Identifying Key Harbingers for Ethyl Acetate Future Use
JUN 27, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Overview
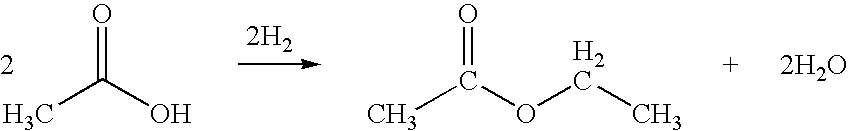
Ethyl acetate, a versatile organic compound with the formula CH3COOC2H5, plays a crucial role in various industries due to its unique properties and wide-ranging applications. This colorless liquid, characterized by its fruity odor, is primarily produced through the esterification of ethanol and acetic acid. Its low toxicity, high solvency, and moderate volatility make it an attractive choice for numerous industrial processes.
In the chemical industry, ethyl acetate serves as a key intermediate in the synthesis of various compounds, including pharmaceuticals, plastics, and other specialty chemicals. Its ability to dissolve a wide range of organic substances makes it an excellent solvent for paints, coatings, and adhesives. The printing industry relies heavily on ethyl acetate for its fast-drying properties, which are essential in the production of inks and varnishes.
The food and beverage sector utilizes ethyl acetate as a flavoring agent and extraction solvent. It is commonly used in the decaffeination of coffee and tea, as well as in the production of artificial fruit flavors. In the pharmaceutical industry, ethyl acetate finds applications in the formulation of drugs and as a solvent in various manufacturing processes.
The global ethyl acetate market has been experiencing steady growth, driven by increasing demand from end-use industries such as packaging, automotive, and electronics. The Asia-Pacific region, particularly China and India, has emerged as a major consumer and producer of ethyl acetate, owing to rapid industrialization and economic growth. North America and Europe also maintain significant market shares, with a focus on high-quality, specialty applications.
Environmental concerns and regulatory pressures have led to the development of bio-based ethyl acetate, derived from renewable resources such as corn and sugarcane. This eco-friendly alternative is gaining traction in various industries, aligning with the growing trend towards sustainable and green chemistry solutions.
As we look towards the future use of ethyl acetate, several key factors are likely to shape its trajectory. Technological advancements in production processes, such as reactive distillation and membrane separation, are expected to improve efficiency and reduce costs. The ongoing shift towards bio-based alternatives and circular economy principles may drive innovation in ethyl acetate production and application methods.
In the chemical industry, ethyl acetate serves as a key intermediate in the synthesis of various compounds, including pharmaceuticals, plastics, and other specialty chemicals. Its ability to dissolve a wide range of organic substances makes it an excellent solvent for paints, coatings, and adhesives. The printing industry relies heavily on ethyl acetate for its fast-drying properties, which are essential in the production of inks and varnishes.
The food and beverage sector utilizes ethyl acetate as a flavoring agent and extraction solvent. It is commonly used in the decaffeination of coffee and tea, as well as in the production of artificial fruit flavors. In the pharmaceutical industry, ethyl acetate finds applications in the formulation of drugs and as a solvent in various manufacturing processes.
The global ethyl acetate market has been experiencing steady growth, driven by increasing demand from end-use industries such as packaging, automotive, and electronics. The Asia-Pacific region, particularly China and India, has emerged as a major consumer and producer of ethyl acetate, owing to rapid industrialization and economic growth. North America and Europe also maintain significant market shares, with a focus on high-quality, specialty applications.
Environmental concerns and regulatory pressures have led to the development of bio-based ethyl acetate, derived from renewable resources such as corn and sugarcane. This eco-friendly alternative is gaining traction in various industries, aligning with the growing trend towards sustainable and green chemistry solutions.
As we look towards the future use of ethyl acetate, several key factors are likely to shape its trajectory. Technological advancements in production processes, such as reactive distillation and membrane separation, are expected to improve efficiency and reduce costs. The ongoing shift towards bio-based alternatives and circular economy principles may drive innovation in ethyl acetate production and application methods.
