by increasing applications in water treatment, pharmaceuticals, and bioprocessing industries. The global hollow fiber membrane market was valued at approximately 5.2 billion USD in 2022 and is projected to reach 8.7 billion USD by 2028, growing at a CAGR of around 9% during the forecast period.
Water treatment applications currently dominate the market share, accounting for nearly 45% of the total hollow fiber membrane demand. This is attributed to growing water scarcity concerns and stringent regulations regarding water quality across developed and developing nations. The pharmaceutical and biotechnology sectors follow closely, representing about 30% of the market, where hollow fiber membranes are extensively used in drug purification, protein separation, and cell culture applications.
Geographically, North America and Europe hold the largest market shares at approximately 32% and 28% respectively, due to advanced healthcare infrastructure and strict environmental regulations. However, the Asia-Pacific region is witnessing the fastest growth rate at 11.5% annually, driven by rapid industrialization, increasing healthcare expenditure, and growing awareness about water purification technologies in countries like China, India, and Japan.
The bubble-point/integrity testing segment specifically represents a critical component of the quality assurance market for hollow fiber membranes, valued at approximately 380 million USD globally. This testing methodology ensures membrane reliability across various applications, particularly in industries where membrane failure could lead to contamination or process inefficiencies.
Key market drivers include increasing demand for quality assurance in pharmaceutical manufacturing, growing adoption of single-use technologies in bioprocessing, and rising emphasis on water reuse and recycling initiatives. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of reliable filtration systems in vaccine production and medical applications.
Market challenges include high initial investment costs for advanced testing equipment, technical complexity requiring specialized training, and varying regulatory standards across different regions. Additionally, the lack of standardization in bubble-point testing protocols across different manufacturing lines creates market fragmentation and potential barriers to entry for smaller manufacturers.
Emerging trends include the integration of automated integrity testing systems, development of non-destructive testing methods, and increasing demand for real-time monitoring solutions that can be implemented across multiple production lines while maintaining consistent quality standards.
Water treatment applications currently dominate the market share, accounting for nearly 45% of the total hollow fiber membrane demand. This is attributed to growing water scarcity concerns and stringent regulations regarding water quality across developed and developing nations. The pharmaceutical and biotechnology sectors follow closely, representing about 30% of the market, where hollow fiber membranes are extensively used in drug purification, protein separation, and cell culture applications.
Geographically, North America and Europe hold the largest market shares at approximately 32% and 28% respectively, due to advanced healthcare infrastructure and strict environmental regulations. However, the Asia-Pacific region is witnessing the fastest growth rate at 11.5% annually, driven by rapid industrialization, increasing healthcare expenditure, and growing awareness about water purification technologies in countries like China, India, and Japan.
The bubble-point/integrity testing segment specifically represents a critical component of the quality assurance market for hollow fiber membranes, valued at approximately 380 million USD globally. This testing methodology ensures membrane reliability across various applications, particularly in industries where membrane failure could lead to contamination or process inefficiencies.
Key market drivers include increasing demand for quality assurance in pharmaceutical manufacturing, growing adoption of single-use technologies in bioprocessing, and rising emphasis on water reuse and recycling initiatives. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of reliable filtration systems in vaccine production and medical applications.
Market challenges include high initial investment costs for advanced testing equipment, technical complexity requiring specialized training, and varying regulatory standards across different regions. Additionally, the lack of standardization in bubble-point testing protocols across different manufacturing lines creates market fragmentation and potential barriers to entry for smaller manufacturers.
Emerging trends include the integration of automated integrity testing systems, development of non-destructive testing methods, and increasing demand for real-time monitoring solutions that can be implemented across multiple production lines while maintaining consistent quality standards.
Current Challenges in Membrane Integrity Testing
Despite significant advancements in hollow fiber membrane technology, membrane integrity testing continues to face several critical challenges that impact quality control processes across production lines. The bubble-point/integrity testing method, while widely adopted, encounters substantial obstacles in standardization and reliability across different manufacturing environments.
One primary challenge is the variability in test parameters across different production lines. Manufacturers often struggle to establish consistent bubble-point pressure thresholds that accurately reflect membrane integrity while accommodating variations in membrane composition, pore size distribution, and structural characteristics. This inconsistency leads to difficulties in comparing test results between different production batches or facilities.
