How Ethyl Acetate Simplifies Laboratory Solvent Systems?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Background and Objectives
Ethyl acetate, a versatile organic compound with the formula CH3COOC2H5, has emerged as a crucial solvent in laboratory settings. Its history dates back to the early 19th century when it was first synthesized through the esterification of ethanol and acetic acid. Since then, ethyl acetate has played an increasingly significant role in simplifying laboratory solvent systems.
The evolution of ethyl acetate as a laboratory solvent has been driven by its unique properties and advantages over traditional solvents. Its low toxicity, moderate volatility, and excellent solvency for a wide range of organic compounds have made it an attractive choice for various applications. The growing emphasis on green chemistry and sustainable practices in recent years has further propelled the adoption of ethyl acetate as an environmentally friendly alternative to more hazardous solvents.
In the context of simplifying laboratory solvent systems, ethyl acetate offers several key benefits. Its ability to dissolve both polar and non-polar substances makes it a versatile solvent for diverse chemical processes. This characteristic reduces the need for multiple solvents in many experimental setups, streamlining procedures and minimizing waste. Additionally, ethyl acetate's relatively low boiling point (77.1°C) facilitates easy removal and recovery, contributing to more efficient and cost-effective laboratory operations.
The primary objective of utilizing ethyl acetate in laboratory solvent systems is to enhance efficiency, safety, and sustainability. By replacing more toxic or environmentally harmful solvents, ethyl acetate helps reduce the overall environmental impact of laboratory processes. It also aims to simplify solvent handling and storage procedures, potentially lowering the risk of accidents and exposure to hazardous substances.
Another crucial goal is to improve the reproducibility and reliability of experimental results. Ethyl acetate's consistent performance across various applications can lead to more standardized protocols, facilitating better comparison of results between different laboratories and research groups. This standardization is particularly valuable in fields such as pharmaceutical research, where reproducibility is paramount.
Looking ahead, the continued development and optimization of ethyl acetate-based solvent systems are expected to focus on further enhancing its properties and expanding its applications. Research efforts may target the development of novel ethyl acetate formulations or blends that can address specific laboratory needs while maintaining its core advantages. The integration of ethyl acetate into emerging technologies, such as microfluidics and automated synthesis platforms, represents another exciting frontier in its evolution as a key laboratory solvent.
The evolution of ethyl acetate as a laboratory solvent has been driven by its unique properties and advantages over traditional solvents. Its low toxicity, moderate volatility, and excellent solvency for a wide range of organic compounds have made it an attractive choice for various applications. The growing emphasis on green chemistry and sustainable practices in recent years has further propelled the adoption of ethyl acetate as an environmentally friendly alternative to more hazardous solvents.
In the context of simplifying laboratory solvent systems, ethyl acetate offers several key benefits. Its ability to dissolve both polar and non-polar substances makes it a versatile solvent for diverse chemical processes. This characteristic reduces the need for multiple solvents in many experimental setups, streamlining procedures and minimizing waste. Additionally, ethyl acetate's relatively low boiling point (77.1°C) facilitates easy removal and recovery, contributing to more efficient and cost-effective laboratory operations.
The primary objective of utilizing ethyl acetate in laboratory solvent systems is to enhance efficiency, safety, and sustainability. By replacing more toxic or environmentally harmful solvents, ethyl acetate helps reduce the overall environmental impact of laboratory processes. It also aims to simplify solvent handling and storage procedures, potentially lowering the risk of accidents and exposure to hazardous substances.
Another crucial goal is to improve the reproducibility and reliability of experimental results. Ethyl acetate's consistent performance across various applications can lead to more standardized protocols, facilitating better comparison of results between different laboratories and research groups. This standardization is particularly valuable in fields such as pharmaceutical research, where reproducibility is paramount.
