How Do Metal Mesh Dimensions Affect Catalytic Efficiency
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metal Mesh Catalysis Background and Objectives
Metal mesh catalysis represents a significant advancement in heterogeneous catalysis, evolving from traditional powder catalysts to structured catalytic systems. The development of metal mesh catalysts began in the mid-20th century but has seen accelerated innovation in the past two decades due to advances in materials science and nanotechnology. These mesh structures offer unique advantages in terms of mechanical stability, flow characteristics, and catalytic performance compared to conventional catalyst forms.
The dimensional properties of metal meshes—including wire diameter, mesh opening size, weave pattern, and overall thickness—fundamentally influence their catalytic efficiency. Historical progression shows a clear trend from simple woven meshes toward precisely engineered structures with optimized geometrical parameters. This evolution has been driven by the increasing understanding of how mesh dimensions affect critical factors such as surface area, mass transfer, and reaction kinetics.
Current research indicates that mesh dimensions directly impact several key performance indicators in catalytic processes. Finer meshes typically provide higher specific surface area for catalyst deposition but may introduce flow restrictions and pressure drops in continuous processes. Conversely, coarser meshes offer improved flow dynamics but potentially reduced active surface area. The optimal balance between these competing factors represents a central challenge in metal mesh catalyst design.
The primary objective of research in this field is to establish quantitative relationships between mesh dimensional parameters and catalytic performance metrics across different reaction environments. This includes understanding how mesh geometry affects catalyst loading capacity, active site accessibility, heat transfer properties, and long-term stability under reaction conditions. Additionally, researchers aim to develop predictive models that can guide the rational design of mesh-based catalytic systems for specific applications.
Recent technological advances have enabled the fabrication of hierarchical mesh structures with precisely controlled dimensions at multiple scales, from macro to nano. These developments open new possibilities for tailoring mesh properties to specific catalytic requirements. The integration of computational fluid dynamics with catalytic reaction modeling has further enhanced our ability to predict how dimensional modifications will affect overall system performance.
Looking forward, the field is moving toward adaptive and responsive mesh designs that can dynamically adjust their dimensional characteristics in response to changing reaction conditions. This represents a paradigm shift from static catalyst supports to dynamic systems capable of maintaining optimal performance across varying operational parameters. The ultimate goal is to develop a comprehensive framework for dimension-optimized metal mesh catalysts that can significantly improve efficiency across chemical manufacturing, environmental remediation, and energy conversion applications.
The dimensional properties of metal meshes—including wire diameter, mesh opening size, weave pattern, and overall thickness—fundamentally influence their catalytic efficiency. Historical progression shows a clear trend from simple woven meshes toward precisely engineered structures with optimized geometrical parameters. This evolution has been driven by the increasing understanding of how mesh dimensions affect critical factors such as surface area, mass transfer, and reaction kinetics.
Current research indicates that mesh dimensions directly impact several key performance indicators in catalytic processes. Finer meshes typically provide higher specific surface area for catalyst deposition but may introduce flow restrictions and pressure drops in continuous processes. Conversely, coarser meshes offer improved flow dynamics but potentially reduced active surface area. The optimal balance between these competing factors represents a central challenge in metal mesh catalyst design.
The primary objective of research in this field is to establish quantitative relationships between mesh dimensional parameters and catalytic performance metrics across different reaction environments. This includes understanding how mesh geometry affects catalyst loading capacity, active site accessibility, heat transfer properties, and long-term stability under reaction conditions. Additionally, researchers aim to develop predictive models that can guide the rational design of mesh-based catalytic systems for specific applications.
Recent technological advances have enabled the fabrication of hierarchical mesh structures with precisely controlled dimensions at multiple scales, from macro to nano. These developments open new possibilities for tailoring mesh properties to specific catalytic requirements. The integration of computational fluid dynamics with catalytic reaction modeling has further enhanced our ability to predict how dimensional modifications will affect overall system performance.
Looking forward, the field is moving toward adaptive and responsive mesh designs that can dynamically adjust their dimensional characteristics in response to changing reaction conditions. This represents a paradigm shift from static catalyst supports to dynamic systems capable of maintaining optimal performance across varying operational parameters. The ultimate goal is to develop a comprehensive framework for dimension-optimized metal mesh catalysts that can significantly improve efficiency across chemical manufacturing, environmental remediation, and energy conversion applications.