The sensitivity of bubble-point testing presents another significant hurdle. Environmental factors such as temperature fluctuations, humidity levels, and testing fluid properties can substantially influence test outcomes. Even minor variations in these conditions can lead to false positives or negatives, compromising the reliability of integrity assessments and potentially allowing defective membranes to pass quality control.
Automation integration remains problematic in many manufacturing settings. While automated bubble-point testing systems offer improved precision and reduced operator dependency, their implementation across multiple production lines requires substantial investment and technical expertise. The calibration and validation of these automated systems across different manufacturing environments present ongoing challenges for quality assurance teams.
Non-destructive testing limitations constitute another major concern. Current bubble-point methods often require test samples to be sacrificed, reducing production efficiency and increasing costs. Developing non-destructive testing protocols that maintain accuracy while preserving the tested membranes remains an industry-wide challenge.
Data interpretation complexity further complicates integrity testing processes. The correlation between bubble-point measurements and actual membrane performance in field applications is not always straightforward. Quality control engineers frequently struggle to translate test results into meaningful predictions about membrane filtration efficiency, selectivity, and longevity under various operating conditions.
Regulatory compliance adds another layer of complexity. Different regions and applications may have varying standards for membrane integrity, requiring manufacturers to adapt testing protocols to meet diverse regulatory requirements while maintaining consistent quality across production lines. This regulatory diversity creates significant challenges for companies operating in global markets with multiple production facilities.
One primary challenge is the variability in test parameters across different production lines. Manufacturers often struggle to establish consistent bubble-point pressure thresholds that accurately reflect membrane integrity while accommodating variations in membrane composition, pore size distribution, and structural characteristics. This inconsistency leads to difficulties in comparing test results between different production batches or facilities.
The sensitivity of bubble-point testing presents another significant hurdle. Environmental factors such as temperature fluctuations, humidity levels, and testing fluid properties can substantially influence test outcomes. Even minor variations in these conditions can lead to false positives or negatives, compromising the reliability of integrity assessments and potentially allowing defective membranes to pass quality control.
Automation integration remains problematic in many manufacturing settings. While automated bubble-point testing systems offer improved precision and reduced operator dependency, their implementation across multiple production lines requires substantial investment and technical expertise. The calibration and validation of these automated systems across different manufacturing environments present ongoing challenges for quality assurance teams.
Non-destructive testing limitations constitute another major concern. Current bubble-point methods often require test samples to be sacrificed, reducing production efficiency and increasing costs. Developing non-destructive testing protocols that maintain accuracy while preserving the tested membranes remains an industry-wide challenge.
Data interpretation complexity further complicates integrity testing processes. The correlation between bubble-point measurements and actual membrane performance in field applications is not always straightforward. Quality control engineers frequently struggle to translate test results into meaningful predictions about membrane filtration efficiency, selectivity, and longevity under various operating conditions.
Regulatory compliance adds another layer of complexity. Different regions and applications may have varying standards for membrane integrity, requiring manufacturers to adapt testing protocols to meet diverse regulatory requirements while maintaining consistent quality across production lines. This regulatory diversity creates significant challenges for companies operating in global markets with multiple production facilities.
Bubble-Point Testing Protocols and Implementation
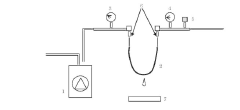
01 Bubble-point testing methods for hollow fiber membranes
Bubble-point testing is a critical method for evaluating the integrity of hollow fiber membranes. This non-destructive test involves applying gas pressure to one side of a wetted membrane until bubbles form at the largest pores, indicating the minimum pressure required to overcome surface tension. The test helps determine pore size distribution and identify defects that could compromise filtration performance. Advanced testing protocols include automated pressure ramping and precise detection systems to ensure accurate and reproducible results.- Bubble-point testing methods for hollow fiber membranes: Bubble-point testing is a critical method for evaluating the integrity of hollow fiber membranes. This technique involves applying gas pressure to one side of a wetted membrane until bubbles form at the largest pores, indicating the minimum pressure required to overcome surface tension. The test provides quantitative data on membrane pore size distribution and integrity, which is essential for quality control in manufacturing processes. Advanced testing protocols may include automated pressure ramping and precise detection systems to ensure accurate and reproducible results.
