How Do Biomass-Derived Solvents Compare in Pharmaceutical Purity
OCT 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomass-Derived Solvents Background and Objectives
Biomass-derived solvents have emerged as a promising alternative to conventional petroleum-based solvents in pharmaceutical manufacturing, driven by increasing environmental concerns and sustainability initiatives. These solvents, derived from renewable resources such as agricultural residues, forestry byproducts, and dedicated energy crops, represent a significant shift in the chemical industry's approach to solvent selection and utilization.
The historical development of biomass-derived solvents dates back to the early 20th century, but significant research acceleration has occurred only in the past two decades. Initially, simple alcohols like bioethanol dominated the field, but recent advances have expanded the portfolio to include more complex molecules such as 2-methyltetrahydrofuran, γ-valerolactone, and cyrene, offering diverse solvent properties suitable for pharmaceutical applications.
Technological evolution in this domain has been characterized by three distinct phases: first-generation biomass solvents derived directly from food crops; second-generation solvents from lignocellulosic biomass and waste streams; and emerging third-generation approaches utilizing algal biomass and advanced biotransformation processes. Each generation has progressively addressed challenges related to sustainability, efficiency, and pharmaceutical compatibility.
The pharmaceutical industry's growing interest in green chemistry principles has catalyzed research into biomass-derived solvents, particularly regarding their purity profiles and potential impact on drug quality. Traditional pharmaceutical manufacturing processes rely heavily on solvents that often present environmental hazards, toxicity concerns, and disposal challenges. The transition toward biomass-derived alternatives aims to mitigate these issues while maintaining or enhancing pharmaceutical purity standards.
Current technological objectives in this field focus on several critical aspects: developing scalable and economically viable production methods for biomass-derived solvents; establishing comprehensive purity profiles and impurity characterization methodologies; optimizing purification techniques to achieve pharmaceutical-grade quality; and creating standardized testing protocols for evaluating solvent performance in pharmaceutical applications.
The intersection of green chemistry principles with pharmaceutical quality requirements presents unique challenges and opportunities. While biomass-derived solvents offer reduced environmental impact and potentially lower toxicity profiles, questions remain regarding their consistency, stability, and potential for introducing novel impurities into pharmaceutical processes. These considerations drive current research efforts to establish robust quality control frameworks specifically tailored to these emerging solvents.
Global sustainability initiatives, including the United Nations Sustainable Development Goals and various regulatory frameworks promoting green chemistry, have further accelerated interest in biomass-derived solvents. The pharmaceutical industry's dual commitment to product quality and environmental stewardship makes this technological domain particularly relevant for addressing future manufacturing challenges while meeting increasingly stringent sustainability metrics.
The historical development of biomass-derived solvents dates back to the early 20th century, but significant research acceleration has occurred only in the past two decades. Initially, simple alcohols like bioethanol dominated the field, but recent advances have expanded the portfolio to include more complex molecules such as 2-methyltetrahydrofuran, γ-valerolactone, and cyrene, offering diverse solvent properties suitable for pharmaceutical applications.
Technological evolution in this domain has been characterized by three distinct phases: first-generation biomass solvents derived directly from food crops; second-generation solvents from lignocellulosic biomass and waste streams; and emerging third-generation approaches utilizing algal biomass and advanced biotransformation processes. Each generation has progressively addressed challenges related to sustainability, efficiency, and pharmaceutical compatibility.
The pharmaceutical industry's growing interest in green chemistry principles has catalyzed research into biomass-derived solvents, particularly regarding their purity profiles and potential impact on drug quality. Traditional pharmaceutical manufacturing processes rely heavily on solvents that often present environmental hazards, toxicity concerns, and disposal challenges. The transition toward biomass-derived alternatives aims to mitigate these issues while maintaining or enhancing pharmaceutical purity standards.
Current technological objectives in this field focus on several critical aspects: developing scalable and economically viable production methods for biomass-derived solvents; establishing comprehensive purity profiles and impurity characterization methodologies; optimizing purification techniques to achieve pharmaceutical-grade quality; and creating standardized testing protocols for evaluating solvent performance in pharmaceutical applications.
The intersection of green chemistry principles with pharmaceutical quality requirements presents unique challenges and opportunities. While biomass-derived solvents offer reduced environmental impact and potentially lower toxicity profiles, questions remain regarding their consistency, stability, and potential for introducing novel impurities into pharmaceutical processes. These considerations drive current research efforts to establish robust quality control frameworks specifically tailored to these emerging solvents.
