Metal Mesh Design Standards for Pharmaceutical Applications
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Pharmaceutical Metal Mesh Technology Background and Objectives
Metal mesh technology in pharmaceutical applications has evolved significantly over the past several decades, transitioning from basic filtration systems to sophisticated precision-engineered components. Initially developed for simple separation processes in the 1950s, metal mesh technology has progressively incorporated advanced materials science and precision manufacturing techniques to meet the increasingly stringent requirements of pharmaceutical production.
The evolution of pharmaceutical regulations, particularly Good Manufacturing Practice (GMP) guidelines established by regulatory bodies such as the FDA and EMA, has been a primary driver in metal mesh technology advancement. These regulations have necessitated the development of materials and designs that minimize contamination risks, ensure batch consistency, and provide documented validation of performance parameters.
Current metal mesh technologies in pharmaceutical applications encompass a diverse range of products including filter screens, tablet press components, powder classification systems, and sterile processing equipment. The technical specifications for these components have become increasingly precise, with tolerances now commonly measured in microns rather than millimeters, reflecting the industry's movement toward nanoscale medicine production.
Material innovation represents another significant aspect of the technology's evolution. While traditional stainless steel (primarily 316L grade) remains prevalent, specialized alloys, titanium-based materials, and composite metal meshes have emerged to address specific pharmaceutical processing challenges such as corrosion resistance, biocompatibility, and enhanced durability under aggressive cleaning protocols.
The primary objective of metal mesh design standards in pharmaceutical applications is to establish comprehensive guidelines that ensure consistent product quality, patient safety, and regulatory compliance. These standards must address critical parameters including mesh aperture uniformity, material composition, surface finish requirements, mechanical integrity, and cleanability characteristics.
Additional objectives include the development of standardized testing methodologies to validate mesh performance across various pharmaceutical processes, from API production to finished dosage form manufacturing. These testing protocols must verify critical attributes such as particle retention efficiency, flow characteristics, and resistance to degradation under repeated sterilization cycles.
Looking forward, the technology roadmap for pharmaceutical metal mesh standards must anticipate emerging trends in pharmaceutical manufacturing, including continuous processing technologies, personalized medicine production, and advanced therapy medicinal products. Standards development must also consider the increasing implementation of Process Analytical Technology (PAT) and Quality by Design (QbD) approaches that require real-time monitoring capabilities and predictable performance characteristics.
The evolution of pharmaceutical regulations, particularly Good Manufacturing Practice (GMP) guidelines established by regulatory bodies such as the FDA and EMA, has been a primary driver in metal mesh technology advancement. These regulations have necessitated the development of materials and designs that minimize contamination risks, ensure batch consistency, and provide documented validation of performance parameters.
Current metal mesh technologies in pharmaceutical applications encompass a diverse range of products including filter screens, tablet press components, powder classification systems, and sterile processing equipment. The technical specifications for these components have become increasingly precise, with tolerances now commonly measured in microns rather than millimeters, reflecting the industry's movement toward nanoscale medicine production.
Material innovation represents another significant aspect of the technology's evolution. While traditional stainless steel (primarily 316L grade) remains prevalent, specialized alloys, titanium-based materials, and composite metal meshes have emerged to address specific pharmaceutical processing challenges such as corrosion resistance, biocompatibility, and enhanced durability under aggressive cleaning protocols.
The primary objective of metal mesh design standards in pharmaceutical applications is to establish comprehensive guidelines that ensure consistent product quality, patient safety, and regulatory compliance. These standards must address critical parameters including mesh aperture uniformity, material composition, surface finish requirements, mechanical integrity, and cleanability characteristics.
Additional objectives include the development of standardized testing methodologies to validate mesh performance across various pharmaceutical processes, from API production to finished dosage form manufacturing. These testing protocols must verify critical attributes such as particle retention efficiency, flow characteristics, and resistance to degradation under repeated sterilization cycles.