- Integrity acceptance criteria for membrane filtration systems: Establishing appropriate integrity acceptance criteria is crucial for ensuring the performance of hollow fiber membrane systems. These criteria typically include minimum bubble-point pressure values, maximum allowable pressure decay rates, and diffusive flow limits. The acceptance standards are determined based on the membrane's intended application, such as pharmaceutical processing, water purification, or bioprocessing. Regulatory compliance often requires documentation of integrity test results against predetermined acceptance criteria to validate that membrane systems maintain their filtration capabilities and prevent contamination.
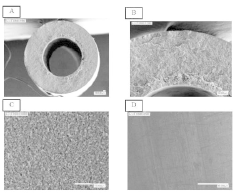
- Manufacturing techniques affecting membrane integrity: The manufacturing process significantly impacts the integrity and bubble-point characteristics of hollow fiber membranes. Key factors include polymer composition, spinning conditions, coagulation bath chemistry, and post-treatment procedures. Techniques such as controlled phase inversion, precise fiber drawing, and optimized annealing can enhance pore uniformity and mechanical strength. Advanced manufacturing methods may incorporate additives or surface modifications to improve membrane performance while maintaining consistent integrity test results. Quality control during production involves continuous monitoring of process parameters to ensure membranes meet predetermined bubble-point specifications.
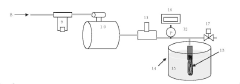
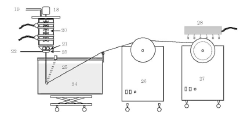
- Automated integrity testing systems: Automated systems for integrity testing of hollow fiber membranes provide enhanced accuracy, reproducibility, and efficiency compared to manual methods. These systems typically incorporate computerized pressure control, flow measurement, and data analysis capabilities. Features may include programmable test sequences, real-time monitoring, automatic pass/fail determination based on predefined acceptance criteria, and comprehensive data logging for regulatory compliance. Advanced systems may also integrate multiple testing methodologies, such as bubble point, pressure hold, and diffusive flow tests, to provide comprehensive membrane characterization.
- Correlation between bubble-point and filtration performance: The bubble-point value of hollow fiber membranes directly correlates with their filtration performance characteristics. Higher bubble-point pressures generally indicate smaller maximum pore sizes, which translate to better retention of particles and microorganisms. Research has established relationships between bubble-point measurements and critical performance parameters such as log reduction values for bacteria, virus retention capabilities, and filtrate clarity. Understanding these correlations allows manufacturers to use bubble-point testing as a non-destructive predictor of membrane performance in various applications, from water treatment to pharmaceutical processing.
02 Integrity acceptance criteria for membrane filtration systems
Establishing appropriate integrity acceptance criteria is essential for quality control of hollow fiber membrane systems. These criteria typically include minimum bubble-point pressure values that correspond to specific pore size requirements and filtration ratings. Acceptance standards may vary based on the membrane's intended application, with more stringent criteria for critical applications like pharmaceutical or medical filtration. Standardized testing protocols ensure that membranes meet predetermined specifications before deployment, with documentation requirements for regulatory compliance.Expand Specific Solutions03 Manufacturing techniques affecting membrane integrity
The manufacturing process significantly impacts the integrity and bubble-point characteristics of hollow fiber membranes. Key factors include polymer composition, spinning conditions, coagulation parameters, and post-treatment processes. Techniques such as controlled phase inversion, precise temperature regulation during extrusion, and specialized coating methods can enhance pore uniformity and mechanical strength. Advanced manufacturing approaches focus on creating consistent membrane structures with predictable bubble-point values and minimal defects to ensure reliable filtration performance.Expand Specific Solutions04 Automated integrity testing systems for quality control
Automated systems for integrity testing of hollow fiber membranes provide efficient and reliable quality control in production environments. These systems incorporate computerized pressure control, flow measurement, and data analysis capabilities to perform consistent bubble-point testing. Real-time monitoring allows for immediate detection of membranes that fail to meet acceptance criteria, reducing waste and ensuring product quality. Advanced systems may include image analysis of bubble formation patterns and automated documentation for regulatory compliance purposes.Expand Specific Solutions05 Correlation between bubble-point and filtration performance