Market Demand Analysis
The global market for ethyl acetate has been experiencing steady growth, driven by its versatile applications across various industries. The compound's unique properties, including its low toxicity, pleasant odor, and excellent solvency, have made it a preferred choice in many sectors. In the paints and coatings industry, ethyl acetate is widely used as a solvent due to its fast evaporation rate and ability to dissolve a wide range of resins. This sector remains a significant contributor to the overall demand for ethyl acetate.
The adhesives industry is another major consumer of ethyl acetate, particularly in the production of flexible packaging adhesives. With the increasing demand for packaged goods and the growth of e-commerce, the need for high-performance adhesives is expected to drive the ethyl acetate market further. Additionally, the pharmaceutical industry utilizes ethyl acetate in the production of various drugs and as an extraction solvent, contributing to the compound's market growth.
In recent years, there has been a noticeable shift towards more environmentally friendly and sustainable products. This trend has both positive and negative implications for the ethyl acetate market. On one hand, ethyl acetate is considered a more eco-friendly alternative to some other solvents, which could potentially increase its demand. On the other hand, the push for even greener alternatives might pose a challenge to its long-term growth in certain applications.
The food and beverage industry represents another significant market for ethyl acetate. Its use as a flavoring agent and in the decaffeination of coffee and tea continues to drive demand. However, regulatory scrutiny and consumer preferences for natural ingredients may impact its growth in this sector.
Geographically, Asia-Pacific has emerged as the largest market for ethyl acetate, primarily due to the rapid industrialization and economic growth in countries like China and India. The region's expanding manufacturing sector, particularly in paints, coatings, and adhesives, is expected to maintain strong demand for ethyl acetate in the coming years.
Looking ahead, the market for ethyl acetate is projected to continue its growth trajectory, albeit at a moderate pace. Factors such as increasing urbanization, rising disposable incomes, and growing industrial activities in developing economies are likely to sustain demand. However, the market may face challenges from stricter environmental regulations and the development of bio-based alternatives. Manufacturers and stakeholders in the ethyl acetate market will need to closely monitor these trends and adapt their strategies accordingly to capitalize on future opportunities and mitigate potential risks.
The adhesives industry is another major consumer of ethyl acetate, particularly in the production of flexible packaging adhesives. With the increasing demand for packaged goods and the growth of e-commerce, the need for high-performance adhesives is expected to drive the ethyl acetate market further. Additionally, the pharmaceutical industry utilizes ethyl acetate in the production of various drugs and as an extraction solvent, contributing to the compound's market growth.
In recent years, there has been a noticeable shift towards more environmentally friendly and sustainable products. This trend has both positive and negative implications for the ethyl acetate market. On one hand, ethyl acetate is considered a more eco-friendly alternative to some other solvents, which could potentially increase its demand. On the other hand, the push for even greener alternatives might pose a challenge to its long-term growth in certain applications.
The food and beverage industry represents another significant market for ethyl acetate. Its use as a flavoring agent and in the decaffeination of coffee and tea continues to drive demand. However, regulatory scrutiny and consumer preferences for natural ingredients may impact its growth in this sector.
Geographically, Asia-Pacific has emerged as the largest market for ethyl acetate, primarily due to the rapid industrialization and economic growth in countries like China and India. The region's expanding manufacturing sector, particularly in paints, coatings, and adhesives, is expected to maintain strong demand for ethyl acetate in the coming years.
Looking ahead, the market for ethyl acetate is projected to continue its growth trajectory, albeit at a moderate pace. Factors such as increasing urbanization, rising disposable incomes, and growing industrial activities in developing economies are likely to sustain demand. However, the market may face challenges from stricter environmental regulations and the development of bio-based alternatives. Manufacturers and stakeholders in the ethyl acetate market will need to closely monitor these trends and adapt their strategies accordingly to capitalize on future opportunities and mitigate potential risks.
Technical Challenges
The ethyl acetate industry faces several significant technical challenges that could impact its future use and market growth. One of the primary concerns is the environmental impact of traditional production methods. The conventional process for manufacturing ethyl acetate relies heavily on petrochemical feedstocks and generates substantial amounts of greenhouse gas emissions. This has led to increased pressure from regulatory bodies and environmentally conscious consumers to develop more sustainable production techniques.