Looking forward, the technology roadmap for pharmaceutical metal mesh standards must anticipate emerging trends in pharmaceutical manufacturing, including continuous processing technologies, personalized medicine production, and advanced therapy medicinal products. Standards development must also consider the increasing implementation of Process Analytical Technology (PAT) and Quality by Design (QbD) approaches that require real-time monitoring capabilities and predictable performance characteristics.
Market Demand Analysis for Pharmaceutical Metal Mesh Solutions
The pharmaceutical industry's demand for specialized metal mesh solutions has experienced significant growth over the past decade, driven primarily by increasing regulatory requirements and the need for enhanced production efficiency. Market research indicates that the global pharmaceutical processing equipment market, which includes metal mesh components, is currently valued at approximately 10.3 billion USD and is projected to grow at a compound annual growth rate of 5.7% through 2028.
Metal mesh solutions serve critical functions in pharmaceutical manufacturing processes, including filtration, separation, containment, and support structures for various production stages. The demand is particularly strong in solid dosage form manufacturing, which accounts for nearly 60% of all pharmaceutical products globally. Within this segment, tablet production lines require specialized mesh designs for sieving, classification, and quality control.
Regulatory changes have substantially influenced market demand patterns. The implementation of more stringent Good Manufacturing Practice (GMP) standards by the FDA, EMA, and other regulatory bodies has necessitated pharmaceutical manufacturers to upgrade their equipment to meet higher cleanliness and cross-contamination prevention requirements. This regulatory pressure has created a significant replacement market for older mesh systems that no longer comply with current standards.
Geographically, North America and Europe currently represent the largest markets for pharmaceutical metal mesh solutions, collectively accounting for approximately 65% of global demand. However, the Asia-Pacific region, particularly China and India, is experiencing the fastest growth rate at nearly 8.5% annually, reflecting the rapid expansion of pharmaceutical manufacturing capacity in these regions.
The continuous drive toward process optimization and cost reduction in pharmaceutical manufacturing has created specific demand for advanced metal mesh designs. Manufacturers are seeking solutions that minimize product loss, reduce cleaning time between batches, and extend operational lifespan. Market surveys indicate that pharmaceutical companies are willing to invest 15-20% more in premium metal mesh solutions that demonstrably improve production efficiency and reduce total cost of ownership.
Customization has emerged as a key market requirement, with over 70% of pharmaceutical manufacturers expressing preference for tailored mesh solutions designed for specific applications rather than generic products. This trend is particularly evident in specialized pharmaceutical segments such as biologics manufacturing, where unique process requirements necessitate purpose-designed filtration and separation systems.
The growing focus on continuous manufacturing processes in pharmaceuticals is creating new opportunities for metal mesh applications. As the industry gradually shifts from batch processing to continuous production, demand is increasing for mesh designs capable of supporting higher throughput rates while maintaining strict quality standards.
Metal mesh solutions serve critical functions in pharmaceutical manufacturing processes, including filtration, separation, containment, and support structures for various production stages. The demand is particularly strong in solid dosage form manufacturing, which accounts for nearly 60% of all pharmaceutical products globally. Within this segment, tablet production lines require specialized mesh designs for sieving, classification, and quality control.
Regulatory changes have substantially influenced market demand patterns. The implementation of more stringent Good Manufacturing Practice (GMP) standards by the FDA, EMA, and other regulatory bodies has necessitated pharmaceutical manufacturers to upgrade their equipment to meet higher cleanliness and cross-contamination prevention requirements. This regulatory pressure has created a significant replacement market for older mesh systems that no longer comply with current standards.
Geographically, North America and Europe currently represent the largest markets for pharmaceutical metal mesh solutions, collectively accounting for approximately 65% of global demand. However, the Asia-Pacific region, particularly China and India, is experiencing the fastest growth rate at nearly 8.5% annually, reflecting the rapid expansion of pharmaceutical manufacturing capacity in these regions.