The bubble-point value of hollow fiber membranes directly correlates with their filtration performance characteristics. Higher bubble-point pressures generally indicate smaller maximum pore sizes and better retention of particles and microorganisms. Research has established relationships between bubble-point measurements and critical performance parameters such as log reduction values for bacteria and viruses, particle retention efficiency, and flow rates. Understanding these correlations allows manufacturers to use bubble-point testing as a predictive tool for membrane performance in various applications.Expand Specific Solutions
Leading Manufacturers and Industry Standards
The hollow fiber membrane market is currently in a growth phase, driven by increasing applications in water treatment, healthcare, and industrial processes. The global market size is estimated to exceed $5 billion, with projected annual growth of 8-10%. Leading players include established Japanese corporations like Toray Industries, Asahi Kasei, and Toyobo, who possess advanced manufacturing capabilities and extensive patent portfolios. Western companies such as Pall Corporation and SeaStar Medical focus on specialized medical applications, while emerging Asian players like Beijing Bishuiyuan and Hainan Litree are rapidly gaining market share through cost-competitive offerings. Technical qualification standards vary across regions, with bubble-point/integrity testing becoming the industry standard for quality assurance across production lines, ensuring consistent membrane performance and reliability.
Toray Industries, Inc.
Technical Solution: Toray Industries has developed a comprehensive hollow fiber membrane qualification system that centers on automated multi-point bubble testing integrated across their global production facilities. Their approach employs a standardized testing protocol where precisely controlled gas pressure is applied to wetted membrane modules while monitoring for the first appearance of bubbles through both visual and sensor-based detection methods. Toray's system incorporates automated pressure ramping with high-precision digital pressure monitoring capable of detecting pressure changes as small as 0.002 bar. Their qualification protocol includes testing at multiple points along each production batch with statistical process control to identify trends before they result in quality issues. Toray has established correlation models between bubble point measurements and actual filtration performance metrics such as bacterial retention, allowing them to set science-based acceptance criteria. Their system includes automated documentation and traceability features that link test results to specific production batches, enabling comprehensive quality assurance across their manufacturing network.
Strengths: Globally standardized testing protocols ensure consistency across multiple production facilities; high-precision measurement systems provide reliable quality assessment; established correlation between bubble point and filtration performance enables science-based acceptance criteria. Weaknesses: Extensive testing infrastructure requires significant capital investment; system complexity necessitates specialized technical support; conservative acceptance criteria may impact production yields.
Toyobo Co., Ltd.
Technical Solution: Toyobo has implemented an advanced hollow fiber membrane qualification system based on automated bubble point testing integrated with comprehensive data analytics. Their approach utilizes a multi-stage integrity testing protocol where membranes undergo both non-destructive bubble point testing and selective destructive testing to validate correlation models. Toyobo's system employs precision-controlled pressure application with digital monitoring systems that can detect pressure changes as small as 0.003 bar while simultaneously measuring gas flow rates through the membrane. Their qualification protocol includes testing at standardized intervals throughout production with automated sampling systems that maintain testing consistency. Toyobo has developed proprietary software that analyzes test data across production batches to identify subtle trends and correlations between manufacturing parameters and bubble point results. This allows for predictive quality control and continuous process improvement. Their system includes automated documentation with electronic signatures that comply with pharmaceutical industry requirements for GMP (Good Manufacturing Practice).
Strengths: Integration of bubble point testing with comprehensive data analytics enables predictive quality control; automated documentation system meets pharmaceutical industry compliance requirements; established correlation models between test results and filtration performance. Weaknesses: Complex data analytics require specialized expertise to interpret results; system validation requires extensive initial testing; higher initial implementation costs compared to simpler testing approaches.
Critical Patents in Membrane Integrity Verification
Polyamide hollow fiber membrane
PatentActiveJP2016068005A
Innovation
- A polyamide hollow fiber membrane produced via a thermally induced phase separation method, incorporating a polyamide resin with a fatty acid amide in the polymer solution, achieves high bubble point and water permeability by controlling pore size and enhancing structural strength.