Another major challenge lies in the optimization of production efficiency. Current manufacturing processes often suffer from low yields and high energy consumption, which directly affects the cost-effectiveness of ethyl acetate production. Improving catalytic systems and reaction conditions to enhance conversion rates and reduce energy requirements remains a key focus area for researchers and industry professionals.
The volatility of raw material prices, particularly ethanol and acetic acid, poses a significant challenge to the stability of ethyl acetate production. Fluctuations in these key ingredients can lead to unpredictable production costs, making it difficult for manufacturers to maintain consistent pricing and profit margins. This volatility also complicates long-term planning and investment decisions in the industry.
Quality control and product purity present ongoing technical challenges. Ethyl acetate is used in a wide range of applications, from pharmaceuticals to food packaging, each with its own stringent purity requirements. Ensuring consistent high-quality output while managing impurities and byproducts demands sophisticated purification techniques and robust quality assurance processes.
The development of bio-based alternatives to traditional ethyl acetate production is an emerging challenge and opportunity. While promising in terms of sustainability, these bio-based routes face hurdles in scalability, cost-competitiveness, and product consistency. Overcoming these obstacles requires significant research and development efforts to make bio-based ethyl acetate a viable alternative to petrochemical-derived products.
Storage and transportation of ethyl acetate present additional technical challenges due to its flammability and volatility. Developing safer storage solutions and more efficient transportation methods that minimize losses due to evaporation are crucial for improving the overall supply chain efficiency and reducing environmental risks associated with ethyl acetate handling.
Lastly, the industry must address the challenge of adapting to changing regulatory landscapes. As environmental and safety regulations become more stringent globally, manufacturers must continuously innovate to comply with new standards while maintaining operational efficiency and product quality. This often requires significant investments in new technologies and process modifications, posing both financial and technical challenges for industry players.
Another major challenge lies in the optimization of production efficiency. Current manufacturing processes often suffer from low yields and high energy consumption, which directly affects the cost-effectiveness of ethyl acetate production. Improving catalytic systems and reaction conditions to enhance conversion rates and reduce energy requirements remains a key focus area for researchers and industry professionals.
The volatility of raw material prices, particularly ethanol and acetic acid, poses a significant challenge to the stability of ethyl acetate production. Fluctuations in these key ingredients can lead to unpredictable production costs, making it difficult for manufacturers to maintain consistent pricing and profit margins. This volatility also complicates long-term planning and investment decisions in the industry.
Quality control and product purity present ongoing technical challenges. Ethyl acetate is used in a wide range of applications, from pharmaceuticals to food packaging, each with its own stringent purity requirements. Ensuring consistent high-quality output while managing impurities and byproducts demands sophisticated purification techniques and robust quality assurance processes.
The development of bio-based alternatives to traditional ethyl acetate production is an emerging challenge and opportunity. While promising in terms of sustainability, these bio-based routes face hurdles in scalability, cost-competitiveness, and product consistency. Overcoming these obstacles requires significant research and development efforts to make bio-based ethyl acetate a viable alternative to petrochemical-derived products.
Storage and transportation of ethyl acetate present additional technical challenges due to its flammability and volatility. Developing safer storage solutions and more efficient transportation methods that minimize losses due to evaporation are crucial for improving the overall supply chain efficiency and reducing environmental risks associated with ethyl acetate handling.
Lastly, the industry must address the challenge of adapting to changing regulatory landscapes. As environmental and safety regulations become more stringent globally, manufacturers must continuously innovate to comply with new standards while maintaining operational efficiency and product quality. This often requires significant investments in new technologies and process modifications, posing both financial and technical challenges for industry players.
Current EA Solutions
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and separation methods. These processes aim to improve the yield and purity of ethyl acetate for industrial applications.- Production and purification of ethyl acetate: Various methods are employed for the production and purification of ethyl acetate, including distillation, extraction, and reactive distillation processes. These techniques aim to improve the yield and purity of ethyl acetate while reducing energy consumption and production costs.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is widely used as a solvent and reagent in various chemical processes. It finds applications in the production of pharmaceuticals, polymers, and other organic compounds. Its properties make it suitable for extraction, purification, and synthesis reactions.