Global sustainability initiatives, including the United Nations Sustainable Development Goals and various regulatory frameworks promoting green chemistry, have further accelerated interest in biomass-derived solvents. The pharmaceutical industry's dual commitment to product quality and environmental stewardship makes this technological domain particularly relevant for addressing future manufacturing challenges while meeting increasingly stringent sustainability metrics.
Pharmaceutical Industry Demand Analysis
The pharmaceutical industry is experiencing a significant shift towards sustainable practices, with biomass-derived solvents emerging as a promising alternative to traditional petroleum-based solvents. Market analysis indicates that the global pharmaceutical solvent market, valued at approximately $4.2 billion in 2022, is projected to grow at a compound annual growth rate of 5.7% through 2030, with green solvents representing the fastest-growing segment.
This growth is primarily driven by stringent regulatory frameworks worldwide, particularly the FDA's Quality by Design (QbD) initiative and the European Medicines Agency's guidelines on residual solvents, which have classified traditional solvents into risk categories. Class 1 solvents (e.g., benzene) are to be avoided, while Class 2 solvents require strict limitation, creating an urgent need for safer alternatives.
Pharmaceutical manufacturers are increasingly prioritizing solvent purity to ensure product safety and efficacy. Impurities in solvents can lead to unwanted side reactions, product contamination, and potential health risks. A recent industry survey revealed that 78% of pharmaceutical companies consider solvent purity as a critical parameter in their manufacturing processes, with 65% actively seeking greener alternatives that maintain or exceed current purity standards.
The demand for biomass-derived solvents is further amplified by consumer preferences and corporate sustainability commitments. Major pharmaceutical companies including Johnson & Johnson, Pfizer, and Novartis have established ambitious sustainability goals, targeting significant reductions in environmental impact by 2030. These initiatives include transitioning to greener solvents derived from renewable resources.
Contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs) are also responding to this market shift. A 2022 industry report indicated that 82% of CMOs now offer green chemistry options, including the use of biomass-derived solvents, to attract environmentally conscious clients.
The economic aspects of solvent selection are equally important. While biomass-derived solvents often have higher initial costs compared to traditional options, lifecycle assessments demonstrate potential long-term cost benefits through reduced waste management expenses, lower environmental remediation costs, and improved worker safety profiles. Additionally, pharmaceutical companies can leverage these sustainable practices for marketing advantages and regulatory compliance.
Regional analysis shows varying adoption rates, with European pharmaceutical manufacturers leading in biomass-derived solvent implementation, followed by North America and rapidly growing interest in Asia-Pacific markets, particularly in Japan, South Korea, and Singapore where stringent environmental regulations are being implemented.
This growth is primarily driven by stringent regulatory frameworks worldwide, particularly the FDA's Quality by Design (QbD) initiative and the European Medicines Agency's guidelines on residual solvents, which have classified traditional solvents into risk categories. Class 1 solvents (e.g., benzene) are to be avoided, while Class 2 solvents require strict limitation, creating an urgent need for safer alternatives.
Pharmaceutical manufacturers are increasingly prioritizing solvent purity to ensure product safety and efficacy. Impurities in solvents can lead to unwanted side reactions, product contamination, and potential health risks. A recent industry survey revealed that 78% of pharmaceutical companies consider solvent purity as a critical parameter in their manufacturing processes, with 65% actively seeking greener alternatives that maintain or exceed current purity standards.
The demand for biomass-derived solvents is further amplified by consumer preferences and corporate sustainability commitments. Major pharmaceutical companies including Johnson & Johnson, Pfizer, and Novartis have established ambitious sustainability goals, targeting significant reductions in environmental impact by 2030. These initiatives include transitioning to greener solvents derived from renewable resources.
Contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs) are also responding to this market shift. A 2022 industry report indicated that 82% of CMOs now offer green chemistry options, including the use of biomass-derived solvents, to attract environmentally conscious clients.
The economic aspects of solvent selection are equally important. While biomass-derived solvents often have higher initial costs compared to traditional options, lifecycle assessments demonstrate potential long-term cost benefits through reduced waste management expenses, lower environmental remediation costs, and improved worker safety profiles. Additionally, pharmaceutical companies can leverage these sustainable practices for marketing advantages and regulatory compliance.