The continuous drive toward process optimization and cost reduction in pharmaceutical manufacturing has created specific demand for advanced metal mesh designs. Manufacturers are seeking solutions that minimize product loss, reduce cleaning time between batches, and extend operational lifespan. Market surveys indicate that pharmaceutical companies are willing to invest 15-20% more in premium metal mesh solutions that demonstrably improve production efficiency and reduce total cost of ownership.
Customization has emerged as a key market requirement, with over 70% of pharmaceutical manufacturers expressing preference for tailored mesh solutions designed for specific applications rather than generic products. This trend is particularly evident in specialized pharmaceutical segments such as biologics manufacturing, where unique process requirements necessitate purpose-designed filtration and separation systems.
The growing focus on continuous manufacturing processes in pharmaceuticals is creating new opportunities for metal mesh applications. As the industry gradually shifts from batch processing to continuous production, demand is increasing for mesh designs capable of supporting higher throughput rates while maintaining strict quality standards.
Current Standards and Technical Challenges in Pharmaceutical Metal Mesh
The pharmaceutical industry employs metal mesh components in various critical applications, including filtration systems, tablet press tooling, and containment solutions. Currently, several international standards govern the design and implementation of metal mesh in pharmaceutical settings. The American Society for Testing and Materials (ASTM) provides comprehensive guidelines for metal mesh specifications, particularly ASTM E2016 which addresses particle size analysis and mesh calibration requirements. Similarly, the International Organization for Standardization (ISO) has established ISO 9044 for industrial woven wire cloth specifications, which pharmaceutical manufacturers must adhere to for quality assurance.
Despite these established frameworks, significant technical challenges persist in pharmaceutical metal mesh applications. Material compatibility represents a primary concern, as mesh materials must resist corrosion from aggressive pharmaceutical compounds while avoiding contamination of the product. The industry struggles with standardizing alloy compositions that balance mechanical strength with chemical resistance across diverse pharmaceutical processes.
Precision and consistency in mesh aperture size present another substantial challenge. Current manufacturing techniques struggle to maintain uniform openings at microscale dimensions (below 10 microns), leading to variability in filtration efficiency and product quality. This inconsistency becomes particularly problematic for high-potency active pharmaceutical ingredients (HPAPIs) where precise particle control is essential for safety and efficacy.
Cleanability and sterilization compatibility create additional complications for metal mesh design. The intricate geometries of mesh structures often form crevices where pharmaceutical residues can accumulate, complicating validation of cleaning procedures. Current standards inadequately address the relationship between mesh design parameters and cleanability metrics, leaving manufacturers to develop proprietary solutions.
Regulatory fragmentation further complicates standardization efforts. Different regions maintain varying requirements for metal mesh certification in pharmaceutical applications. The FDA's requirements for materials in contact with pharmaceuticals differ from those of the European Medicines Agency, creating compliance challenges for global manufacturers and hindering the development of universally accepted design standards.
Emerging manufacturing technologies, particularly additive manufacturing, are disrupting traditional mesh design paradigms. While these technologies offer unprecedented control over mesh geometry and material properties, existing standards have not evolved sufficiently to accommodate these innovations. The lack of standardized testing protocols for additively manufactured mesh components creates regulatory uncertainty and slows industry adoption.
Cross-contamination prevention remains a persistent challenge, particularly in multi-product facilities. Current standards provide limited guidance on mesh design features that minimize product carryover between batches, leaving manufacturers to develop custom solutions through trial and error rather than following established design principles.
Despite these established frameworks, significant technical challenges persist in pharmaceutical metal mesh applications. Material compatibility represents a primary concern, as mesh materials must resist corrosion from aggressive pharmaceutical compounds while avoiding contamination of the product. The industry struggles with standardizing alloy compositions that balance mechanical strength with chemical resistance across diverse pharmaceutical processes.
Precision and consistency in mesh aperture size present another substantial challenge. Current manufacturing techniques struggle to maintain uniform openings at microscale dimensions (below 10 microns), leading to variability in filtration efficiency and product quality. This inconsistency becomes particularly problematic for high-potency active pharmaceutical ingredients (HPAPIs) where precise particle control is essential for safety and efficacy.