Integrity test method of hollow fiber membrane module
PatentActiveJP2018196861A
Innovation
- A method involving repeated cycles of air pressure and water flow under pressure to fill hollow fiber membrane pores, ensuring complete water filling and accurate defect detection in a short time without complex operations.
Regulatory Compliance for Membrane Manufacturing
Regulatory compliance forms the cornerstone of membrane manufacturing processes, particularly for hollow fiber membranes used in critical applications such as pharmaceutical filtration, medical devices, and water purification systems. The regulatory landscape governing these manufacturing processes is complex and multifaceted, encompassing standards from various international bodies including the FDA, EMA, ISO, and ASTM International.
For hollow fiber membrane qualification using bubble-point/integrity testing, manufacturers must adhere to FDA's 21 CFR Part 210/211 for pharmaceutical applications, which mandates consistent quality control procedures across production lines. The FDA guidance document "Sterile Drug Products Produced by Aseptic Processing" specifically addresses integrity testing requirements for filtration systems, emphasizing the need for validated bubble-point tests as a non-destructive method to verify membrane integrity.
The European Medicines Agency (EMA) provides complementary guidelines through EudraLex Volume 4, particularly Annex 1, which outlines requirements for sterile medicinal products. These regulations require documented evidence that membrane integrity testing procedures are standardized across manufacturing lines, with clear acceptance criteria established for bubble-point values.
ISO 13408-2 specifically addresses aseptic processing of health care products and provides detailed requirements for filtration processes, including integrity testing methodologies. This standard requires manufacturers to establish scientifically sound acceptance criteria for bubble-point tests and maintain consistency across production lines.
Compliance with these regulations necessitates robust documentation systems. Manufacturers must maintain detailed records of bubble-point test procedures, calibration of testing equipment, training of personnel, and validation studies demonstrating the correlation between bubble-point values and bacterial retention capabilities. These records are subject to regulatory inspection and must demonstrate consistency across different manufacturing lines.
Risk management principles, as outlined in ICH Q9, must be incorporated into the qualification process. Manufacturers need to identify potential failure modes in the integrity testing process and implement appropriate control strategies. This includes establishing appropriate sampling plans, defining acceptance criteria based on statistical analysis, and implementing corrective and preventive action (CAPA) systems for addressing deviations.
The qualification of new manufacturing lines requires comprehensive validation protocols that demonstrate equivalence to established lines. This typically involves comparative studies of bubble-point values across different production lines, with statistical analysis to establish acceptance ranges that ensure consistent product quality while accommodating normal manufacturing variability.
For hollow fiber membrane qualification using bubble-point/integrity testing, manufacturers must adhere to FDA's 21 CFR Part 210/211 for pharmaceutical applications, which mandates consistent quality control procedures across production lines. The FDA guidance document "Sterile Drug Products Produced by Aseptic Processing" specifically addresses integrity testing requirements for filtration systems, emphasizing the need for validated bubble-point tests as a non-destructive method to verify membrane integrity.
The European Medicines Agency (EMA) provides complementary guidelines through EudraLex Volume 4, particularly Annex 1, which outlines requirements for sterile medicinal products. These regulations require documented evidence that membrane integrity testing procedures are standardized across manufacturing lines, with clear acceptance criteria established for bubble-point values.
ISO 13408-2 specifically addresses aseptic processing of health care products and provides detailed requirements for filtration processes, including integrity testing methodologies. This standard requires manufacturers to establish scientifically sound acceptance criteria for bubble-point tests and maintain consistency across production lines.
Compliance with these regulations necessitates robust documentation systems. Manufacturers must maintain detailed records of bubble-point test procedures, calibration of testing equipment, training of personnel, and validation studies demonstrating the correlation between bubble-point values and bacterial retention capabilities. These records are subject to regulatory inspection and must demonstrate consistency across different manufacturing lines.
Risk management principles, as outlined in ICH Q9, must be incorporated into the qualification process. Manufacturers need to identify potential failure modes in the integrity testing process and implement appropriate control strategies. This includes establishing appropriate sampling plans, defining acceptance criteria based on statistical analysis, and implementing corrective and preventive action (CAPA) systems for addressing deviations.