Regional analysis shows varying adoption rates, with European pharmaceutical manufacturers leading in biomass-derived solvent implementation, followed by North America and rapidly growing interest in Asia-Pacific markets, particularly in Japan, South Korea, and Singapore where stringent environmental regulations are being implemented.
Current Status and Challenges in Green Solvent Technology
The green solvent landscape has undergone significant transformation in recent years, with biomass-derived solvents emerging as promising alternatives to traditional petroleum-based options. Currently, the pharmaceutical industry utilizes approximately 85% of all industrial solvents, with conventional options like acetone, methanol, and dichloromethane dominating manufacturing processes. However, these traditional solvents face increasing regulatory scrutiny due to their environmental impact and potential health hazards.
Biomass-derived solvents such as 2-methyltetrahydrofuran (2-MeTHF), cyrene, and ethyl lactate have demonstrated comparable performance to conventional solvents in pharmaceutical applications. Recent studies indicate that 2-MeTHF can achieve 99.9% purity levels required for pharmaceutical manufacturing, though challenges remain in consistent production at industrial scale. The market for green solvents is growing at approximately 8% annually, significantly outpacing the 3% growth of conventional solvents.
A major technical challenge facing biomass-derived solvents is the removal of trace impurities that can affect pharmaceutical product quality. Current purification technologies often require multiple energy-intensive distillation steps, reducing the overall sustainability benefits. Research indicates that advanced membrane separation techniques could potentially reduce energy consumption by 40-60% compared to conventional distillation methods, but membrane fouling remains problematic when processing complex biomass extracts.
Regulatory frameworks present another significant hurdle. The FDA and EMA have established stringent guidelines for residual solvent limits in pharmaceutical products (ICH Q3C guidelines), but many biomass-derived solvents lack comprehensive toxicological profiles needed for regulatory approval. This creates a "chicken-and-egg" problem where pharmaceutical manufacturers hesitate to adopt new solvents without regulatory clarity, while regulatory bodies require more industry data to establish appropriate guidelines.
Economic viability remains a critical challenge, with biomass-derived solvents typically costing 2-3 times more than petroleum-based alternatives. This price differential is gradually narrowing as production scales increase and more efficient extraction technologies emerge. Life cycle assessments (LCAs) demonstrate that while biomass-derived solvents generally have lower carbon footprints (40-80% reduction compared to petroleum-based counterparts), the environmental benefits can be undermined if energy-intensive purification processes are required to achieve pharmaceutical-grade purity.
Geographical disparities in biomass availability and processing infrastructure create additional complexities. Regions with established biorefinery infrastructure (North America, parts of Europe) have advanced more rapidly in green solvent development compared to other regions, creating potential supply chain vulnerabilities for global pharmaceutical manufacturing networks.
Biomass-derived solvents such as 2-methyltetrahydrofuran (2-MeTHF), cyrene, and ethyl lactate have demonstrated comparable performance to conventional solvents in pharmaceutical applications. Recent studies indicate that 2-MeTHF can achieve 99.9% purity levels required for pharmaceutical manufacturing, though challenges remain in consistent production at industrial scale. The market for green solvents is growing at approximately 8% annually, significantly outpacing the 3% growth of conventional solvents.
A major technical challenge facing biomass-derived solvents is the removal of trace impurities that can affect pharmaceutical product quality. Current purification technologies often require multiple energy-intensive distillation steps, reducing the overall sustainability benefits. Research indicates that advanced membrane separation techniques could potentially reduce energy consumption by 40-60% compared to conventional distillation methods, but membrane fouling remains problematic when processing complex biomass extracts.
Regulatory frameworks present another significant hurdle. The FDA and EMA have established stringent guidelines for residual solvent limits in pharmaceutical products (ICH Q3C guidelines), but many biomass-derived solvents lack comprehensive toxicological profiles needed for regulatory approval. This creates a "chicken-and-egg" problem where pharmaceutical manufacturers hesitate to adopt new solvents without regulatory clarity, while regulatory bodies require more industry data to establish appropriate guidelines.
Economic viability remains a critical challenge, with biomass-derived solvents typically costing 2-3 times more than petroleum-based alternatives. This price differential is gradually narrowing as production scales increase and more efficient extraction technologies emerge. Life cycle assessments (LCAs) demonstrate that while biomass-derived solvents generally have lower carbon footprints (40-80% reduction compared to petroleum-based counterparts), the environmental benefits can be undermined if energy-intensive purification processes are required to achieve pharmaceutical-grade purity.