Cleanability and sterilization compatibility create additional complications for metal mesh design. The intricate geometries of mesh structures often form crevices where pharmaceutical residues can accumulate, complicating validation of cleaning procedures. Current standards inadequately address the relationship between mesh design parameters and cleanability metrics, leaving manufacturers to develop proprietary solutions.
Regulatory fragmentation further complicates standardization efforts. Different regions maintain varying requirements for metal mesh certification in pharmaceutical applications. The FDA's requirements for materials in contact with pharmaceuticals differ from those of the European Medicines Agency, creating compliance challenges for global manufacturers and hindering the development of universally accepted design standards.
Emerging manufacturing technologies, particularly additive manufacturing, are disrupting traditional mesh design paradigms. While these technologies offer unprecedented control over mesh geometry and material properties, existing standards have not evolved sufficiently to accommodate these innovations. The lack of standardized testing protocols for additively manufactured mesh components creates regulatory uncertainty and slows industry adoption.
Cross-contamination prevention remains a persistent challenge, particularly in multi-product facilities. Current standards provide limited guidance on mesh design features that minimize product carryover between batches, leaving manufacturers to develop custom solutions through trial and error rather than following established design principles.
Current Metal Mesh Design Solutions for Pharmaceutical Processing
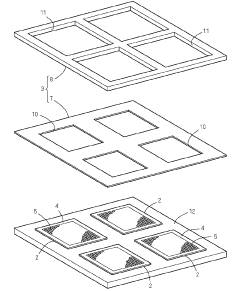
01 Metal mesh for electromagnetic shielding
Metal mesh structures are used for electromagnetic shielding applications, providing protection against electromagnetic interference (EMI) and radio frequency interference (RFI). These meshes are designed with specific patterns and dimensions to block or attenuate electromagnetic waves while maintaining other properties such as optical transparency when required. The mesh can be incorporated into various devices including electronic displays, windows, and enclosures to prevent electromagnetic radiation from entering or escaping.- Metal mesh for touch screen applications: Metal mesh structures are used in touch screen technologies to create transparent conductive patterns. These meshes provide excellent electrical conductivity while maintaining optical transparency, making them ideal for touchscreens in various electronic devices. The metal mesh patterns can be designed with specific geometries to optimize touch sensitivity and reduce visibility of the conductive pattern to users.
- Manufacturing methods for metal mesh structures: Various manufacturing techniques are employed to produce metal mesh structures with precise dimensions and properties. These methods include photolithography, etching processes, printing techniques, and deposition methods. Advanced manufacturing approaches allow for the creation of fine metal patterns with controlled thickness, width, and spacing, which are critical for the performance of the resulting mesh in different applications.
- Metal mesh for electromagnetic shielding: Metal mesh structures are utilized for electromagnetic interference (EMI) shielding in electronic devices and components. The mesh design allows for effective blocking of electromagnetic radiation while maintaining other necessary properties such as ventilation or visibility. The mesh density, material composition, and structural configuration can be optimized for specific frequency ranges and shielding requirements.
- Metal mesh for battery and electrode applications: Metal mesh structures serve as current collectors and structural supports in batteries and electrochemical devices. The mesh design provides high surface area, good mechanical stability, and efficient electron transport pathways. These properties make metal meshes valuable components in energy storage technologies, where they can enhance performance metrics such as power density, cycle life, and charge/discharge rates.
- Specialized coatings and treatments for metal mesh: Metal mesh structures can be enhanced through various surface treatments and coatings to improve their properties for specific applications. These treatments may include corrosion-resistant layers, conductive coatings, or functional materials that add properties such as hydrophobicity or antimicrobial activity. Surface modifications can significantly extend the lifespan and functionality of metal mesh components in challenging environments.