Looking ahead, the continued development and optimization of ethyl acetate-based solvent systems are expected to focus on further enhancing its properties and expanding its applications. Research efforts may target the development of novel ethyl acetate formulations or blends that can address specific laboratory needs while maintaining its core advantages. The integration of ethyl acetate into emerging technologies, such as microfluidics and automated synthesis platforms, represents another exciting frontier in its evolution as a key laboratory solvent.
Market Demand Analysis for Ethyl Acetate in Labs
The market demand for ethyl acetate in laboratory settings has been steadily increasing due to its versatile properties and wide range of applications. As a solvent, ethyl acetate offers a unique combination of characteristics that make it highly desirable for various laboratory processes. Its low toxicity, high volatility, and excellent solvency power have positioned it as a preferred choice in many research and analytical procedures.
In recent years, there has been a growing trend towards simplifying solvent systems in laboratories, driven by the need for cost-effectiveness, improved safety, and environmental considerations. Ethyl acetate has emerged as a key player in this shift, as it can often replace more hazardous or expensive solvents in many applications. This has led to a significant increase in demand across academic, industrial, and pharmaceutical research laboratories.
The pharmaceutical industry, in particular, has been a major driver of ethyl acetate demand in laboratory settings. As drug discovery and development processes become more complex, the need for efficient and versatile solvents has grown. Ethyl acetate's ability to dissolve a wide range of organic compounds makes it invaluable in extraction, purification, and synthesis processes, contributing to its increased usage in pharmaceutical research laboratories.
Environmental regulations and safety concerns have also played a crucial role in boosting the demand for ethyl acetate. As stricter guidelines are implemented regarding the use and disposal of laboratory chemicals, many institutions are seeking safer alternatives to traditional solvents. Ethyl acetate's relatively low toxicity and biodegradability make it an attractive option for laboratories looking to reduce their environmental impact and improve worker safety.
The analytical chemistry sector has also contributed to the rising demand for ethyl acetate in labs. Its use in chromatography, particularly in high-performance liquid chromatography (HPLC) and gas chromatography (GC), has become increasingly common. The solvent's properties allow for efficient separation and analysis of various compounds, making it a valuable tool in both research and quality control applications.
Furthermore, the growing emphasis on green chemistry principles has led to increased interest in ethyl acetate as a more sustainable solvent option. Its production from renewable resources and its potential for recycling align well with the goals of reducing the environmental footprint of laboratory operations. This trend is expected to continue driving demand in the coming years as more laboratories adopt sustainable practices.
The market for ethyl acetate in laboratory applications is projected to expand further as research and development activities intensify across various scientific disciplines. The ongoing need for efficient, safe, and environmentally friendly solvents in laboratories is likely to sustain the growth trajectory of ethyl acetate demand in the foreseeable future.
In recent years, there has been a growing trend towards simplifying solvent systems in laboratories, driven by the need for cost-effectiveness, improved safety, and environmental considerations. Ethyl acetate has emerged as a key player in this shift, as it can often replace more hazardous or expensive solvents in many applications. This has led to a significant increase in demand across academic, industrial, and pharmaceutical research laboratories.
The pharmaceutical industry, in particular, has been a major driver of ethyl acetate demand in laboratory settings. As drug discovery and development processes become more complex, the need for efficient and versatile solvents has grown. Ethyl acetate's ability to dissolve a wide range of organic compounds makes it invaluable in extraction, purification, and synthesis processes, contributing to its increased usage in pharmaceutical research laboratories.
Environmental regulations and safety concerns have also played a crucial role in boosting the demand for ethyl acetate. As stricter guidelines are implemented regarding the use and disposal of laboratory chemicals, many institutions are seeking safer alternatives to traditional solvents. Ethyl acetate's relatively low toxicity and biodegradability make it an attractive option for laboratories looking to reduce their environmental impact and improve worker safety.
The analytical chemistry sector has also contributed to the rising demand for ethyl acetate in labs. Its use in chromatography, particularly in high-performance liquid chromatography (HPLC) and gas chromatography (GC), has become increasingly common. The solvent's properties allow for efficient separation and analysis of various compounds, making it a valuable tool in both research and quality control applications.