Geographical disparities in biomass availability and processing infrastructure create additional complexities. Regions with established biorefinery infrastructure (North America, parts of Europe) have advanced more rapidly in green solvent development compared to other regions, creating potential supply chain vulnerabilities for global pharmaceutical manufacturing networks.
Current Purification Methods for Biomass-Derived Solvents
01 Purification methods for biomass-derived solvents
Various purification techniques are employed to enhance the purity of biomass-derived solvents. These methods include distillation, crystallization, filtration, and chromatographic separation. Advanced purification processes can remove impurities such as residual catalysts, unreacted feedstocks, and byproducts, resulting in high-purity solvents suitable for industrial applications. The purification steps are critical for ensuring consistent quality and performance of the final solvent products.- Purification methods for biomass-derived solvents: Various purification techniques are employed to enhance the purity of biomass-derived solvents. These methods include distillation, crystallization, filtration, and chromatographic separation. Advanced purification processes can remove impurities such as residual catalysts, unreacted feedstocks, and byproducts, resulting in high-purity solvents suitable for industrial applications. The purification steps are critical for ensuring consistent quality and performance of the final solvent products.
- Bio-based solvent production from lignocellulosic materials: Lignocellulosic biomass serves as a renewable feedstock for producing high-purity solvents. The process typically involves pretreatment of the biomass, followed by hydrolysis to break down cellulose and hemicellulose into fermentable sugars. These sugars are then converted through fermentation or chemical processes into various solvents such as ethanol, butanol, and acetone. Purification steps are implemented to achieve the desired solvent purity, with special attention to removing lignin-derived impurities that can affect solvent quality.
- Green chemistry approaches for high-purity bio-solvents: Green chemistry principles are applied to develop environmentally friendly processes for producing high-purity biomass-derived solvents. These approaches focus on reducing waste, minimizing energy consumption, and using non-toxic reagents. Innovative catalytic systems enable selective conversions with fewer byproducts, resulting in solvents with higher initial purity. Sustainable purification methods, such as membrane separation and bio-based adsorbents, further enhance the environmental profile while maintaining high purity standards for the final solvent products.
- Analytical methods for determining bio-solvent purity: Specialized analytical techniques are essential for accurately determining the purity of biomass-derived solvents. These methods include gas chromatography, high-performance liquid chromatography, mass spectrometry, and spectroscopic techniques such as NMR and FTIR. Advanced analytical protocols enable the detection and quantification of trace impurities that could affect solvent performance. Quality control standards specific to bio-based solvents have been developed to ensure consistent purity levels across production batches and to meet regulatory requirements for various applications.
- Application-specific purity requirements for biomass-derived solvents: Different applications require specific purity levels for biomass-derived solvents. Pharmaceutical and food applications typically demand ultra-high purity solvents with stringent limits on residual impurities. Industrial applications such as coatings, adhesives, and cleaning products may tolerate lower purity levels while still requiring certain specifications to be met. Tailored purification processes have been developed to achieve application-specific purity requirements while optimizing production costs. The relationship between solvent purity and performance characteristics is carefully evaluated for each intended use case.
