How Ethyl Acetate Innovates in Cosmetic Formulations?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate in Cosmetics: Background and Objectives
Ethyl acetate, a versatile organic compound, has been a staple in the cosmetics industry for decades. Its journey from a simple solvent to a multifunctional ingredient in cosmetic formulations reflects the evolving landscape of beauty product development. Initially utilized primarily for its solvent properties, ethyl acetate has gradually emerged as a key player in innovative cosmetic formulations, offering a unique blend of benefits that cater to both manufacturers and consumers.
The historical use of ethyl acetate in cosmetics can be traced back to the mid-20th century when it was predominantly employed as a solvent in nail polish removers. Its ability to effectively dissolve nail polish without causing excessive dryness to the nails and surrounding skin made it a preferred choice over harsher alternatives. This initial application paved the way for further exploration of ethyl acetate's potential in various cosmetic products.
As the cosmetics industry progressed, formulators began to recognize the broader potential of ethyl acetate beyond its solvent capabilities. Its low toxicity, pleasant fruity odor, and rapid evaporation rate opened up new possibilities for its use in a wide range of cosmetic products. From fragrances to skincare formulations, ethyl acetate started to find its place in diverse applications, contributing to improved product performance and user experience.
The evolution of ethyl acetate's role in cosmetics aligns with the industry's growing focus on multifunctional ingredients. As consumers demand more from their beauty products, formulators are challenged to create innovative solutions that address multiple concerns simultaneously. Ethyl acetate's unique properties position it as a valuable tool in meeting these complex demands, driving its integration into advanced cosmetic formulations.
In recent years, the cosmetics industry has witnessed a surge in research and development efforts aimed at harnessing the full potential of ethyl acetate. The primary objective of these initiatives is to explore novel applications and enhance the efficacy of existing formulations. Researchers are investigating ways to leverage ethyl acetate's properties to improve product stability, enhance active ingredient delivery, and create new textures and sensory experiences.
Looking ahead, the future of ethyl acetate in cosmetics appears promising. As sustainability becomes an increasingly critical factor in product development, ethyl acetate's potential as a bio-based ingredient is garnering attention. Efforts are underway to develop more environmentally friendly production methods, aligning with the industry's shift towards greener alternatives. Additionally, ongoing research aims to uncover new synergies between ethyl acetate and other cosmetic ingredients, potentially leading to breakthrough formulations that redefine beauty product performance.
The historical use of ethyl acetate in cosmetics can be traced back to the mid-20th century when it was predominantly employed as a solvent in nail polish removers. Its ability to effectively dissolve nail polish without causing excessive dryness to the nails and surrounding skin made it a preferred choice over harsher alternatives. This initial application paved the way for further exploration of ethyl acetate's potential in various cosmetic products.
As the cosmetics industry progressed, formulators began to recognize the broader potential of ethyl acetate beyond its solvent capabilities. Its low toxicity, pleasant fruity odor, and rapid evaporation rate opened up new possibilities for its use in a wide range of cosmetic products. From fragrances to skincare formulations, ethyl acetate started to find its place in diverse applications, contributing to improved product performance and user experience.
The evolution of ethyl acetate's role in cosmetics aligns with the industry's growing focus on multifunctional ingredients. As consumers demand more from their beauty products, formulators are challenged to create innovative solutions that address multiple concerns simultaneously. Ethyl acetate's unique properties position it as a valuable tool in meeting these complex demands, driving its integration into advanced cosmetic formulations.
In recent years, the cosmetics industry has witnessed a surge in research and development efforts aimed at harnessing the full potential of ethyl acetate. The primary objective of these initiatives is to explore novel applications and enhance the efficacy of existing formulations. Researchers are investigating ways to leverage ethyl acetate's properties to improve product stability, enhance active ingredient delivery, and create new textures and sensory experiences.