- Ethyl acetate in coating and adhesive formulations: Ethyl acetate is a key component in many coating and adhesive formulations. It is used as a solvent in paints, varnishes, and adhesives due to its excellent solvency and fast evaporation rate. This allows for quick drying and improved film formation in various applications.
- Ethyl acetate in the food and beverage industry: In the food and beverage industry, ethyl acetate is used as a flavoring agent and extraction solvent. It is employed in the production of artificial flavors and in the extraction of caffeine from coffee and tea. Its low toxicity and pleasant fruity odor make it suitable for these applications.
- Environmental and safety considerations for ethyl acetate: As ethyl acetate is a volatile organic compound, there are environmental and safety considerations associated with its use. Research focuses on developing more sustainable production methods, improving handling and storage practices, and exploring alternatives to reduce environmental impact and enhance worker safety.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes as a solvent, reactant, or intermediate. It finds applications in the production of other chemicals, pharmaceuticals, and materials, showcasing its versatility in industrial settings.Expand Specific Solutions03 Ethyl acetate in extraction and separation processes
Ethyl acetate is employed in extraction and separation processes for various compounds. Its properties make it suitable for liquid-liquid extraction, chromatography, and other separation techniques in chemical and pharmaceutical industries.Expand Specific Solutions04 Ethyl acetate as a green solvent
The use of ethyl acetate as an environmentally friendly solvent is explored in various applications. Its relatively low toxicity and biodegradability make it an attractive alternative to more harmful solvents in industrial processes and consumer products.Expand Specific Solutions05 Ethyl acetate in polymer and material science
Ethyl acetate plays a role in polymer and material science applications. It is used in the synthesis, processing, or modification of various materials, including plastics, coatings, and adhesives, contributing to the development of new materials with specific properties.Expand Specific Solutions
Key Industry Players
The market for ethyl acetate future use is in a growth phase, driven by increasing demand across various industries. The global market size is projected to expand significantly in the coming years, with a compound annual growth rate expected to be in the high single digits. Technologically, the field is moderately mature but continues to evolve, with key players like Celanese International Corp., Eastman Chemical Co., and BASF Corp. leading innovation. These companies, along with others such as China Petroleum & Chemical Corp. and Resonac Corp., are investing in research and development to improve production processes and explore new applications, indicating a competitive and dynamic landscape.
Celanese International Corp.
Technical Solution: Celanese has developed a proprietary acetyl technology platform for ethyl acetate production, focusing on sustainability and efficiency. Their process utilizes advanced catalysts and optimized reaction conditions to achieve higher yields and lower energy consumption. The company has implemented a closed-loop system that recycles byproducts, reducing waste and improving overall resource utilization[1]. Additionally, Celanese is exploring bio-based feedstocks for ethyl acetate production, aiming to decrease reliance on fossil fuels and reduce carbon footprint[2].
Strengths: Advanced proprietary technology, high efficiency, and focus on sustainability. Weaknesses: Potential higher initial investment costs and dependence on specific raw materials.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has developed a novel approach to ethyl acetate production using their Gasification Technology. This process converts biomass and waste plastics into syngas, which is then used to produce ethyl acetate and other chemicals[3]. The company has also invested in advanced separation techniques, such as membrane technology, to improve the purity of ethyl acetate and reduce energy consumption during the purification process[4]. Eastman is actively researching catalysts that can operate at lower temperatures, potentially reducing the overall energy requirements of ethyl acetate production[5].
Strengths: Innovative use of waste materials, potential for circular economy integration. Weaknesses: Complex technology that may require significant infrastructure changes.
Innovative EA Research
Direct and selective production of ethyl acetate from acetic acid utilizing a bimetal supported catalyst
PatentInactiveUS20100029980A1
Innovation
- A process utilizing a hydrogenating catalyst composed of metals like nickel, platinum, or palladium in combination with molybdenum, rhenium, zirconium, copper, or cobalt supported on catalysts such as silica or zeolites, which selectively converts acetic acid to ethyl acetate with high yield and selectivity.