02 Touch screen applications with metal mesh
Metal mesh structures are utilized in touch screen technologies, providing conductive patterns that can detect touch inputs while maintaining optical transparency. These meshes are fabricated with precise geometries to balance conductivity, visibility, and touch sensitivity. The metal mesh electrodes can be patterned on substrates using various deposition and etching techniques, creating invisible conductive networks that enable touch functionality in displays for smartphones, tablets, and other interactive devices.Expand Specific Solutions03 Manufacturing methods for metal mesh
Various manufacturing techniques are employed to produce metal mesh structures with precise specifications. These methods include photolithography, etching, electroplating, screen printing, and roll-to-roll processing. Advanced fabrication approaches allow for the creation of ultra-fine mesh patterns with controlled line width, spacing, and thickness. The manufacturing processes can be optimized to achieve specific electrical, optical, and mechanical properties required for different applications, while also considering factors such as production efficiency and cost-effectiveness.Expand Specific Solutions04 Metal mesh for filtration and separation
Metal mesh structures serve as effective filtration and separation media across various industries. These meshes are designed with specific pore sizes, wire diameters, and weave patterns to filter particles, separate materials, or support catalysts. Applications include industrial sieves, filters for liquids and gases, catalyst supports, and separation membranes. The meshes can be fabricated from various metals and alloys to provide resistance to corrosion, high temperatures, and mechanical stress while maintaining filtration efficiency.Expand Specific Solutions05 Structural applications of metal mesh
Metal mesh is utilized in various structural applications to provide reinforcement, support, or protection. These applications include reinforcement in composite materials, architectural elements, safety barriers, and protective enclosures. The mesh structures can be designed with specific patterns, wire diameters, and spacing to achieve desired mechanical properties such as strength, flexibility, and impact resistance. Metal meshes can also be incorporated into building facades, providing both aesthetic appeal and functional benefits such as sun shading or ventilation.Expand Specific Solutions
Key Industry Players in Pharmaceutical Metal Mesh Manufacturing
The metal mesh design standards for pharmaceutical applications market is in a growth phase, characterized by increasing demand for standardized solutions in drug manufacturing and delivery systems. The market size is expanding due to pharmaceutical industry's focus on quality control and regulatory compliance. Technologically, the field is moderately mature with established players like Atrium Medical, Fresenius Kabi, and Genentech leading innovation in mesh materials for drug delivery systems. Research institutions including Zhengzhou University and Tianjin University contribute significantly to technical advancements. Companies like Merck, F. Hoffmann-La Roche, and Janssen Pharmaceutica are integrating these technologies into pharmaceutical manufacturing processes, while specialized firms such as LifeCell and FibroGen focus on tissue engineering applications utilizing metal mesh technologies.
Atrium Medical Corp.
Technical Solution: Atrium Medical has developed specialized metal mesh technologies for pharmaceutical and medical device applications, with particular focus on implantable drug delivery systems. Their proprietary designs utilize precision-woven stainless steel and nitinol meshes with controlled porosity ranging from 20-200 microns. These meshes incorporate surface treatments that enhance biocompatibility while minimizing inflammatory responses in vivo. Atrium's technology includes specialized coating processes that enable controlled elution of pharmaceutical agents from the metal substrate over extended periods. Their metal mesh platforms have been implemented in various medical devices including vascular stents and tissue reinforcement applications where drug delivery capabilities enhance therapeutic outcomes. The company maintains comprehensive design controls compliant with ISO 10993 biocompatibility standards.
Strengths: Excellent in vivo performance with minimal foreign body response and proven long-term stability in physiological environments. Established regulatory pathway with multiple approved products. Weaknesses: Limited throughput capacity for high-volume pharmaceutical manufacturing applications and relatively narrow range of compatible pharmaceutical compounds.