Looking ahead, the future of ethyl acetate in cosmetics appears promising. As sustainability becomes an increasingly critical factor in product development, ethyl acetate's potential as a bio-based ingredient is garnering attention. Efforts are underway to develop more environmentally friendly production methods, aligning with the industry's shift towards greener alternatives. Additionally, ongoing research aims to uncover new synergies between ethyl acetate and other cosmetic ingredients, potentially leading to breakthrough formulations that redefine beauty product performance.
Market Demand Analysis for Ethyl Acetate-based Cosmetics
The global cosmetics market has witnessed a significant shift towards innovative and sustainable ingredients, with ethyl acetate emerging as a key player in this transformation. Market analysis reveals a growing demand for ethyl acetate-based cosmetic formulations, driven by consumer preferences for eco-friendly and multifunctional products. The compound's versatility as a solvent and fragrance ingredient has positioned it as a valuable component in various cosmetic applications.
Consumer awareness regarding product ingredients has led to increased scrutiny of cosmetic formulations. Ethyl acetate, being a naturally occurring substance found in fruits and vegetables, aligns well with the clean beauty trend. This has resulted in a surge in demand for ethyl acetate in natural and organic cosmetic products, particularly in skincare and nail care segments.
The nail care industry has been a major driver of ethyl acetate demand, with the compound being a crucial ingredient in nail polish removers. The global nail care market's steady growth, coupled with the rising popularity of at-home manicure kits, has further boosted the demand for ethyl acetate-based products. Additionally, the compound's mild, fruity odor makes it an attractive alternative to harsh-smelling solvents traditionally used in nail care products.
In the skincare sector, ethyl acetate's properties as a gentle solvent and its ability to enhance the penetration of active ingredients have led to its increased incorporation in various formulations. Anti-aging creams, serums, and facial masks utilizing ethyl acetate as a carrier for active ingredients have gained traction among consumers seeking effective and safe skincare solutions.
The fragrance industry has also contributed to the rising demand for ethyl acetate. Its role as a fixative and solvent in perfumes and colognes has made it an essential component in fragrance formulations. The compound's ability to enhance the longevity and stability of fragrances has made it particularly valuable in the luxury perfume segment.
Market trends indicate a growing preference for multifunctional cosmetic products, where ethyl acetate's diverse properties can be leveraged. This has led to the development of innovative formulations that combine skincare, fragrance, and color cosmetics, further expanding the potential applications of ethyl acetate in the cosmetics industry.
Geographically, North America and Europe lead in the adoption of ethyl acetate-based cosmetics, driven by stringent regulations on cosmetic ingredients and a strong consumer base for natural and organic products. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by increasing disposable incomes and a rising beauty-conscious population.
Consumer awareness regarding product ingredients has led to increased scrutiny of cosmetic formulations. Ethyl acetate, being a naturally occurring substance found in fruits and vegetables, aligns well with the clean beauty trend. This has resulted in a surge in demand for ethyl acetate in natural and organic cosmetic products, particularly in skincare and nail care segments.
The nail care industry has been a major driver of ethyl acetate demand, with the compound being a crucial ingredient in nail polish removers. The global nail care market's steady growth, coupled with the rising popularity of at-home manicure kits, has further boosted the demand for ethyl acetate-based products. Additionally, the compound's mild, fruity odor makes it an attractive alternative to harsh-smelling solvents traditionally used in nail care products.
In the skincare sector, ethyl acetate's properties as a gentle solvent and its ability to enhance the penetration of active ingredients have led to its increased incorporation in various formulations. Anti-aging creams, serums, and facial masks utilizing ethyl acetate as a carrier for active ingredients have gained traction among consumers seeking effective and safe skincare solutions.
The fragrance industry has also contributed to the rising demand for ethyl acetate. Its role as a fixative and solvent in perfumes and colognes has made it an essential component in fragrance formulations. The compound's ability to enhance the longevity and stability of fragrances has made it particularly valuable in the luxury perfume segment.