Market Applications and Demand Analysis
The global market for catalytic technologies is experiencing robust growth, driven by stringent environmental regulations and increasing industrial applications. Metal mesh catalysts represent a significant segment within this market, with their efficiency directly impacting economic and environmental outcomes across multiple industries. The relationship between metal mesh dimensions and catalytic efficiency has become a critical factor influencing market demand and application development.
In the automotive sector, demand for optimized metal mesh catalysts in catalytic converters continues to rise as emission standards become more stringent worldwide. The European Union's Euro 7 standards and similar regulations in North America and Asia have created substantial market pressure for catalysts that can achieve higher conversion rates with minimal precious metal loading. This has intensified research into how mesh dimensions affect performance, with manufacturers seeking the optimal surface area-to-volume ratio.
The chemical processing industry represents another major market driver, where reaction selectivity and yield are directly influenced by catalyst design. Companies in this sector are increasingly investing in customized metal mesh catalysts with precisely engineered dimensions to maximize throughput while minimizing energy consumption. Market analysis indicates that even marginal improvements in catalytic efficiency can translate to millions in operational savings for large-scale chemical plants.
Renewable energy applications, particularly in hydrogen production and fuel cell technologies, have emerged as rapidly growing markets for advanced metal mesh catalysts. The hydrogen economy's projected growth rate of 9.2% annually through 2030 is creating significant demand for catalysts that can improve electrolysis efficiency and reduce costs. Research indicates that nano-structured metal meshes with optimized dimensions can substantially enhance hydrogen production rates while reducing precious metal requirements.
Environmental remediation represents another expanding application area, with metal mesh catalysts being deployed for air purification, wastewater treatment, and soil decontamination. The global environmental technology market, valued at $552.1 billion in 2021, is projected to reach $890.5 billion by 2026, with catalytic technologies playing a crucial role in this growth.
Market research indicates regional variations in demand patterns. Asia-Pacific leads in terms of volume demand, driven by rapid industrialization and automotive sector growth in China and India. However, North America and Europe lead in terms of innovation and high-performance applications, with greater emphasis on catalyst longevity and efficiency rather than initial cost.
Customer requirements are increasingly sophisticated, with end-users demanding catalysts that not only offer high initial activity but also demonstrate resistance to poisoning, thermal stability, and predictable performance degradation patterns. This has shifted market dynamics toward value-based pricing models where premium products with optimized mesh dimensions command significantly higher prices due to their superior lifetime performance metrics.
In the automotive sector, demand for optimized metal mesh catalysts in catalytic converters continues to rise as emission standards become more stringent worldwide. The European Union's Euro 7 standards and similar regulations in North America and Asia have created substantial market pressure for catalysts that can achieve higher conversion rates with minimal precious metal loading. This has intensified research into how mesh dimensions affect performance, with manufacturers seeking the optimal surface area-to-volume ratio.
The chemical processing industry represents another major market driver, where reaction selectivity and yield are directly influenced by catalyst design. Companies in this sector are increasingly investing in customized metal mesh catalysts with precisely engineered dimensions to maximize throughput while minimizing energy consumption. Market analysis indicates that even marginal improvements in catalytic efficiency can translate to millions in operational savings for large-scale chemical plants.
Renewable energy applications, particularly in hydrogen production and fuel cell technologies, have emerged as rapidly growing markets for advanced metal mesh catalysts. The hydrogen economy's projected growth rate of 9.2% annually through 2030 is creating significant demand for catalysts that can improve electrolysis efficiency and reduce costs. Research indicates that nano-structured metal meshes with optimized dimensions can substantially enhance hydrogen production rates while reducing precious metal requirements.
Environmental remediation represents another expanding application area, with metal mesh catalysts being deployed for air purification, wastewater treatment, and soil decontamination. The global environmental technology market, valued at $552.1 billion in 2021, is projected to reach $890.5 billion by 2026, with catalytic technologies playing a crucial role in this growth.
Market research indicates regional variations in demand patterns. Asia-Pacific leads in terms of volume demand, driven by rapid industrialization and automotive sector growth in China and India. However, North America and Europe lead in terms of innovation and high-performance applications, with greater emphasis on catalyst longevity and efficiency rather than initial cost.