Furthermore, the growing emphasis on green chemistry principles has led to increased interest in ethyl acetate as a more sustainable solvent option. Its production from renewable resources and its potential for recycling align well with the goals of reducing the environmental footprint of laboratory operations. This trend is expected to continue driving demand in the coming years as more laboratories adopt sustainable practices.
The market for ethyl acetate in laboratory applications is projected to expand further as research and development activities intensify across various scientific disciplines. The ongoing need for efficient, safe, and environmentally friendly solvents in laboratories is likely to sustain the growth trajectory of ethyl acetate demand in the foreseeable future.
Current Status and Challenges in Lab Solvent Systems
Laboratory solvent systems have undergone significant evolution in recent years, with a growing emphasis on simplification and sustainability. The current status of these systems is characterized by a shift towards more efficient, cost-effective, and environmentally friendly solutions. Ethyl acetate has emerged as a key player in this transformation, offering numerous advantages over traditional solvent systems.
One of the primary challenges in conventional lab solvent systems is the complexity of multi-solvent mixtures. These systems often require precise ratios of various solvents, leading to increased preparation time, potential errors, and higher costs. Ethyl acetate's versatility addresses this issue by serving as a single-solvent solution for many applications, significantly simplifying laboratory processes.
Another major challenge is the environmental impact of traditional solvents. Many commonly used solvents are toxic, flammable, or contribute to air pollution. Ethyl acetate, while not entirely benign, offers a more environmentally friendly alternative. It has lower toxicity compared to many other organic solvents and is biodegradable, aligning with the growing focus on green chemistry principles in laboratory practices.
The adoption of ethyl acetate in laboratory solvent systems faces some obstacles. One challenge is the need for method validation and standardization. As laboratories transition from established multi-solvent systems to ethyl acetate-based solutions, extensive testing and validation are required to ensure comparable or improved results across various applications.
Additionally, the physical properties of ethyl acetate present both advantages and challenges. Its relatively low boiling point (77.1°C) makes it easy to remove from samples, but also requires careful handling to prevent evaporation during use. This characteristic necessitates the development of new protocols and potentially specialized equipment for optimal utilization.
The current status of ethyl acetate in lab solvent systems is marked by increasing adoption across various fields, including pharmaceutical research, environmental analysis, and organic synthesis. Its ability to dissolve a wide range of polar and non-polar compounds makes it a versatile choice for extraction, purification, and chromatography applications. However, the transition is not uniform across all laboratory settings, with some areas showing faster adoption rates than others.
As the scientific community continues to seek more sustainable and efficient practices, the role of ethyl acetate in simplifying laboratory solvent systems is likely to expand. Ongoing research focuses on optimizing its use in different applications, developing novel formulations, and addressing any limitations. The challenge lies in balancing the benefits of simplification with the need for maintaining or improving analytical precision and experimental reproducibility.
One of the primary challenges in conventional lab solvent systems is the complexity of multi-solvent mixtures. These systems often require precise ratios of various solvents, leading to increased preparation time, potential errors, and higher costs. Ethyl acetate's versatility addresses this issue by serving as a single-solvent solution for many applications, significantly simplifying laboratory processes.
Another major challenge is the environmental impact of traditional solvents. Many commonly used solvents are toxic, flammable, or contribute to air pollution. Ethyl acetate, while not entirely benign, offers a more environmentally friendly alternative. It has lower toxicity compared to many other organic solvents and is biodegradable, aligning with the growing focus on green chemistry principles in laboratory practices.
The adoption of ethyl acetate in laboratory solvent systems faces some obstacles. One challenge is the need for method validation and standardization. As laboratories transition from established multi-solvent systems to ethyl acetate-based solutions, extensive testing and validation are required to ensure comparable or improved results across various applications.
Additionally, the physical properties of ethyl acetate present both advantages and challenges. Its relatively low boiling point (77.1°C) makes it easy to remove from samples, but also requires careful handling to prevent evaporation during use. This characteristic necessitates the development of new protocols and potentially specialized equipment for optimal utilization.