Market trends indicate a growing preference for multifunctional cosmetic products, where ethyl acetate's diverse properties can be leveraged. This has led to the development of innovative formulations that combine skincare, fragrance, and color cosmetics, further expanding the potential applications of ethyl acetate in the cosmetics industry.
Geographically, North America and Europe lead in the adoption of ethyl acetate-based cosmetics, driven by stringent regulations on cosmetic ingredients and a strong consumer base for natural and organic products. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by increasing disposable incomes and a rising beauty-conscious population.
Current Applications and Challenges in Cosmetic Formulations
Ethyl acetate has emerged as a versatile and innovative ingredient in cosmetic formulations, offering a range of applications and benefits. Currently, it is widely used as a solvent in nail polish removers, demonstrating excellent efficacy in dissolving nail polish without causing excessive dryness to the nails and surrounding skin. Its low toxicity and mild odor make it a preferred choice over harsher alternatives like acetone.
In skincare products, ethyl acetate serves as an effective emollient and penetration enhancer. It helps to improve the texture and spreadability of creams and lotions while facilitating the absorption of active ingredients into the skin. This property has made it particularly valuable in formulations targeting deep skin hydration and anti-aging effects.
The fragrance industry has also embraced ethyl acetate for its role as a fixative in perfumes and colognes. It helps to stabilize volatile scent compounds, extending the longevity of fragrances on the skin. Additionally, its fruity aroma contributes to the overall scent profile of many cosmetic products.
Despite its widespread use, ethyl acetate faces several challenges in cosmetic formulations. One primary concern is its potential to cause skin irritation in some individuals, particularly those with sensitive skin. Formulators must carefully balance its concentration to maximize benefits while minimizing adverse reactions.
Another challenge lies in the volatile nature of ethyl acetate. Its rapid evaporation can lead to changes in product consistency and efficacy over time. This necessitates careful packaging considerations and the development of stabilizing formulations to maintain product integrity throughout its shelf life.
Environmental concerns also pose a challenge to the continued use of ethyl acetate in cosmetics. As consumers become more eco-conscious, there is growing pressure to find sustainable alternatives or develop bio-based sources of ethyl acetate. This has spurred research into green chemistry approaches for its production and incorporation into cosmetic formulations.
Regulatory compliance presents an ongoing challenge, with varying restrictions on ethyl acetate usage across different regions. Cosmetic manufacturers must navigate these regulatory landscapes to ensure their products meet global standards while maintaining the desired functionality provided by ethyl acetate.
In skincare products, ethyl acetate serves as an effective emollient and penetration enhancer. It helps to improve the texture and spreadability of creams and lotions while facilitating the absorption of active ingredients into the skin. This property has made it particularly valuable in formulations targeting deep skin hydration and anti-aging effects.
The fragrance industry has also embraced ethyl acetate for its role as a fixative in perfumes and colognes. It helps to stabilize volatile scent compounds, extending the longevity of fragrances on the skin. Additionally, its fruity aroma contributes to the overall scent profile of many cosmetic products.
Despite its widespread use, ethyl acetate faces several challenges in cosmetic formulations. One primary concern is its potential to cause skin irritation in some individuals, particularly those with sensitive skin. Formulators must carefully balance its concentration to maximize benefits while minimizing adverse reactions.
Another challenge lies in the volatile nature of ethyl acetate. Its rapid evaporation can lead to changes in product consistency and efficacy over time. This necessitates careful packaging considerations and the development of stabilizing formulations to maintain product integrity throughout its shelf life.
Environmental concerns also pose a challenge to the continued use of ethyl acetate in cosmetics. As consumers become more eco-conscious, there is growing pressure to find sustainable alternatives or develop bio-based sources of ethyl acetate. This has spurred research into green chemistry approaches for its production and incorporation into cosmetic formulations.