Customer requirements are increasingly sophisticated, with end-users demanding catalysts that not only offer high initial activity but also demonstrate resistance to poisoning, thermal stability, and predictable performance degradation patterns. This has shifted market dynamics toward value-based pricing models where premium products with optimized mesh dimensions command significantly higher prices due to their superior lifetime performance metrics.
Current Challenges in Metal Mesh Catalytic Technology
Despite significant advancements in metal mesh catalytic technology, several critical challenges continue to impede optimal performance and widespread industrial adoption. The relationship between mesh dimensions and catalytic efficiency remains incompletely understood, creating a fundamental barrier to design optimization. Current manufacturing processes struggle to consistently produce metal meshes with precise dimensional specifications at industrial scales, resulting in performance variability across production batches.
Surface area optimization presents another significant challenge. While smaller mesh openings generally provide greater surface area for catalytic reactions, they simultaneously restrict flow dynamics and increase pressure drops across the system. This trade-off between surface area and flow efficiency has not been fully resolved in current designs, particularly for applications requiring high throughput.
Material degradation under operational conditions continues to plague metal mesh catalysts. The dimensional stability of meshes often deteriorates during extended exposure to high temperatures, corrosive environments, and mechanical stress. This degradation alters the critical dimensions that determine catalytic performance, leading to efficiency losses over time and increasing maintenance requirements.
Computational modeling capabilities, while advancing, still fall short in accurately predicting how specific mesh dimensions will perform across diverse reaction environments. Current models struggle to simultaneously account for surface chemistry, fluid dynamics, heat transfer, and structural mechanics at different dimensional scales, limiting their predictive value for design optimization.
Standardization across the industry presents another obstacle. The lack of universally accepted testing protocols for evaluating how mesh dimensions affect catalytic performance makes direct comparisons between different research findings and commercial products difficult. This hampers knowledge transfer and slows technological advancement in the field.
Cost-effective scaling of optimized mesh designs from laboratory to industrial applications remains problematic. Manufacturing techniques capable of producing precisely dimensioned meshes often become prohibitively expensive at larger scales, creating barriers to commercial implementation of theoretically superior designs.
The integration of advanced materials (such as nanomaterials and composites) with traditional metal mesh structures introduces additional dimensional complexity that current fabrication methods struggle to control precisely. This limits the potential performance gains from these material innovations.
Finally, real-time monitoring and adaptive control of mesh dimensional parameters during operation remain underdeveloped, preventing dynamic optimization of catalytic systems in response to changing reaction conditions or catalyst aging. This represents a significant gap in the technology's capability to maintain peak efficiency throughout its operational lifecycle.
Surface area optimization presents another significant challenge. While smaller mesh openings generally provide greater surface area for catalytic reactions, they simultaneously restrict flow dynamics and increase pressure drops across the system. This trade-off between surface area and flow efficiency has not been fully resolved in current designs, particularly for applications requiring high throughput.
Material degradation under operational conditions continues to plague metal mesh catalysts. The dimensional stability of meshes often deteriorates during extended exposure to high temperatures, corrosive environments, and mechanical stress. This degradation alters the critical dimensions that determine catalytic performance, leading to efficiency losses over time and increasing maintenance requirements.
Computational modeling capabilities, while advancing, still fall short in accurately predicting how specific mesh dimensions will perform across diverse reaction environments. Current models struggle to simultaneously account for surface chemistry, fluid dynamics, heat transfer, and structural mechanics at different dimensional scales, limiting their predictive value for design optimization.
Standardization across the industry presents another obstacle. The lack of universally accepted testing protocols for evaluating how mesh dimensions affect catalytic performance makes direct comparisons between different research findings and commercial products difficult. This hampers knowledge transfer and slows technological advancement in the field.
Cost-effective scaling of optimized mesh designs from laboratory to industrial applications remains problematic. Manufacturing techniques capable of producing precisely dimensioned meshes often become prohibitively expensive at larger scales, creating barriers to commercial implementation of theoretically superior designs.
The integration of advanced materials (such as nanomaterials and composites) with traditional metal mesh structures introduces additional dimensional complexity that current fabrication methods struggle to control precisely. This limits the potential performance gains from these material innovations.