The current status of ethyl acetate in lab solvent systems is marked by increasing adoption across various fields, including pharmaceutical research, environmental analysis, and organic synthesis. Its ability to dissolve a wide range of polar and non-polar compounds makes it a versatile choice for extraction, purification, and chromatography applications. However, the transition is not uniform across all laboratory settings, with some areas showing faster adoption rates than others.
As the scientific community continues to seek more sustainable and efficient practices, the role of ethyl acetate in simplifying laboratory solvent systems is likely to expand. Ongoing research focuses on optimizing its use in different applications, developing novel formulations, and addressing any limitations. The challenge lies in balancing the benefits of simplification with the need for maintaining or improving analytical precision and experimental reproducibility.
Existing Applications of Ethyl Acetate in Labs
01 Distillation and purification methods
Various distillation and purification techniques are employed to simplify and purify ethyl acetate. These methods include fractional distillation, azeotropic distillation, and the use of specialized distillation columns. These processes help to separate ethyl acetate from other components and improve its purity.- Distillation and purification methods: Various distillation and purification techniques are employed to simplify and improve the production of ethyl acetate. These methods include fractional distillation, azeotropic distillation, and the use of specific catalysts or additives to enhance separation efficiency and product purity.
- Continuous production processes: Continuous production processes have been developed to streamline ethyl acetate manufacturing. These processes often involve specialized reactor designs, optimized reaction conditions, and integrated separation systems to improve efficiency and reduce energy consumption.
- Catalytic synthesis methods: Novel catalytic synthesis methods have been introduced to simplify ethyl acetate production. These approaches utilize advanced catalysts, such as heterogeneous or supported catalysts, to facilitate the reaction between ethanol and acetic acid or other precursors, improving yield and selectivity.
- Green chemistry approaches: Environmentally friendly approaches to ethyl acetate production have been developed, focusing on the use of renewable feedstocks, bio-based catalysts, and low-impact solvents. These methods aim to reduce the environmental footprint of the production process while maintaining or improving efficiency.
- Process intensification techniques: Process intensification techniques have been applied to ethyl acetate production, including the use of microreactors, reactive distillation, and membrane-based separation processes. These approaches aim to combine multiple unit operations, reduce equipment size, and improve overall process efficiency.
02 Catalytic processes for ethyl acetate production
Catalytic processes are used to simplify the production of ethyl acetate. These methods involve the use of specific catalysts to facilitate the reaction between ethanol and acetic acid or other precursors. The catalysts help to improve reaction efficiency and selectivity, resulting in a simpler and more cost-effective production process.Expand Specific Solutions03 Membrane separation techniques
Membrane separation technologies are applied to simplify the purification of ethyl acetate. These techniques use selective membranes to separate ethyl acetate from other components in the mixture. This approach can be more energy-efficient and environmentally friendly compared to traditional distillation methods.Expand Specific Solutions04 Continuous flow processes
Continuous flow processes are developed to simplify ethyl acetate production and purification. These methods involve the continuous feeding of reactants and removal of products, allowing for more efficient and streamlined operations. Continuous processes can lead to improved product quality and reduced energy consumption.Expand Specific Solutions05 Green chemistry approaches
Green chemistry principles are applied to simplify ethyl acetate production and reduce environmental impact. These approaches include the use of renewable feedstocks, bio-based catalysts, and environmentally friendly solvents. Such methods aim to minimize waste generation and energy consumption while maintaining product quality.Expand Specific Solutions
Key Players in Ethyl Acetate Production and Lab Equipment
The ethyl acetate solvent system market is in a mature growth phase, with a global market size estimated at over $3 billion. The technology is well-established, with major chemical companies like BASF, Eastman Chemical, and Celanese dominating production. These industry leaders have optimized manufacturing processes and supply chains, creating high barriers to entry. However, emerging players like Viridis Chemical are innovating with bio-based ethyl acetate production, signaling potential shifts towards more sustainable solutions. The widespread use of ethyl acetate in various industries, from pharmaceuticals to coatings, ensures steady demand and continued refinement of purification and application techniques by both established and newer market entrants.