Regulatory compliance presents an ongoing challenge, with varying restrictions on ethyl acetate usage across different regions. Cosmetic manufacturers must navigate these regulatory landscapes to ensure their products meet global standards while maintaining the desired functionality provided by ethyl acetate.
Existing Ethyl Acetate Formulation Techniques
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The production methods aim to optimize the synthesis of ethyl acetate from its precursors, typically ethanol and acetic acid.- Production and purification of ethyl acetate: Various methods are employed for the production and purification of ethyl acetate, including esterification reactions, distillation processes, and separation techniques. These methods aim to improve yield, purity, and efficiency in the manufacturing of ethyl acetate for industrial applications.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is widely used as a solvent and reagent in various chemical processes. It finds applications in extraction, synthesis, and as a reaction medium in different industries, including pharmaceuticals, polymers, and fine chemicals.
- Ethyl acetate in coating and adhesive formulations: Ethyl acetate is utilized as a solvent in coating and adhesive formulations. It provides desirable properties such as fast evaporation, low toxicity, and compatibility with various resins and polymers, making it suitable for use in paints, varnishes, and adhesives.
- Recovery and recycling of ethyl acetate: Processes for recovering and recycling ethyl acetate from industrial waste streams and spent solvents are developed to improve sustainability and reduce environmental impact. These methods often involve distillation, membrane separation, or adsorption techniques.
- Ethyl acetate as a green solvent alternative: Ethyl acetate is explored as a more environmentally friendly solvent alternative in various applications. Its relatively low toxicity, biodegradability, and favorable properties make it a suitable replacement for more hazardous solvents in certain processes and products.
02 Applications of ethyl acetate in industrial processes
Ethyl acetate finds diverse applications in industrial processes. It is used as a solvent in various industries, including pharmaceuticals, coatings, and adhesives. The compound is also utilized in extraction processes, particularly in the food and beverage industry. Its low toxicity and favorable properties make it a versatile chemical in many manufacturing processes.Expand Specific Solutions03 Ethyl acetate in polymer and material science
Ethyl acetate plays a significant role in polymer and material science. It is used in the production of various polymers and resins, serving as a solvent or reaction medium. The compound is also employed in the development of advanced materials with specific properties, such as improved durability or enhanced performance characteristics.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
The use of ethyl acetate in industrial processes necessitates careful consideration of environmental and safety aspects. This includes developing methods for its safe handling, storage, and disposal. Efforts are also made to reduce emissions and improve the overall environmental impact of processes involving ethyl acetate, such as implementing closed-loop systems or exploring alternative, more environmentally friendly solvents.Expand Specific Solutions05 Novel synthesis routes and derivatives of ethyl acetate
Research into novel synthesis routes for ethyl acetate and its derivatives is ongoing. This includes exploring new catalysts, reaction conditions, and precursor materials to improve efficiency and yield. Additionally, the development of ethyl acetate derivatives with enhanced properties or specific functionalities for targeted applications is an area of active investigation in the field of organic chemistry.Expand Specific Solutions
Key Players in Ethyl Acetate Production and Cosmetic Industry
The ethyl acetate market in cosmetic formulations is in a growth phase, driven by increasing demand for innovative and sustainable ingredients. The market size is expanding, with major players like L'Oréal, Shiseido, and Unilever investing in research and development. Technological maturity varies, with established companies like BASF and Croda leading in advanced formulations, while newer entrants like Botanix Pharmaceuticals are exploring novel applications. The competitive landscape is diverse, featuring both multinational corporations and specialized chemical companies, indicating a dynamic and evolving market with opportunities for innovation and differentiation.
L'Oréal SA
Technical Solution: L'Oréal has innovated in using ethyl acetate in cosmetic formulations, particularly in nail care products. They have developed a range of nail polishes and removers that utilize ethyl acetate as a key solvent. The company has also explored the use of ethyl acetate in hair care products, where it acts as a carrier for active ingredients and contributes to the product's texture. L'Oréal's research has focused on optimizing the concentration of ethyl acetate to balance efficacy and safety, resulting in formulations that provide quick-drying properties for nail products and enhanced penetration of active ingredients in hair care[1][3]. Additionally, they have investigated the potential of ethyl acetate as a green solvent in eco-friendly cosmetic formulations, aligning with their sustainability initiatives[5].