Finally, real-time monitoring and adaptive control of mesh dimensional parameters during operation remain underdeveloped, preventing dynamic optimization of catalytic systems in response to changing reaction conditions or catalyst aging. This represents a significant gap in the technology's capability to maintain peak efficiency throughout its operational lifecycle.
Current Dimensional Optimization Approaches
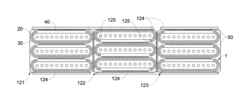
01 Metal mesh structure for enhanced catalytic efficiency
Metal mesh structures can be designed to optimize catalytic efficiency by providing increased surface area for reactions. The specific geometry, pore size, and material composition of the mesh influence the catalytic performance. These structures allow for better contact between reactants and catalysts while facilitating efficient mass transfer and heat distribution, which are crucial factors in catalytic processes.- Metal mesh structure for enhanced catalytic efficiency: The design and structure of metal meshes significantly impact catalytic efficiency. Specific mesh configurations, including pore size, thickness, and surface area, can optimize the contact between reactants and catalytic surfaces. Advanced mesh designs incorporate features that enhance mass transfer and reaction kinetics, leading to improved catalytic performance in various chemical processes.
- Surface modification of metal meshes for catalysis: Surface treatments and modifications of metal meshes can substantially improve their catalytic properties. Techniques such as coating with active materials, surface roughening, and chemical functionalization create more reactive sites on the mesh surface. These modifications enhance the adsorption of reactants and facilitate electron transfer during catalytic reactions, resulting in higher efficiency and selectivity.
- Metal mesh composition and alloy effects on catalysis: The composition of metal meshes, including the use of specific alloys and composite materials, plays a crucial role in determining catalytic efficiency. Different metals and their combinations exhibit varying catalytic activities for specific reactions. Noble metal meshes, transition metal alloys, and bimetallic compositions can be tailored to achieve optimal catalytic performance while considering factors such as cost, durability, and resistance to poisoning.
- Operating conditions for metal mesh catalysts: The optimization of operating conditions significantly affects the catalytic efficiency of metal meshes. Parameters such as temperature, pressure, flow rate, and reactant concentration must be carefully controlled to maximize performance. The relationship between these operating variables and catalytic activity is often complex, requiring systematic investigation to determine optimal conditions for specific reactions and catalyst systems.
- Innovative applications of metal mesh catalysts: Metal mesh catalysts are being applied in innovative ways across various industries. These applications include environmental remediation, energy conversion systems, chemical synthesis, and automotive emission control. The unique properties of metal meshes, such as high mechanical strength combined with catalytic activity, make them suitable for demanding applications where traditional catalyst forms might be inadequate. Recent developments focus on integrating these catalysts into microreactors, fuel cells, and sustainable energy technologies.
02 Surface modification of metal mesh catalysts
Surface modifications of metal mesh catalysts can significantly improve catalytic efficiency. Techniques include coating with active materials, creating nano-structured surfaces, and introducing specific functional groups. These modifications enhance the number of active sites, improve selectivity, and increase the stability of catalysts under reaction conditions, leading to higher conversion rates and better product yields.Expand Specific Solutions03 Metal mesh composition and alloy effects on catalysis
The composition of metal meshes and the use of specific alloys can dramatically affect catalytic efficiency. Different metals and their combinations exhibit varying catalytic properties for different reactions. Noble metals, transition metals, and their alloys can be strategically selected and combined in mesh form to achieve optimal catalytic performance while potentially reducing the amount of precious metals required.Expand Specific Solutions04 Temperature and pressure control in metal mesh catalytic systems
Effective temperature and pressure management in metal mesh catalytic systems is essential for maintaining optimal catalytic efficiency. Metal mesh structures can be designed to withstand high temperatures and pressures while providing efficient heat transfer. Proper control of these parameters prevents catalyst deactivation, sintering, and other degradation mechanisms, extending catalyst lifetime and maintaining high conversion rates.Expand Specific Solutions05 Reactor design incorporating metal mesh catalysts