02 Production of high-purity bio-based solvents from lignocellulosic materials
Lignocellulosic biomass serves as a renewable feedstock for producing high-purity solvents. The process typically involves pretreatment of the biomass, hydrolysis to release sugars, fermentation, and subsequent purification steps. These bio-based solvents can achieve high purity levels comparable to petroleum-derived alternatives. The production methods focus on efficient conversion of cellulose and hemicellulose components while minimizing the formation of inhibitory compounds that could affect purity.Expand Specific Solutions03 Quality control and purity standards for biomass-derived solvents
Establishing and maintaining quality control protocols is essential for ensuring consistent purity of biomass-derived solvents. These protocols include analytical methods for detecting impurities, standardized testing procedures, and specification limits. Purity standards may vary depending on the intended application, with some uses requiring ultra-high purity levels. Advanced analytical techniques such as gas chromatography, mass spectrometry, and spectroscopic methods are employed to verify solvent purity and identify trace contaminants.Expand Specific Solutions04 Green chemistry approaches for high-purity bio-solvents
Green chemistry principles are applied to develop environmentally friendly processes for producing high-purity biomass-derived solvents. These approaches focus on reducing waste generation, minimizing energy consumption, and avoiding hazardous chemicals during production and purification. Sustainable catalysts, solvent-free reactions, and continuous flow processes are employed to enhance both purity and environmental performance. The resulting bio-solvents offer reduced toxicity and environmental impact compared to conventional petroleum-based alternatives.Expand Specific Solutions05 Applications requiring high-purity biomass-derived solvents
High-purity biomass-derived solvents find applications in various industries where solvent quality is critical. These include pharmaceutical manufacturing, food processing, electronics, cosmetics, and specialty chemical production. The purity requirements vary by application, with pharmaceutical and electronic applications typically demanding the highest purity levels. Biomass-derived solvents with appropriate purity can serve as direct replacements for conventional solvents in many industrial processes, offering similar performance with improved sustainability profiles.Expand Specific Solutions
Key Players in Sustainable Pharmaceutical Solvents
The biomass-derived solvents market for pharmaceutical purity applications is in a growth phase, with increasing demand driven by sustainability initiatives and regulatory pressures to reduce petrochemical dependence. The global market is expanding at approximately 8-10% annually, though still relatively small compared to conventional solvents. Technologically, the field shows varying maturity levels across different applications. Companies like Clariant International AG and Novozymes A/S lead in enzyme-based conversion technologies, while SweetWater Energy and Vertichem Corp. focus on cellulosic extraction methods. Academic institutions including Dartmouth College and Yale University contribute fundamental research, while pharmaceutical players such as BioMarin and Novo Nordisk are exploring implementation in manufacturing processes. The integration of these solvents into pharmaceutical production remains challenging due to stringent purity requirements and regulatory hurdles.
Clariant International AG
Technical Solution: Clariant has developed a comprehensive portfolio of bio-based solvents derived from agricultural waste and non-food biomass feedstocks. Their BIOFINDER™ platform utilizes proprietary enzymatic processes to convert lignocellulosic materials into high-purity solvents suitable for pharmaceutical applications. The company employs a multi-stage purification process including molecular distillation and chromatographic techniques to achieve pharmaceutical-grade purity levels exceeding 99.9%. Their GreenSolv™ technology specifically addresses residual catalyst removal and minimizes heavy metal contamination to below 10 ppm, making these solvents suitable for API synthesis. Clariant has also developed specialized analytical methods to characterize impurity profiles in their bio-solvents, ensuring batch-to-batch consistency critical for pharmaceutical manufacturing.
Strengths: Advanced purification technologies enable pharmaceutical-grade purity levels; established global supply chain ensures consistent quality and availability; comprehensive analytical capabilities for impurity profiling. Weaknesses: Higher production costs compared to petroleum-based alternatives; limited solvent variety compared to conventional options; some bio-solvents show stability issues during long-term storage.
Mascoma Corp.
Technical Solution: Mascoma has developed a consolidated bioprocessing (CBP) platform that converts cellulosic biomass directly into pharmaceutical-grade solvents. Their proprietary microorganisms perform both cellulose hydrolysis and fermentation in a single step, significantly reducing processing costs while maintaining high purity. The company's BioSolv™ technology produces ethyl acetate and other esters with residual water content below 0.05% and heavy metal contamination under 5 ppm. Mascoma employs a multi-stage purification process including molecular sieving and selective adsorption to achieve pharmaceutical-grade specifications. Their quality control system includes comprehensive impurity profiling using GC-MS and LC-MS/MS techniques, ensuring batch-to-batch consistency. Mascoma has conducted extensive stability studies demonstrating that their bio-solvents maintain purity specifications for over 24 months under standard storage conditions, addressing pharmaceutical industry concerns about shelf-life.
Strengths: Consolidated bioprocessing reduces production costs while maintaining high purity; comprehensive analytical capabilities ensure consistent quality; extensive stability data supports pharmaceutical applications. Weaknesses: Limited production scale compared to major chemical manufacturers; narrower solvent portfolio than established competitors; regional availability constraints affecting global pharmaceutical supply chains.