Strengths: Extensive research capabilities, global market presence, and a strong focus on innovation. Weaknesses: Potential environmental concerns associated with volatile organic compounds (VOCs) in some formulations.
Shiseido Co., Ltd.
Technical Solution: Shiseido has incorporated ethyl acetate into their cosmetic formulations, focusing on its properties as a solvent and fragrance ingredient. The company has developed innovative skincare products that use ethyl acetate to enhance the delivery of active ingredients. Shiseido's research has led to the creation of lightweight, fast-absorbing serums and essences that utilize ethyl acetate's ability to dissolve both oil-soluble and water-soluble components[2]. They have also explored the use of ethyl acetate in fragrance formulations, taking advantage of its fruity, ethereal scent profile to create unique olfactory experiences. Shiseido has invested in developing proprietary blending techniques that optimize the stability and performance of ethyl acetate in their products, ensuring consistent quality and efficacy[4].
Strengths: Strong focus on R&D, expertise in Asian skincare market, and advanced formulation technologies. Weaknesses: Limited presence in some global markets compared to other major cosmetic companies.
Innovative Uses of Ethyl Acetate in Cosmetics
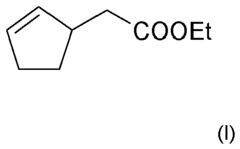
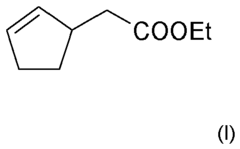
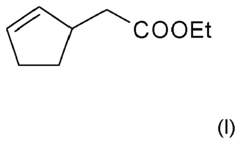
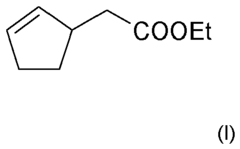
Perfume mixtures containing Cyclopent-2-Enyl-ethyl acetate
PatentActiveEP2474301A1
Innovation
- A fragrance mixture comprising cyclopent-2-enyl-acetate ethyl ester combined with one or more floral odor notes from alcohols or aldehydes, and ketones, ethers, and esters, which effectively emphasizes or masks specific odor aspects, particularly fatty and technical notes, without dominating the overall sensory impression.
Novel esters and compositions and uses thereof
PatentWO2009083735A1
Innovation
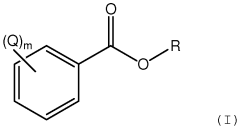
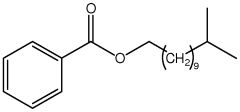
- The development of aromatic esters of branched C13 primary alcohols, specifically esters of optionally substituted benzoic acid and branched C13 primary alcohols, which provide enhanced emolliency, solubility, and sunscreen properties when incorporated into cosmetic compositions, acting as both emollients and solvents.
Safety and Regulatory Considerations for Ethyl Acetate Use
The use of ethyl acetate in cosmetic formulations is subject to stringent safety and regulatory considerations. Regulatory bodies worldwide, including the U.S. Food and Drug Administration (FDA) and the European Chemicals Agency (ECHA), have established guidelines for its safe use in cosmetic products.
In the United States, ethyl acetate is generally recognized as safe (GRAS) by the FDA for use in food and cosmetics. However, its concentration in cosmetic formulations is typically limited to ensure consumer safety. The Cosmetic Ingredient Review (CIR) Expert Panel has evaluated ethyl acetate and concluded that it is safe for use in cosmetic products when formulated to be non-irritating.
The European Union, through its Cosmetic Regulation (EC) No. 1223/2009, allows the use of ethyl acetate in cosmetic products. However, it must be listed in the ingredients when its concentration exceeds 0.001% in leave-on products and 0.01% in rinse-off products. This regulation ensures transparency and allows consumers to make informed choices.