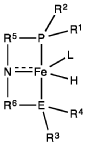
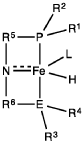
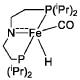
Homogeneous iron catalysts for the conversion of ethanol to ethyl acetate and hydrogen
PatentWO2019027965A1
Innovation
- A process utilizing a homogeneous iron catalyst with a tridentate pincer ligand for dehydrogenative coupling of ethanol at moderate temperatures, producing ethyl acetate efficiently and selectively, with iron loadings as low as 0.001 mol%, allowing for continuous operation and easy separation of ethyl acetate from the catalyst.
Regulatory Landscape
The regulatory landscape surrounding ethyl acetate is complex and dynamic, reflecting the compound's widespread use across various industries and its potential environmental and health impacts. At the global level, organizations such as the World Health Organization (WHO) and the International Agency for Research on Cancer (IARC) provide guidelines and assessments that influence national and regional regulations. These bodies have classified ethyl acetate as a low-toxicity substance, which has generally led to less stringent controls compared to more hazardous chemicals.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA) and the Clean Air Act. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for workers, while the Food and Drug Administration (FDA) oversees its use in food packaging and pharmaceutical applications. The European Union, through its REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation, requires manufacturers and importers to register ethyl acetate and provide safety data.
Many countries have implemented specific regulations governing the use of ethyl acetate in consumer products, particularly in cosmetics and personal care items. For instance, Japan's Ministry of Health, Labour and Welfare has set maximum concentration limits for ethyl acetate in certain cosmetic formulations. Similarly, Health Canada has guidelines for its use in various consumer goods.
The transportation and storage of ethyl acetate are subject to strict regulations due to its flammability. The United Nations' Recommendations on the Transport of Dangerous Goods classify it as a Class 3 Flammable Liquid, which informs international shipping and handling requirements. National agencies, such as the U.S. Department of Transportation, have adopted these guidelines into their own regulatory frameworks.
Environmental regulations are becoming increasingly important in shaping the future use of ethyl acetate. Many jurisdictions have implemented or are considering volatile organic compound (VOC) emission controls, which could impact industrial processes using ethyl acetate. For example, California's Air Resources Board has stringent VOC regulations that affect the use of ethyl acetate in coatings and adhesives.
As sustainability concerns grow, there is a trend towards promoting bio-based alternatives to petrochemical-derived ethyl acetate. The European Union's Renewable Energy Directive and similar policies in other regions are incentivizing the development and use of bio-based chemicals, which could influence future regulatory approaches to ethyl acetate production and use.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA) and the Clean Air Act. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for workers, while the Food and Drug Administration (FDA) oversees its use in food packaging and pharmaceutical applications. The European Union, through its REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation, requires manufacturers and importers to register ethyl acetate and provide safety data.
Many countries have implemented specific regulations governing the use of ethyl acetate in consumer products, particularly in cosmetics and personal care items. For instance, Japan's Ministry of Health, Labour and Welfare has set maximum concentration limits for ethyl acetate in certain cosmetic formulations. Similarly, Health Canada has guidelines for its use in various consumer goods.
The transportation and storage of ethyl acetate are subject to strict regulations due to its flammability. The United Nations' Recommendations on the Transport of Dangerous Goods classify it as a Class 3 Flammable Liquid, which informs international shipping and handling requirements. National agencies, such as the U.S. Department of Transportation, have adopted these guidelines into their own regulatory frameworks.
Environmental regulations are becoming increasingly important in shaping the future use of ethyl acetate. Many jurisdictions have implemented or are considering volatile organic compound (VOC) emission controls, which could impact industrial processes using ethyl acetate. For example, California's Air Resources Board has stringent VOC regulations that affect the use of ethyl acetate in coatings and adhesives.