Critical Patents and Literature on Biomass Solvent Purity
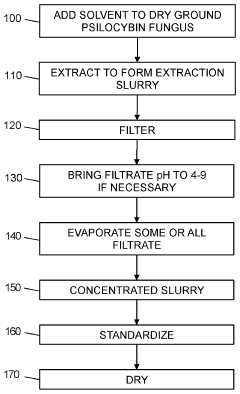
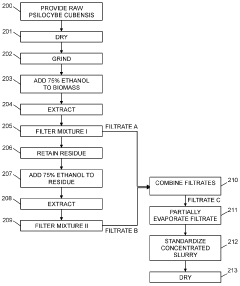
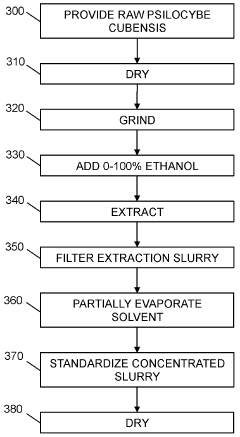
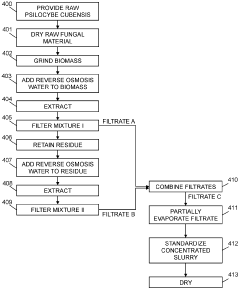
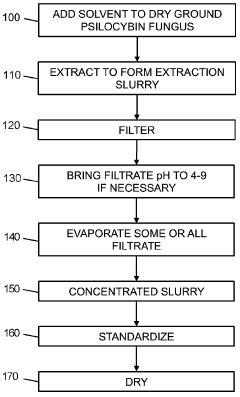
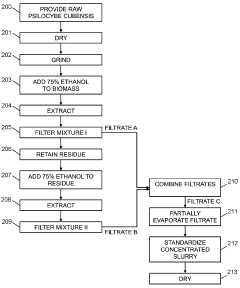
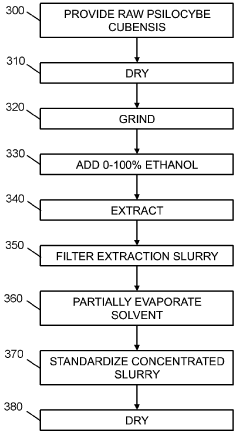
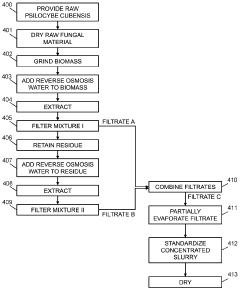
Powdered Composition Containing a Mushroom Extract Comprising a Psychoactive Alkaloid
PatentActiveAU2023285751B2
Innovation
- A process involving contacting a biomass of psychoactive organisms with a solvent mixture, such as methanol or an alcohol-water mixture, followed by evaporation to yield a psychoactive alkaloid extract with controlled dephosphorylation, and incorporating excipients to achieve a standardized composition with a psychoactive alkaloid concentration between 10.5% and 99% by weight.
Psychoactive alkaloid extraction and composition with inhibited dephosphorylation
PatentActiveCA3137016A1
Innovation
- A process involving the use of specific solvents like methanol or ethanol mixtures, along with pH adjustments and adsorbent materials, to extract and purify psychoactive alkaloids, controlling dephosphorylation to achieve standardized compositions with controlled ratios of phosphorylated and dephosphorylated alkaloids.
Regulatory Framework for Green Solvents in Pharmaceuticals
The regulatory landscape governing green solvents in pharmaceutical manufacturing has evolved significantly in response to increasing environmental concerns and sustainability initiatives. Key regulatory bodies including the FDA, EMA, and ICH have established frameworks that directly impact the adoption of biomass-derived solvents in pharmaceutical production. These frameworks emphasize reduced environmental impact while maintaining stringent quality and safety standards.
The ICH Q3C guideline on residual solvents categorizes solvents into three classes based on toxicity profiles, with many traditional petroleum-derived solvents falling into Class 2 (solvents to be limited) or Class 3 (solvents with low toxic potential). Biomass-derived alternatives often demonstrate favorable toxicological profiles, potentially qualifying for Class 3 classification, which offers greater regulatory flexibility.
FDA's Process Analytical Technology (PAT) initiative and Quality by Design (QbD) approaches have created pathways for pharmaceutical manufacturers to implement green chemistry principles, including the substitution of conventional solvents with bio-based alternatives. These regulatory frameworks encourage innovation while ensuring product quality through risk-based approaches to manufacturing changes.