Safety assessments for ethyl acetate in cosmetics focus on several key aspects. Skin irritation and sensitization potential are primary concerns, as ethyl acetate can cause dryness and irritation at high concentrations. Manufacturers must conduct thorough testing to determine safe concentration levels for different product types.
Inhalation toxicity is another critical consideration, particularly for products like nail polish removers where ethyl acetate is used in higher concentrations. Adequate ventilation during use and proper packaging to minimize vapor exposure are essential safety measures.
Environmental impact is also a growing concern in cosmetic regulations. While ethyl acetate is biodegradable and not considered highly toxic to aquatic life, its production and disposal still require careful management to minimize environmental footprint.
Manufacturers must adhere to Good Manufacturing Practices (GMP) when using ethyl acetate in cosmetic formulations. This includes proper handling, storage, and quality control measures to prevent contamination and ensure product stability.
As regulations evolve, cosmetic companies must stay updated on changes in safety guidelines and adjust their formulations accordingly. Ongoing research into the long-term effects of ethyl acetate exposure and its interactions with other cosmetic ingredients continues to inform regulatory decisions and industry practices.
In the United States, ethyl acetate is generally recognized as safe (GRAS) by the FDA for use in food and cosmetics. However, its concentration in cosmetic formulations is typically limited to ensure consumer safety. The Cosmetic Ingredient Review (CIR) Expert Panel has evaluated ethyl acetate and concluded that it is safe for use in cosmetic products when formulated to be non-irritating.
The European Union, through its Cosmetic Regulation (EC) No. 1223/2009, allows the use of ethyl acetate in cosmetic products. However, it must be listed in the ingredients when its concentration exceeds 0.001% in leave-on products and 0.01% in rinse-off products. This regulation ensures transparency and allows consumers to make informed choices.
Safety assessments for ethyl acetate in cosmetics focus on several key aspects. Skin irritation and sensitization potential are primary concerns, as ethyl acetate can cause dryness and irritation at high concentrations. Manufacturers must conduct thorough testing to determine safe concentration levels for different product types.
Inhalation toxicity is another critical consideration, particularly for products like nail polish removers where ethyl acetate is used in higher concentrations. Adequate ventilation during use and proper packaging to minimize vapor exposure are essential safety measures.
Environmental impact is also a growing concern in cosmetic regulations. While ethyl acetate is biodegradable and not considered highly toxic to aquatic life, its production and disposal still require careful management to minimize environmental footprint.
Manufacturers must adhere to Good Manufacturing Practices (GMP) when using ethyl acetate in cosmetic formulations. This includes proper handling, storage, and quality control measures to prevent contamination and ensure product stability.
As regulations evolve, cosmetic companies must stay updated on changes in safety guidelines and adjust their formulations accordingly. Ongoing research into the long-term effects of ethyl acetate exposure and its interactions with other cosmetic ingredients continues to inform regulatory decisions and industry practices.
Environmental Impact of Ethyl Acetate in Cosmetics
The environmental impact of ethyl acetate in cosmetics is a crucial consideration as the industry strives for sustainability. Ethyl acetate, widely used as a solvent in cosmetic formulations, presents both advantages and challenges from an environmental perspective.
One of the primary environmental concerns associated with ethyl acetate is its volatile organic compound (VOC) status. VOCs contribute to air pollution and can potentially form ground-level ozone when released into the atmosphere. However, compared to other solvents used in cosmetics, ethyl acetate has a relatively low ozone-forming potential, making it a preferable choice in terms of air quality impact.
The production of ethyl acetate also raises environmental considerations. Traditionally synthesized from ethanol and acetic acid, the manufacturing process can be energy-intensive and rely on petrochemical feedstocks. However, recent innovations have led to more sustainable production methods, including bio-based ethyl acetate derived from renewable resources such as sugarcane or corn. These eco-friendly alternatives significantly reduce the carbon footprint associated with ethyl acetate production.