Innovative reactor designs that incorporate metal mesh catalysts can significantly improve overall catalytic efficiency. These designs focus on optimizing flow patterns, minimizing pressure drops, and ensuring uniform distribution of reactants across the catalyst surface. Fixed-bed, fluidized-bed, and structured reactors utilizing metal mesh catalysts can be tailored for specific reactions to maximize throughput and selectivity while minimizing energy consumption.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The catalytic efficiency of metal mesh dimensions is currently in a growth phase, with the market expanding due to increasing applications in energy, automotive, and environmental sectors. The global market size for metal mesh catalysts is projected to reach significant scale as industries seek more efficient catalytic solutions. Technologically, this field is advancing rapidly with varying maturity levels across applications. Leading players like China Petroleum & Chemical Corp. and Air Liquide demonstrate advanced capabilities in industrial catalysis, while automotive companies including Nissan, Honda, and Mazda focus on emissions control applications. Research institutions such as CNRS and Korea Institute of Energy Research are driving fundamental innovations, while specialized firms like Directa Plus and AFC Energy are developing next-generation catalytic technologies for emerging applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced metal mesh catalysts with precisely controlled dimensions for petroleum refining processes. Their technology focuses on optimizing mesh aperture size (typically 20-100 μm) and wire diameter (5-30 μm) to enhance mass transfer efficiency. Sinopec's research demonstrates that decreasing mesh aperture size while maintaining optimal wire diameter significantly improves catalytic efficiency by increasing the specific surface area available for reactions. Their proprietary coating techniques ensure uniform catalyst distribution across the mesh structure, preventing agglomeration and channel blocking. Sinopec has implemented these optimized metal mesh catalysts in their fluid catalytic cracking (FCC) units, where the engineered mesh dimensions have shown to increase conversion rates by up to 15% while reducing coking and extending catalyst lifespan through improved heat distribution properties.
Strengths: Superior mass transfer efficiency due to optimized mesh geometry; excellent durability in high-temperature petroleum processing environments; reduced coking tendency. Weaknesses: Higher manufacturing costs compared to conventional catalyst supports; potential for channel blocking in processes with high particulate content; limited flexibility for rapid dimension adjustments once deployed.
IFP Energies Nouvelles
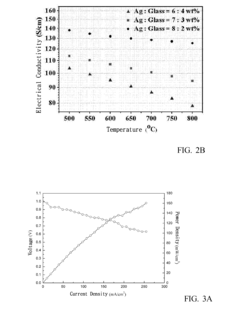
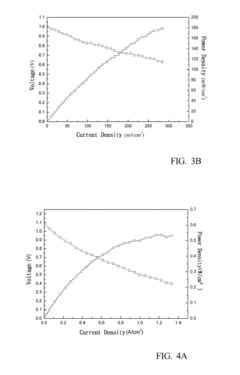
Technical Solution: IFP Energies Nouvelles has pioneered hierarchical metal mesh catalyst structures with multi-scale dimensional control for enhanced catalytic efficiency. Their approach incorporates primary mesh structures (200-500 μm) with secondary micro-meshes (10-50 μm) to create a cascade of catalytic environments. This dimensional hierarchy optimizes both reactant access and residence time distribution. IFP's research demonstrates that the ratio between primary and secondary mesh dimensions critically affects conversion efficiency, with an optimal ratio of approximately 10:1 maximizing performance. Their proprietary manufacturing process allows precise control of mesh thickness (typically 0.2-1.0 mm) and porosity (40-80%), which directly correlates with pressure drop characteristics and catalyst utilization efficiency. For hydrogen production applications, IFP has developed specialized nickel-based metal mesh catalysts where the mesh dimensions are dynamically optimized based on operating temperature profiles, resulting in up to 30% improvement in hydrogen yield compared to conventional catalyst systems.
Strengths: Exceptional control over reaction kinetics through multi-scale dimensional engineering; superior heat management capabilities; excellent resistance to thermal cycling. Weaknesses: Complex and costly manufacturing process; challenging scale-up for industrial implementation; potential for non-uniform aging across different dimensional scales.
Critical Patents in Mesh Geometry Effects
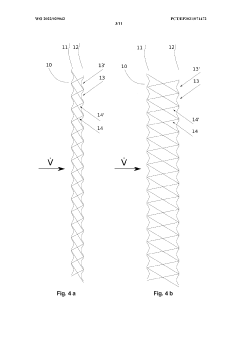
Gauzes having a tertiary structure for the catalytic conversion of fluids
PatentWO2022029042A1
Innovation
- The development of catalytic meshes with a tertiary structure, where two or more mesh layers are connected by pile threads to create a wave-like pattern directly during the knitting process, enhancing surface area and airflow resistance without the need for a rigid, non-catalytic surface, allowing for symmetrical or asymmetrical designs and overcoming size limitations.