As sustainability concerns grow, there is a trend towards promoting bio-based alternatives to petrochemical-derived ethyl acetate. The European Union's Renewable Energy Directive and similar policies in other regions are incentivizing the development and use of bio-based chemicals, which could influence future regulatory approaches to ethyl acetate production and use.
Environmental Impact
The environmental impact of ethyl acetate production and use is a critical consideration for its future applications. As a volatile organic compound (VOC), ethyl acetate contributes to air pollution and the formation of ground-level ozone when released into the atmosphere. However, compared to many other solvents, ethyl acetate is considered relatively less harmful due to its lower toxicity and faster biodegradability.
In terms of production, the traditional method of synthesizing ethyl acetate through the esterification of ethanol and acetic acid has a moderate environmental footprint. The process requires energy input and generates some waste products, but advancements in catalytic processes and reactor designs have improved efficiency and reduced emissions. Alternative production methods, such as the Tishchenko reaction using acetaldehyde, are being explored to further minimize environmental impact.
The use phase of ethyl acetate presents both challenges and opportunities for environmental sustainability. In industrial applications, proper handling and containment systems are essential to prevent fugitive emissions. Many industries have implemented solvent recovery systems to capture and recycle ethyl acetate, reducing both environmental impact and raw material costs. However, in consumer products like nail polish removers, emissions during use remain a concern.
End-of-life considerations for ethyl acetate are generally favorable. Its high volatility means that most of the compound evaporates during use or disposal, leaving minimal residues. In the atmosphere, ethyl acetate undergoes photochemical degradation with a half-life of about 10 days. When released into water or soil, it biodegrades relatively quickly under both aerobic and anaerobic conditions.
Looking towards the future, several trends are likely to influence the environmental impact of ethyl acetate. The push towards green chemistry is driving research into bio-based production methods, potentially using renewable feedstocks instead of petrochemical sources. This could significantly reduce the carbon footprint of ethyl acetate production. Additionally, ongoing efforts to develop more efficient application technologies and closed-loop systems in industrial processes are expected to further minimize emissions and waste.
Regulatory pressures, particularly regarding VOC emissions, will continue to shape the use of ethyl acetate. While it is often considered a preferred alternative to more harmful solvents, stricter environmental regulations may still impact its use in certain applications. This could drive innovation in formulation technologies to reduce ethyl acetate content or develop alternative compounds with even lower environmental impact.
In terms of production, the traditional method of synthesizing ethyl acetate through the esterification of ethanol and acetic acid has a moderate environmental footprint. The process requires energy input and generates some waste products, but advancements in catalytic processes and reactor designs have improved efficiency and reduced emissions. Alternative production methods, such as the Tishchenko reaction using acetaldehyde, are being explored to further minimize environmental impact.
The use phase of ethyl acetate presents both challenges and opportunities for environmental sustainability. In industrial applications, proper handling and containment systems are essential to prevent fugitive emissions. Many industries have implemented solvent recovery systems to capture and recycle ethyl acetate, reducing both environmental impact and raw material costs. However, in consumer products like nail polish removers, emissions during use remain a concern.
End-of-life considerations for ethyl acetate are generally favorable. Its high volatility means that most of the compound evaporates during use or disposal, leaving minimal residues. In the atmosphere, ethyl acetate undergoes photochemical degradation with a half-life of about 10 days. When released into water or soil, it biodegrades relatively quickly under both aerobic and anaerobic conditions.
Looking towards the future, several trends are likely to influence the environmental impact of ethyl acetate. The push towards green chemistry is driving research into bio-based production methods, potentially using renewable feedstocks instead of petrochemical sources. This could significantly reduce the carbon footprint of ethyl acetate production. Additionally, ongoing efforts to develop more efficient application technologies and closed-loop systems in industrial processes are expected to further minimize emissions and waste.
Regulatory pressures, particularly regarding VOC emissions, will continue to shape the use of ethyl acetate. While it is often considered a preferred alternative to more harmful solvents, stricter environmental regulations may still impact its use in certain applications. This could drive innovation in formulation technologies to reduce ethyl acetate content or develop alternative compounds with even lower environmental impact.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!