In terms of biodegradability, ethyl acetate demonstrates favorable characteristics. It is readily biodegradable in both aerobic and anaerobic conditions, breaking down into ethanol and acetic acid, which are naturally occurring substances. This property minimizes its long-term environmental persistence and reduces the risk of accumulation in ecosystems.
Water pollution is another aspect to consider when evaluating the environmental impact of ethyl acetate in cosmetics. While ethyl acetate is somewhat soluble in water, its rapid biodegradation helps mitigate potential aquatic toxicity. Nevertheless, proper waste management practices are essential to prevent large-scale releases into water bodies.
The use of ethyl acetate in cosmetic formulations can indirectly contribute to environmental benefits. Its efficacy as a solvent allows for the creation of water-free or low-water formulations, potentially reducing the overall water footprint of cosmetic products. Additionally, its low toxicity profile compared to some alternative solvents makes it a safer option for both consumers and the environment.
As the cosmetic industry moves towards more sustainable practices, the role of ethyl acetate is evolving. Manufacturers are exploring ways to optimize its use, such as implementing closed-loop systems for solvent recovery and recycling. These efforts not only reduce environmental impact but also improve cost-efficiency in production processes.
In conclusion, while ethyl acetate does have some environmental implications, its overall impact in cosmetic formulations is relatively favorable when compared to many alternative solvents. The industry's ongoing efforts to develop more sustainable sourcing and production methods, coupled with responsible use and disposal practices, are key to further minimizing the environmental footprint of ethyl acetate in cosmetics.
One of the primary environmental concerns associated with ethyl acetate is its volatile organic compound (VOC) status. VOCs contribute to air pollution and can potentially form ground-level ozone when released into the atmosphere. However, compared to other solvents used in cosmetics, ethyl acetate has a relatively low ozone-forming potential, making it a preferable choice in terms of air quality impact.
The production of ethyl acetate also raises environmental considerations. Traditionally synthesized from ethanol and acetic acid, the manufacturing process can be energy-intensive and rely on petrochemical feedstocks. However, recent innovations have led to more sustainable production methods, including bio-based ethyl acetate derived from renewable resources such as sugarcane or corn. These eco-friendly alternatives significantly reduce the carbon footprint associated with ethyl acetate production.
In terms of biodegradability, ethyl acetate demonstrates favorable characteristics. It is readily biodegradable in both aerobic and anaerobic conditions, breaking down into ethanol and acetic acid, which are naturally occurring substances. This property minimizes its long-term environmental persistence and reduces the risk of accumulation in ecosystems.
Water pollution is another aspect to consider when evaluating the environmental impact of ethyl acetate in cosmetics. While ethyl acetate is somewhat soluble in water, its rapid biodegradation helps mitigate potential aquatic toxicity. Nevertheless, proper waste management practices are essential to prevent large-scale releases into water bodies.
The use of ethyl acetate in cosmetic formulations can indirectly contribute to environmental benefits. Its efficacy as a solvent allows for the creation of water-free or low-water formulations, potentially reducing the overall water footprint of cosmetic products. Additionally, its low toxicity profile compared to some alternative solvents makes it a safer option for both consumers and the environment.
As the cosmetic industry moves towards more sustainable practices, the role of ethyl acetate is evolving. Manufacturers are exploring ways to optimize its use, such as implementing closed-loop systems for solvent recovery and recycling. These efforts not only reduce environmental impact but also improve cost-efficiency in production processes.
In conclusion, while ethyl acetate does have some environmental implications, its overall impact in cosmetic formulations is relatively favorable when compared to many alternative solvents. The industry's ongoing efforts to develop more sustainable sourcing and production methods, coupled with responsible use and disposal practices, are key to further minimizing the environmental footprint of ethyl acetate in cosmetics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!