Anode supported flat-tube SOFC and manufacturing method thereof
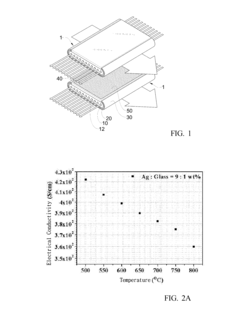
PatentInactiveUS20120045707A1
Innovation
- An anode-supported flat-tube SOFC design with a stack configuration that includes a flow path for fuel gas, an electrolyte, a cathode, and an interconnect layer formed by mixing electrical conductive materials with glass, and a metallic mesh for current collection, which simplifies stack manufacturing and current collection while reducing costs.
Materials Science Considerations
The fundamental properties of metal mesh materials significantly influence catalytic performance through multiple mechanisms. Material selection is paramount, with noble metals like platinum, palladium, and rhodium demonstrating superior catalytic activity due to their electronic configurations that facilitate adsorption and desorption processes. Base metals such as nickel, copper, and iron offer cost-effective alternatives with modified catalytic properties when properly engineered.
Surface characteristics of metal meshes directly impact reaction kinetics. Roughness at the microscale increases the effective surface area available for catalytic reactions, while nanoscale features can create unique electronic environments that alter activation energies. Surface crystallography also plays a crucial role, as different crystal facets exhibit varying catalytic activities toward specific reactions.
Porosity architecture represents another critical dimension affecting catalytic efficiency. Macro-porosity (>50 nm) facilitates mass transport of reactants and products, reducing diffusion limitations. Meso-porosity (2-50 nm) provides an optimal balance between surface area and transport properties. Micro-porosity (<2 nm) maximizes surface area but may introduce diffusion constraints for larger molecules.
Thermal and mechanical stability considerations cannot be overlooked when designing metal mesh catalysts. Operating temperatures in catalytic processes often reach several hundred degrees Celsius, necessitating materials that resist sintering and maintain structural integrity. Mechanical stability under flow conditions prevents catalyst degradation through attrition or fragmentation.
Compositional engineering through alloying or doping introduces electronic and geometric effects that can dramatically enhance catalytic performance. Bimetallic and multimetallic systems often exhibit synergistic effects that surpass the performance of individual components. Surface modification techniques such as atomic layer deposition allow precise control over catalyst composition at the nanoscale.
Advanced characterization techniques including scanning electron microscopy, transmission electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy are essential for understanding structure-property relationships in metal mesh catalysts. These methods provide critical insights into morphology, crystallinity, electronic structure, and surface chemistry that govern catalytic behavior.
Recent developments in computational materials science have enabled rational design of metal mesh catalysts through density functional theory calculations and molecular dynamics simulations. These approaches allow prediction of adsorption energies, reaction pathways, and catalytic activities before experimental validation, accelerating the discovery of high-performance catalytic materials with optimized mesh dimensions.
Surface characteristics of metal meshes directly impact reaction kinetics. Roughness at the microscale increases the effective surface area available for catalytic reactions, while nanoscale features can create unique electronic environments that alter activation energies. Surface crystallography also plays a crucial role, as different crystal facets exhibit varying catalytic activities toward specific reactions.
Porosity architecture represents another critical dimension affecting catalytic efficiency. Macro-porosity (>50 nm) facilitates mass transport of reactants and products, reducing diffusion limitations. Meso-porosity (2-50 nm) provides an optimal balance between surface area and transport properties. Micro-porosity (<2 nm) maximizes surface area but may introduce diffusion constraints for larger molecules.
Thermal and mechanical stability considerations cannot be overlooked when designing metal mesh catalysts. Operating temperatures in catalytic processes often reach several hundred degrees Celsius, necessitating materials that resist sintering and maintain structural integrity. Mechanical stability under flow conditions prevents catalyst degradation through attrition or fragmentation.