The qualification of new manufacturing lines requires comprehensive validation protocols that demonstrate equivalence to established lines. This typically involves comparative studies of bubble-point values across different production lines, with statistical analysis to establish acceptance ranges that ensure consistent product quality while accommodating normal manufacturing variability.
Quality Control Systems Integration
The integration of quality control systems for hollow fiber membrane qualification represents a critical aspect of manufacturing excellence in filtration technology. Modern production facilities require sophisticated integration of bubble-point and integrity testing systems across multiple production lines to ensure consistent product quality and regulatory compliance. This integration encompasses hardware connectivity, software platforms, data management systems, and procedural standardization.
Automated testing equipment for bubble-point and integrity testing must be networked through industrial control systems that allow centralized monitoring and data collection. These systems typically employ programmable logic controllers (PLCs) connected to supervisory control and data acquisition (SCADA) networks, enabling real-time monitoring of testing parameters across multiple production lines. The integration architecture generally follows a hierarchical model with device-level controls feeding into line-level systems, which ultimately connect to plant-wide quality management platforms.
Data standardization represents a fundamental challenge in quality control systems integration. Manufacturers must establish uniform data formats and testing protocols to ensure that integrity test results from different production lines can be meaningfully compared and analyzed. This standardization extends to measurement units, acceptance criteria thresholds, and testing conditions such as temperature and pressure parameters that may affect bubble-point measurements.
Manufacturing execution systems (MES) serve as the backbone for quality control integration, providing the middleware layer that connects shop floor testing equipment with enterprise resource planning (ERP) systems. These MES platforms enable automated documentation of test results, statistical process control (SPC) implementation, and electronic batch record generation that satisfies regulatory requirements for traceability in industries such as pharmaceuticals and medical devices.
Cloud-based quality management solutions are increasingly being deployed to facilitate multi-site integration of hollow fiber membrane qualification processes. These platforms enable global standardization of testing procedures while accommodating site-specific variations in equipment configurations. Advanced analytics capabilities within these systems allow for trend analysis across production lines, facilitating early detection of quality drift before specifications are breached.
Integration challenges frequently arise from equipment heterogeneity across production lines, particularly in facilities that have expanded over time. Legacy testing systems may utilize proprietary communication protocols that require custom interface development to connect with modern quality management platforms. Manufacturers often implement middleware solutions and API-based integration approaches to bridge these technological gaps while maintaining validation status of existing systems.
Automated testing equipment for bubble-point and integrity testing must be networked through industrial control systems that allow centralized monitoring and data collection. These systems typically employ programmable logic controllers (PLCs) connected to supervisory control and data acquisition (SCADA) networks, enabling real-time monitoring of testing parameters across multiple production lines. The integration architecture generally follows a hierarchical model with device-level controls feeding into line-level systems, which ultimately connect to plant-wide quality management platforms.
Data standardization represents a fundamental challenge in quality control systems integration. Manufacturers must establish uniform data formats and testing protocols to ensure that integrity test results from different production lines can be meaningfully compared and analyzed. This standardization extends to measurement units, acceptance criteria thresholds, and testing conditions such as temperature and pressure parameters that may affect bubble-point measurements.
Manufacturing execution systems (MES) serve as the backbone for quality control integration, providing the middleware layer that connects shop floor testing equipment with enterprise resource planning (ERP) systems. These MES platforms enable automated documentation of test results, statistical process control (SPC) implementation, and electronic batch record generation that satisfies regulatory requirements for traceability in industries such as pharmaceuticals and medical devices.
Cloud-based quality management solutions are increasingly being deployed to facilitate multi-site integration of hollow fiber membrane qualification processes. These platforms enable global standardization of testing procedures while accommodating site-specific variations in equipment configurations. Advanced analytics capabilities within these systems allow for trend analysis across production lines, facilitating early detection of quality drift before specifications are breached.
Integration challenges frequently arise from equipment heterogeneity across production lines, particularly in facilities that have expanded over time. Legacy testing systems may utilize proprietary communication protocols that require custom interface development to connect with modern quality management platforms. Manufacturers often implement middleware solutions and API-based integration approaches to bridge these technological gaps while maintaining validation status of existing systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!