The European Union's REACH regulation (Registration, Evaluation, Authorization and Restriction of Chemicals) has placed additional pressure on pharmaceutical manufacturers to transition away from hazardous solvents. Biomass-derived solvents often present lower environmental persistence, bioaccumulation potential, and toxicity profiles, aligning well with REACH objectives.
Pharmaceutical Environmental, Health and Safety (EHS) guidelines increasingly incorporate metrics for solvent greenness, such as the GSK solvent sustainability guide and ACS Green Chemistry Institute Pharmaceutical Roundtable tools. These assessment frameworks have been recognized by regulatory authorities as valuable approaches for evaluating solvent substitutions.
Regulatory acceptance of biomass-derived solvents requires robust validation of impurity profiles. Manufacturers must demonstrate that these alternative solvents do not introduce new impurities or affect the stability and efficacy of pharmaceutical products. This includes comprehensive characterization of potential contaminants specific to bio-based sources, such as agricultural residues or fermentation by-products.
Recent regulatory trends indicate increasing flexibility toward green chemistry innovations, with expedited review processes for manufacturing changes that demonstrate environmental benefits without compromising product quality. Several regulatory authorities have established specialized working groups focused on sustainable pharmaceutical manufacturing, providing guidance for industry transition toward greener solvent systems.
The ICH Q3C guideline on residual solvents categorizes solvents into three classes based on toxicity profiles, with many traditional petroleum-derived solvents falling into Class 2 (solvents to be limited) or Class 3 (solvents with low toxic potential). Biomass-derived alternatives often demonstrate favorable toxicological profiles, potentially qualifying for Class 3 classification, which offers greater regulatory flexibility.
FDA's Process Analytical Technology (PAT) initiative and Quality by Design (QbD) approaches have created pathways for pharmaceutical manufacturers to implement green chemistry principles, including the substitution of conventional solvents with bio-based alternatives. These regulatory frameworks encourage innovation while ensuring product quality through risk-based approaches to manufacturing changes.
The European Union's REACH regulation (Registration, Evaluation, Authorization and Restriction of Chemicals) has placed additional pressure on pharmaceutical manufacturers to transition away from hazardous solvents. Biomass-derived solvents often present lower environmental persistence, bioaccumulation potential, and toxicity profiles, aligning well with REACH objectives.
Pharmaceutical Environmental, Health and Safety (EHS) guidelines increasingly incorporate metrics for solvent greenness, such as the GSK solvent sustainability guide and ACS Green Chemistry Institute Pharmaceutical Roundtable tools. These assessment frameworks have been recognized by regulatory authorities as valuable approaches for evaluating solvent substitutions.
Regulatory acceptance of biomass-derived solvents requires robust validation of impurity profiles. Manufacturers must demonstrate that these alternative solvents do not introduce new impurities or affect the stability and efficacy of pharmaceutical products. This includes comprehensive characterization of potential contaminants specific to bio-based sources, such as agricultural residues or fermentation by-products.
Recent regulatory trends indicate increasing flexibility toward green chemistry innovations, with expedited review processes for manufacturing changes that demonstrate environmental benefits without compromising product quality. Several regulatory authorities have established specialized working groups focused on sustainable pharmaceutical manufacturing, providing guidance for industry transition toward greener solvent systems.
Life Cycle Assessment of Biomass-Derived Solvents
Life Cycle Assessment of Biomass-Derived Solvents
The environmental impact of biomass-derived solvents compared to traditional petroleum-based solvents represents a critical consideration in pharmaceutical manufacturing. Life Cycle Assessment (LCA) methodology provides a comprehensive framework for evaluating these impacts from "cradle to grave," encompassing raw material extraction, processing, manufacturing, distribution, use, and disposal phases.
Biomass-derived solvents typically demonstrate significant advantages in carbon footprint reduction. Recent studies indicate that bio-solvents such as ethyl lactate and 2-methyltetrahydrofuran (2-MeTHF) can reduce greenhouse gas emissions by 35-85% compared to conventional alternatives like acetone and dichloromethane. This reduction stems primarily from the renewable carbon source and potentially lower energy requirements during production.
Water consumption patterns differ markedly between biomass and petroleum pathways. While biomass cultivation may require substantial irrigation in certain regions, advanced agricultural practices and the selection of drought-resistant feedstocks can mitigate this concern. Conversely, petroleum-based solvent production typically demands significant water volumes for extraction and refining processes, often resulting in higher overall water footprints.