Fresenius Kabi Deutschland GmbH
Technical Solution: Fresenius Kabi has developed comprehensive metal mesh filtration systems specifically designed for parenteral pharmaceutical manufacturing. Their technology incorporates multi-layer sintered stainless steel meshes with defined pore structures ranging from 0.2-10 microns that meet stringent particulate control requirements. These mesh systems feature electropolished surfaces that minimize product adhesion and enhance cleanability in pharmaceutical processing environments. Fresenius Kabi's designs include specialized flow distribution patterns that optimize filtration efficiency while reducing pressure drop across the filter media. The company has implemented these technologies in their aseptic filling operations with validated cleaning and sterilization protocols that ensure consistent performance across multiple production cycles while maintaining compliance with EU GMP Annex 1 and FDA aseptic processing guidelines.
Strengths: Exceptional particle retention capabilities with documented endotoxin reduction properties. Comprehensive validation documentation supporting implementation in GMP environments. Weaknesses: Higher initial capital investment compared to disposable filtration systems and more complex validation requirements for implementation.
Critical Patents and Technical Literature in Pharmaceutical Metal Mesh
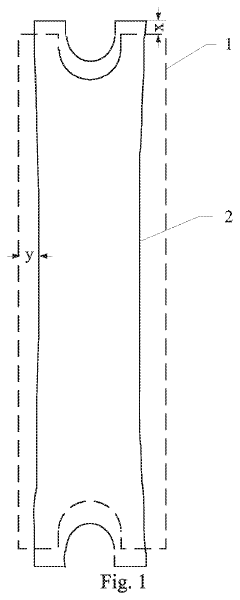
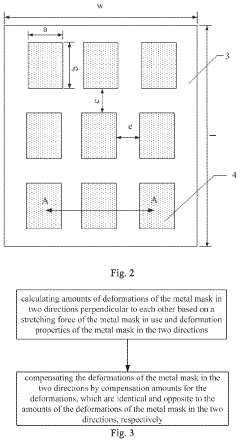
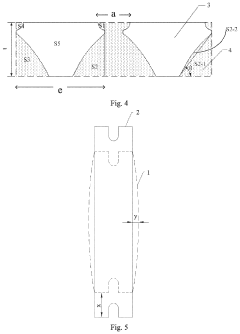
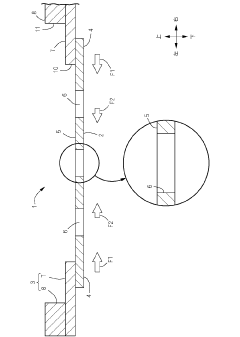
Design method of metal mask and manufacturing method of metal mask
PatentActiveUS20210187576A1
Innovation
- A design method that calculates deformation properties in two perpendicular directions based on stretching force and material properties, including Young's modulus and Poisson's ratio, to determine compensation amounts for deformations, allowing for direct incorporation of these values during the design and manufacturing of the metal mask.
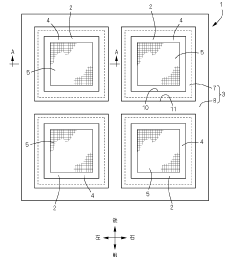
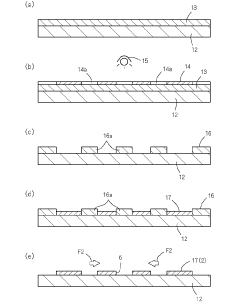
Metal mask and production method of the same
PatentActiveJP2022097146A
Innovation
- A metal mask design with a frame having a smaller thermal expansion coefficient than the mask body, applying both tensile and compressive stresses to counteract thermal distortions, ensuring precise alignment of through-holes by adjusting the thermal expansion coefficients and using a multi-step electroforming process to integrate the frame with the mask body.
Regulatory Compliance and Quality Assurance Standards
Regulatory compliance in pharmaceutical metal mesh applications is governed by a complex framework of international standards and guidelines. The FDA's Current Good Manufacturing Practice (cGMP) regulations serve as the cornerstone for pharmaceutical equipment design in the United States, with specific requirements for materials that contact pharmaceutical products. These regulations mandate that metal mesh components must be non-reactive, non-additive, and non-absorptive to maintain product integrity throughout the manufacturing process.