Celanese International Corp.
Technical Solution: Celanese International Corp. has pioneered an innovative approach to simplifying laboratory solvent systems using ethyl acetate. Their strategy focuses on developing high-performance, ethyl acetate-based solvent blends that can replace multiple traditional solvents in various laboratory applications. Celanese has engineered proprietary formulations that enhance ethyl acetate's solvency power, making it suitable for a broader range of analytical and preparative techniques[9]. The company has also developed advanced purification methods to produce ultra-high purity ethyl acetate, meeting the stringent requirements of sensitive laboratory procedures. Additionally, Celanese has created a line of stabilized ethyl acetate products that offer extended shelf life and improved performance in challenging laboratory environments. Their research has also focused on optimizing ethyl acetate's use in green chemistry applications, promoting more sustainable laboratory practices[10].
Strengths: High-performance blends, ultra-high purity products, extended shelf life, and focus on green chemistry. Weaknesses: May require significant marketing efforts to change established laboratory solvent preferences.
BASF Corp.
Technical Solution: BASF Corp. has developed an innovative approach to simplify laboratory solvent systems using ethyl acetate. Their method involves a green chemistry approach, utilizing ethyl acetate as a more environmentally friendly alternative to traditional solvents. The company has engineered a process that optimizes the use of ethyl acetate in various laboratory applications, including extraction, purification, and synthesis[1]. This system reduces the need for multiple solvents, streamlining laboratory processes and minimizing waste. BASF's technology also incorporates advanced recycling techniques, allowing for the efficient recovery and reuse of ethyl acetate, further enhancing the sustainability of laboratory operations[3].
Strengths: Environmentally friendly, reduces solvent variety, improves process efficiency, and promotes solvent recycling. Weaknesses: May require initial investment in new equipment and training for laboratory personnel.
Core Innovations in Ethyl Acetate-based Solvent Systems
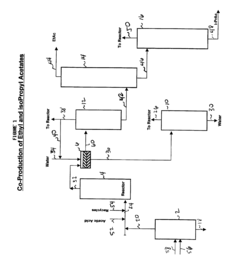
Process for the simultaneous coproduction and purification of ethyl acetate and isopropyl acetate
PatentInactiveUS6765110B2
Innovation
- A process involving the reaction of a mixed alcohol stream of ethanol and isopropanol with acetic acid in the liquid phase using an acidic catalyst at elevated temperatures and pressures, with optional purification of the Fischer Tropsch alcohol mixture before use, followed by a series of distillation towers to separate and purify the ester products, achieving high purity levels of ethyl and isopropyl acetate.
Method for the design of self-emulsifying drug formulations
PatentWO2006112541A1
Innovation
- A method involving the use of a diluent solvent like ethanol to dissolve and dispense viscous or solid components, followed by automated high-throughput formulation screening using Laboratory Automation Systems (LAS) to prepare and evaluate multiple test mixtures, with turbidity measurements for rapid evaluation of emulsion stability and particle size.
Environmental Impact of Ethyl Acetate Use
The use of ethyl acetate in laboratory solvent systems has significant environmental implications that warrant careful consideration. As a widely used organic solvent, ethyl acetate offers several advantages in terms of its environmental profile compared to many traditional solvents.
One of the key environmental benefits of ethyl acetate is its relatively low toxicity. Unlike many other organic solvents, ethyl acetate is not classified as a carcinogen or mutagen, reducing the potential health risks associated with its use. This characteristic makes it a safer option for laboratory personnel and minimizes the environmental impact in case of accidental release.
Furthermore, ethyl acetate is biodegradable, breaking down into ethanol and acetic acid in the environment. This property significantly reduces its long-term environmental persistence compared to more stable solvents. The rapid biodegradation of ethyl acetate helps to mitigate potential soil and water contamination issues.