Compositional engineering through alloying or doping introduces electronic and geometric effects that can dramatically enhance catalytic performance. Bimetallic and multimetallic systems often exhibit synergistic effects that surpass the performance of individual components. Surface modification techniques such as atomic layer deposition allow precise control over catalyst composition at the nanoscale.
Advanced characterization techniques including scanning electron microscopy, transmission electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy are essential for understanding structure-property relationships in metal mesh catalysts. These methods provide critical insights into morphology, crystallinity, electronic structure, and surface chemistry that govern catalytic behavior.
Recent developments in computational materials science have enabled rational design of metal mesh catalysts through density functional theory calculations and molecular dynamics simulations. These approaches allow prediction of adsorption energies, reaction pathways, and catalytic activities before experimental validation, accelerating the discovery of high-performance catalytic materials with optimized mesh dimensions.
Scalability and Industrial Implementation
The scalability of metal mesh catalytic systems represents a critical factor in their industrial adoption and commercial viability. Current laboratory-scale research demonstrates promising catalytic efficiency relationships with mesh dimensions, but translating these findings to industrial scale presents significant engineering challenges. Production facilities must consider the economic feasibility of manufacturing precise mesh dimensions consistently across large surface areas while maintaining structural integrity and catalytic performance.
Industrial implementation requires specialized equipment for precise metal mesh fabrication at scale. Wire drawing and weaving technologies must be adapted to produce meshes with controlled aperture sizes, wire diameters, and surface treatments. The capital investment for such equipment varies significantly based on the required precision, with higher-precision mesh manufacturing demanding substantially greater investment but potentially yielding superior catalytic performance.
Quality control systems become increasingly important at industrial scale, as dimensional variations that might be negligible in laboratory settings can lead to significant performance inconsistencies across large catalytic units. Advanced inspection technologies, including automated optical systems and real-time monitoring, are essential for ensuring dimensional consistency throughout production runs.
Cost-benefit analysis reveals that optimizing mesh dimensions can significantly impact operational economics. While finer meshes with smaller apertures typically demonstrate higher catalytic efficiency due to increased surface area, they also present greater pressure drop across catalytic beds, necessitating higher energy input for fluid transport. This trade-off must be carefully balanced against production throughput requirements and energy costs.
Maintenance considerations also influence industrial implementation strategies. Metal meshes with optimized dimensions for catalytic efficiency may experience different fouling rates or mechanical stress patterns during operation. Preventive maintenance protocols must be developed specifically for each mesh configuration to ensure sustained performance and extended service life.
Scaling production also introduces new opportunities for dimensional customization across different zones of industrial catalytic reactors. Strategic variation of mesh dimensions throughout a reactor can optimize reaction kinetics at different stages of the catalytic process, potentially improving overall system efficiency beyond what uniform mesh applications can achieve.
Industrial implementation requires specialized equipment for precise metal mesh fabrication at scale. Wire drawing and weaving technologies must be adapted to produce meshes with controlled aperture sizes, wire diameters, and surface treatments. The capital investment for such equipment varies significantly based on the required precision, with higher-precision mesh manufacturing demanding substantially greater investment but potentially yielding superior catalytic performance.
Quality control systems become increasingly important at industrial scale, as dimensional variations that might be negligible in laboratory settings can lead to significant performance inconsistencies across large catalytic units. Advanced inspection technologies, including automated optical systems and real-time monitoring, are essential for ensuring dimensional consistency throughout production runs.
Cost-benefit analysis reveals that optimizing mesh dimensions can significantly impact operational economics. While finer meshes with smaller apertures typically demonstrate higher catalytic efficiency due to increased surface area, they also present greater pressure drop across catalytic beds, necessitating higher energy input for fluid transport. This trade-off must be carefully balanced against production throughput requirements and energy costs.
Maintenance considerations also influence industrial implementation strategies. Metal meshes with optimized dimensions for catalytic efficiency may experience different fouling rates or mechanical stress patterns during operation. Preventive maintenance protocols must be developed specifically for each mesh configuration to ensure sustained performance and extended service life.
Scaling production also introduces new opportunities for dimensional customization across different zones of industrial catalytic reactors. Strategic variation of mesh dimensions throughout a reactor can optimize reaction kinetics at different stages of the catalytic process, potentially improving overall system efficiency beyond what uniform mesh applications can achieve.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!