The European Medicines Agency (EMA) provides complementary guidelines through EU GMP Annex 1, which specifically addresses requirements for sterile medicinal products and places stringent demands on filtration materials including metal mesh components. These standards emphasize validation protocols that demonstrate consistent performance under worst-case production conditions.
ISO 9001 and ISO 13485 certification requirements extend quality management principles to metal mesh manufacturing, ensuring documented processes for design, production, and validation. For pharmaceutical applications, additional compliance with ISO 14644 (cleanroom standards) is often necessary, as metal mesh components frequently operate in controlled environments.
Material selection standards represent another critical compliance area. USP <87>, <88>, and <661> establish testing protocols for biocompatibility and extractables/leachables, while ASTM F838-15a provides standardized methods for bacterial retention testing of metal mesh filters. The 3-A Sanitary Standards, though primarily focused on food processing, have been widely adopted in pharmaceutical applications for their rigorous hygienic design principles.
Quality assurance for pharmaceutical metal mesh requires comprehensive documentation systems. This includes Design History Files (DHF), Device Master Records (DMR), and Device History Records (DHR) that track the entire lifecycle from initial design specifications through production and deployment. Validation protocols must follow GAMP 5 guidelines, with particular emphasis on Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
Risk management frameworks such as ICH Q9 provide methodologies for identifying and mitigating potential failure points in metal mesh applications. These approaches typically incorporate Failure Mode and Effects Analysis (FMEA) to systematically evaluate potential risks to product quality and patient safety. Manufacturers must demonstrate ongoing compliance through regular audits and continuous monitoring programs that verify consistent performance against established specifications.
The increasing global harmonization of pharmaceutical regulations through initiatives like the Pharmaceutical Inspection Co-operation Scheme (PIC/S) is gradually standardizing requirements across international markets, though regional variations in metal mesh compliance requirements persist and must be carefully navigated by manufacturers serving multiple markets.
The European Medicines Agency (EMA) provides complementary guidelines through EU GMP Annex 1, which specifically addresses requirements for sterile medicinal products and places stringent demands on filtration materials including metal mesh components. These standards emphasize validation protocols that demonstrate consistent performance under worst-case production conditions.
ISO 9001 and ISO 13485 certification requirements extend quality management principles to metal mesh manufacturing, ensuring documented processes for design, production, and validation. For pharmaceutical applications, additional compliance with ISO 14644 (cleanroom standards) is often necessary, as metal mesh components frequently operate in controlled environments.
Material selection standards represent another critical compliance area. USP <87>, <88>, and <661> establish testing protocols for biocompatibility and extractables/leachables, while ASTM F838-15a provides standardized methods for bacterial retention testing of metal mesh filters. The 3-A Sanitary Standards, though primarily focused on food processing, have been widely adopted in pharmaceutical applications for their rigorous hygienic design principles.
Quality assurance for pharmaceutical metal mesh requires comprehensive documentation systems. This includes Design History Files (DHF), Device Master Records (DMR), and Device History Records (DHR) that track the entire lifecycle from initial design specifications through production and deployment. Validation protocols must follow GAMP 5 guidelines, with particular emphasis on Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
Risk management frameworks such as ICH Q9 provide methodologies for identifying and mitigating potential failure points in metal mesh applications. These approaches typically incorporate Failure Mode and Effects Analysis (FMEA) to systematically evaluate potential risks to product quality and patient safety. Manufacturers must demonstrate ongoing compliance through regular audits and continuous monitoring programs that verify consistent performance against established specifications.
The increasing global harmonization of pharmaceutical regulations through initiatives like the Pharmaceutical Inspection Co-operation Scheme (PIC/S) is gradually standardizing requirements across international markets, though regional variations in metal mesh compliance requirements persist and must be carefully navigated by manufacturers serving multiple markets.