In terms of air quality, ethyl acetate has a lower vapor pressure compared to many other common solvents, resulting in reduced volatile organic compound (VOC) emissions. This characteristic contributes to improved air quality in laboratory settings and reduces the overall atmospheric pollution potential of solvent-based processes.
However, it is important to note that ethyl acetate is still flammable and can contribute to the formation of ground-level ozone when released into the atmosphere. Proper handling and disposal practices are essential to minimize these risks and ensure environmental protection.
From a lifecycle perspective, ethyl acetate can be produced from renewable resources, such as ethanol derived from biomass. This potential for bio-based production offers a more sustainable alternative to petroleum-derived solvents, aligning with global efforts to reduce dependence on fossil fuels.
The simplification of laboratory solvent systems through the use of ethyl acetate can lead to reduced overall solvent consumption. By replacing multiple solvents with a single, versatile option, laboratories can decrease their chemical inventory and minimize waste generation. This streamlining effect contributes to a smaller environmental footprint for research and analytical processes.
In waste management, ethyl acetate's high volatility facilitates easier recovery and recycling processes. Distillation and other separation techniques can be employed to reclaim used ethyl acetate, reducing the volume of hazardous waste requiring disposal and conserving resources.
While ethyl acetate presents several environmental advantages, it is crucial to implement proper engineering controls and safety measures to prevent accidental releases and minimize exposure. Closed systems, efficient fume hoods, and appropriate personal protective equipment are essential components of responsible ethyl acetate use in laboratory settings.
One of the key environmental benefits of ethyl acetate is its relatively low toxicity. Unlike many other organic solvents, ethyl acetate is not classified as a carcinogen or mutagen, reducing the potential health risks associated with its use. This characteristic makes it a safer option for laboratory personnel and minimizes the environmental impact in case of accidental release.
Furthermore, ethyl acetate is biodegradable, breaking down into ethanol and acetic acid in the environment. This property significantly reduces its long-term environmental persistence compared to more stable solvents. The rapid biodegradation of ethyl acetate helps to mitigate potential soil and water contamination issues.
In terms of air quality, ethyl acetate has a lower vapor pressure compared to many other common solvents, resulting in reduced volatile organic compound (VOC) emissions. This characteristic contributes to improved air quality in laboratory settings and reduces the overall atmospheric pollution potential of solvent-based processes.
However, it is important to note that ethyl acetate is still flammable and can contribute to the formation of ground-level ozone when released into the atmosphere. Proper handling and disposal practices are essential to minimize these risks and ensure environmental protection.
From a lifecycle perspective, ethyl acetate can be produced from renewable resources, such as ethanol derived from biomass. This potential for bio-based production offers a more sustainable alternative to petroleum-derived solvents, aligning with global efforts to reduce dependence on fossil fuels.
The simplification of laboratory solvent systems through the use of ethyl acetate can lead to reduced overall solvent consumption. By replacing multiple solvents with a single, versatile option, laboratories can decrease their chemical inventory and minimize waste generation. This streamlining effect contributes to a smaller environmental footprint for research and analytical processes.
In waste management, ethyl acetate's high volatility facilitates easier recovery and recycling processes. Distillation and other separation techniques can be employed to reclaim used ethyl acetate, reducing the volume of hazardous waste requiring disposal and conserving resources.
While ethyl acetate presents several environmental advantages, it is crucial to implement proper engineering controls and safety measures to prevent accidental releases and minimize exposure. Closed systems, efficient fume hoods, and appropriate personal protective equipment are essential components of responsible ethyl acetate use in laboratory settings.
Safety Considerations for Ethyl Acetate in Labs
Ethyl acetate is a widely used solvent in laboratory settings, offering numerous advantages in simplifying solvent systems. However, its use requires careful consideration of safety measures to protect laboratory personnel and maintain a secure working environment. When handling ethyl acetate, it is crucial to be aware of its flammability, as it has a low flash point of -4°C (25°F). This characteristic necessitates proper storage in fire-resistant cabinets and the implementation of stringent fire prevention protocols in laboratories where it is used.