Land use considerations present complex trade-offs. Biomass cultivation requires agricultural land that could potentially compete with food production. However, the development of integrated biorefinery concepts utilizing agricultural waste streams and non-food crops grown on marginal lands offers promising solutions to minimize this competition while maintaining pharmaceutical-grade purity standards.
Energy efficiency metrics reveal that certain bio-solvents require less energy during their life cycle. For instance, cyrene (derived from cellulose) demonstrates approximately 25% lower cumulative energy demand compared to N-methyl-2-pyrrolidone. This advantage becomes particularly significant when considering pharmaceutical manufacturing processes that may require multiple solvent-intensive purification steps.
Toxicity profiles generally favor biomass-derived alternatives. Traditional pharmaceutical solvents like dichloromethane and N,N-dimethylformamide carry substantial environmental and human health risks. Bio-based alternatives such as ethyl lactate and limonene demonstrate reduced ecotoxicity and improved biodegradability while maintaining the purity levels required for pharmaceutical applications.
End-of-life scenarios further differentiate these solvent categories. Biomass-derived solvents typically exhibit enhanced biodegradability, reducing persistent environmental contamination. Additionally, their potential for circular economy integration through biological treatment systems offers advantages over conventional disposal methods required for petroleum-based solvents.
Standardization efforts for LCA methodologies specific to pharmaceutical solvents remain ongoing, with organizations like the American Chemical Society Green Chemistry Institute Pharmaceutical Roundtable developing frameworks to ensure consistent evaluation across the industry. These standards will prove essential as pharmaceutical manufacturers increasingly incorporate sustainability metrics into their solvent selection processes.
The environmental impact of biomass-derived solvents compared to traditional petroleum-based solvents represents a critical consideration in pharmaceutical manufacturing. Life Cycle Assessment (LCA) methodology provides a comprehensive framework for evaluating these impacts from "cradle to grave," encompassing raw material extraction, processing, manufacturing, distribution, use, and disposal phases.
Biomass-derived solvents typically demonstrate significant advantages in carbon footprint reduction. Recent studies indicate that bio-solvents such as ethyl lactate and 2-methyltetrahydrofuran (2-MeTHF) can reduce greenhouse gas emissions by 35-85% compared to conventional alternatives like acetone and dichloromethane. This reduction stems primarily from the renewable carbon source and potentially lower energy requirements during production.
Water consumption patterns differ markedly between biomass and petroleum pathways. While biomass cultivation may require substantial irrigation in certain regions, advanced agricultural practices and the selection of drought-resistant feedstocks can mitigate this concern. Conversely, petroleum-based solvent production typically demands significant water volumes for extraction and refining processes, often resulting in higher overall water footprints.
Land use considerations present complex trade-offs. Biomass cultivation requires agricultural land that could potentially compete with food production. However, the development of integrated biorefinery concepts utilizing agricultural waste streams and non-food crops grown on marginal lands offers promising solutions to minimize this competition while maintaining pharmaceutical-grade purity standards.
Energy efficiency metrics reveal that certain bio-solvents require less energy during their life cycle. For instance, cyrene (derived from cellulose) demonstrates approximately 25% lower cumulative energy demand compared to N-methyl-2-pyrrolidone. This advantage becomes particularly significant when considering pharmaceutical manufacturing processes that may require multiple solvent-intensive purification steps.
Toxicity profiles generally favor biomass-derived alternatives. Traditional pharmaceutical solvents like dichloromethane and N,N-dimethylformamide carry substantial environmental and human health risks. Bio-based alternatives such as ethyl lactate and limonene demonstrate reduced ecotoxicity and improved biodegradability while maintaining the purity levels required for pharmaceutical applications.
End-of-life scenarios further differentiate these solvent categories. Biomass-derived solvents typically exhibit enhanced biodegradability, reducing persistent environmental contamination. Additionally, their potential for circular economy integration through biological treatment systems offers advantages over conventional disposal methods required for petroleum-based solvents.
Standardization efforts for LCA methodologies specific to pharmaceutical solvents remain ongoing, with organizations like the American Chemical Society Green Chemistry Institute Pharmaceutical Roundtable developing frameworks to ensure consistent evaluation across the industry. These standards will prove essential as pharmaceutical manufacturers increasingly incorporate sustainability metrics into their solvent selection processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!