Material Compatibility and Contamination Control Strategies
In pharmaceutical applications, material compatibility is a critical consideration for metal mesh design. Stainless steel, particularly grades 316 and 316L, remains the predominant material due to its excellent corrosion resistance and minimal particle shedding characteristics. These properties are essential in preventing product contamination during pharmaceutical manufacturing processes. Additionally, titanium alloys have gained traction for specialized applications requiring enhanced chemical resistance, though their higher cost often limits widespread adoption.
Material selection must account for compatibility with various pharmaceutical compounds, cleaning agents, and sterilization methods. Incompatible materials can lead to chemical reactions, leaching of metal ions, or degradation of the mesh structure, all of which compromise product integrity. Recent studies indicate that even trace amounts of metal ions can catalyze unwanted reactions in certain pharmaceutical formulations, highlighting the importance of proper material selection.
Contamination control strategies begin with rigorous material testing protocols. The pharmaceutical industry has adopted standardized leachable and extractable testing methodologies to evaluate potential contamination risks. These tests simulate worst-case scenarios by exposing mesh materials to aggressive solvents and conditions exceeding normal operating parameters. Surface treatment technologies, including electropolishing and passivation, have emerged as effective methods for reducing contamination risks by creating smoother surfaces that resist particle adhesion and bacterial colonization.
Cleanability represents another crucial aspect of contamination control. Metal mesh designs must facilitate thorough cleaning and sanitization without harboring residues. This requirement has driven innovations in mesh geometry, with open, uniform structures that minimize dead zones where contaminants might accumulate. The industry trend toward continuous manufacturing has further emphasized the need for mesh designs that support clean-in-place (CIP) and sterilize-in-place (SIP) protocols.
Documentation and traceability systems form the backbone of contamination control strategies. Material certificates, surface roughness measurements, and cleaning validation studies must be maintained throughout the mesh lifecycle. The implementation of digital tracking systems has improved this process, allowing manufacturers to trace specific mesh components from raw material to final installation.
Regulatory compliance frameworks, including FDA and EMA guidelines, have established increasingly stringent requirements for material compatibility documentation. These regulations emphasize risk-based approaches to material selection and contamination control, requiring manufacturers to demonstrate comprehensive understanding of potential interaction mechanisms between metal mesh components and pharmaceutical products.
Material selection must account for compatibility with various pharmaceutical compounds, cleaning agents, and sterilization methods. Incompatible materials can lead to chemical reactions, leaching of metal ions, or degradation of the mesh structure, all of which compromise product integrity. Recent studies indicate that even trace amounts of metal ions can catalyze unwanted reactions in certain pharmaceutical formulations, highlighting the importance of proper material selection.
Contamination control strategies begin with rigorous material testing protocols. The pharmaceutical industry has adopted standardized leachable and extractable testing methodologies to evaluate potential contamination risks. These tests simulate worst-case scenarios by exposing mesh materials to aggressive solvents and conditions exceeding normal operating parameters. Surface treatment technologies, including electropolishing and passivation, have emerged as effective methods for reducing contamination risks by creating smoother surfaces that resist particle adhesion and bacterial colonization.
Cleanability represents another crucial aspect of contamination control. Metal mesh designs must facilitate thorough cleaning and sanitization without harboring residues. This requirement has driven innovations in mesh geometry, with open, uniform structures that minimize dead zones where contaminants might accumulate. The industry trend toward continuous manufacturing has further emphasized the need for mesh designs that support clean-in-place (CIP) and sterilize-in-place (SIP) protocols.
Documentation and traceability systems form the backbone of contamination control strategies. Material certificates, surface roughness measurements, and cleaning validation studies must be maintained throughout the mesh lifecycle. The implementation of digital tracking systems has improved this process, allowing manufacturers to trace specific mesh components from raw material to final installation.
Regulatory compliance frameworks, including FDA and EMA guidelines, have established increasingly stringent requirements for material compatibility documentation. These regulations emphasize risk-based approaches to material selection and contamination control, requiring manufacturers to demonstrate comprehensive understanding of potential interaction mechanisms between metal mesh components and pharmaceutical products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!