Inhalation of ethyl acetate vapors can cause respiratory irritation and, in high concentrations, may lead to dizziness, drowsiness, and other central nervous system effects. To mitigate these risks, adequate ventilation is essential in areas where ethyl acetate is handled. Fume hoods should be utilized during experiments involving this solvent, and personal protective equipment (PPE) such as respirators may be necessary when working with large quantities or in poorly ventilated spaces.
Skin contact with ethyl acetate can result in irritation and defatting of the skin, potentially leading to dermatitis with prolonged exposure. Eye contact may cause severe irritation and possible corneal injury. To prevent these hazards, appropriate PPE, including chemical-resistant gloves, lab coats, and safety goggles, should be worn at all times when handling ethyl acetate.
Proper disposal of ethyl acetate is another critical safety consideration. As an organic solvent, it should never be poured down the drain or released into the environment. Instead, waste ethyl acetate should be collected in designated containers and disposed of through approved chemical waste management procedures.
In terms of chemical incompatibilities, ethyl acetate should be kept away from strong oxidizing agents, strong acids, and strong bases, as these interactions can lead to hazardous reactions. Additionally, it is important to note that ethyl acetate can form explosive peroxides over time, especially when exposed to air and light. Regular testing for peroxide formation and proper rotation of stock can help mitigate this risk.
Emergency response procedures should be well-established and communicated to all laboratory personnel. This includes the location and proper use of eyewash stations, safety showers, fire extinguishers, and spill control equipment. Regular safety training and drills can ensure that all staff members are prepared to respond effectively in case of accidents involving ethyl acetate.
Lastly, proper labeling and storage of ethyl acetate containers are essential for maintaining a safe laboratory environment. All containers should be clearly labeled with the chemical name, hazard warnings, and any necessary precautionary statements. Storage areas should be cool, dry, and well-ventilated, away from sources of heat or ignition.
Inhalation of ethyl acetate vapors can cause respiratory irritation and, in high concentrations, may lead to dizziness, drowsiness, and other central nervous system effects. To mitigate these risks, adequate ventilation is essential in areas where ethyl acetate is handled. Fume hoods should be utilized during experiments involving this solvent, and personal protective equipment (PPE) such as respirators may be necessary when working with large quantities or in poorly ventilated spaces.
Skin contact with ethyl acetate can result in irritation and defatting of the skin, potentially leading to dermatitis with prolonged exposure. Eye contact may cause severe irritation and possible corneal injury. To prevent these hazards, appropriate PPE, including chemical-resistant gloves, lab coats, and safety goggles, should be worn at all times when handling ethyl acetate.
Proper disposal of ethyl acetate is another critical safety consideration. As an organic solvent, it should never be poured down the drain or released into the environment. Instead, waste ethyl acetate should be collected in designated containers and disposed of through approved chemical waste management procedures.
In terms of chemical incompatibilities, ethyl acetate should be kept away from strong oxidizing agents, strong acids, and strong bases, as these interactions can lead to hazardous reactions. Additionally, it is important to note that ethyl acetate can form explosive peroxides over time, especially when exposed to air and light. Regular testing for peroxide formation and proper rotation of stock can help mitigate this risk.
Emergency response procedures should be well-established and communicated to all laboratory personnel. This includes the location and proper use of eyewash stations, safety showers, fire extinguishers, and spill control equipment. Regular safety training and drills can ensure that all staff members are prepared to respond effectively in case of accidents involving ethyl acetate.
Lastly, proper labeling and storage of ethyl acetate containers are essential for maintaining a safe laboratory environment. All containers should be clearly labeled with the chemical name, hazard warnings, and any necessary precautionary statements. Storage areas should be cool, dry, and well-ventilated, away from sources of heat or ignition.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!