Ethyl Acetate’s Emerging Role in Industrial Applications
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Overview
Ethyl acetate, a versatile organic compound with the chemical formula CH3COOC2H5, has been gaining significant attention in various industrial applications. This colorless liquid, characterized by its fruity odor, is produced through the esterification of ethanol and acetic acid. Its unique properties, including low toxicity, high solvency, and rapid evaporation rate, have made it an increasingly popular choice across multiple sectors.
In the chemical industry, ethyl acetate serves as a crucial intermediate in the synthesis of numerous compounds. Its ability to dissolve a wide range of substances has led to its extensive use as a solvent in paints, coatings, and adhesives. The automotive and construction industries, in particular, have seen a surge in ethyl acetate consumption due to its effectiveness in these applications.
The pharmaceutical sector has also embraced ethyl acetate for its role in drug formulation and as an extraction solvent. Its low toxicity profile makes it suitable for use in the production of various medications and active pharmaceutical ingredients. Additionally, the food industry utilizes ethyl acetate as a flavoring agent and in the decaffeination of coffee and tea, leveraging its natural presence in many fruits and wines.
In recent years, the electronics industry has found new applications for ethyl acetate in the manufacturing of circuit boards and semiconductor components. Its rapid evaporation rate and ability to leave minimal residue have made it an excellent choice for cleaning and degreasing electronic parts. This expanding use in high-tech industries has contributed to the compound's growing market demand.
The cosmetics and personal care industry has also recognized the benefits of ethyl acetate. It is commonly used in nail polish removers and as a solvent in perfumes and fragrances. Its mild odor and quick-drying properties make it an ideal component in these products, enhancing their performance and user experience.
As environmental concerns continue to shape industrial practices, ethyl acetate has emerged as a more environmentally friendly alternative to some traditional solvents. Its biodegradability and lower toxicity compared to certain other organic solvents have positioned it as a preferred choice in green chemistry initiatives. This aspect has further boosted its adoption across various industries seeking to improve their sustainability profiles.
The global market for ethyl acetate has been experiencing steady growth, driven by these diverse applications and the compound's favorable characteristics. As industries continue to innovate and seek more efficient and sustainable solutions, ethyl acetate's role is expected to expand further, potentially opening up new avenues for its application in emerging technologies and processes.
In the chemical industry, ethyl acetate serves as a crucial intermediate in the synthesis of numerous compounds. Its ability to dissolve a wide range of substances has led to its extensive use as a solvent in paints, coatings, and adhesives. The automotive and construction industries, in particular, have seen a surge in ethyl acetate consumption due to its effectiveness in these applications.
The pharmaceutical sector has also embraced ethyl acetate for its role in drug formulation and as an extraction solvent. Its low toxicity profile makes it suitable for use in the production of various medications and active pharmaceutical ingredients. Additionally, the food industry utilizes ethyl acetate as a flavoring agent and in the decaffeination of coffee and tea, leveraging its natural presence in many fruits and wines.
In recent years, the electronics industry has found new applications for ethyl acetate in the manufacturing of circuit boards and semiconductor components. Its rapid evaporation rate and ability to leave minimal residue have made it an excellent choice for cleaning and degreasing electronic parts. This expanding use in high-tech industries has contributed to the compound's growing market demand.
The cosmetics and personal care industry has also recognized the benefits of ethyl acetate. It is commonly used in nail polish removers and as a solvent in perfumes and fragrances. Its mild odor and quick-drying properties make it an ideal component in these products, enhancing their performance and user experience.
As environmental concerns continue to shape industrial practices, ethyl acetate has emerged as a more environmentally friendly alternative to some traditional solvents. Its biodegradability and lower toxicity compared to certain other organic solvents have positioned it as a preferred choice in green chemistry initiatives. This aspect has further boosted its adoption across various industries seeking to improve their sustainability profiles.
The global market for ethyl acetate has been experiencing steady growth, driven by these diverse applications and the compound's favorable characteristics. As industries continue to innovate and seek more efficient and sustainable solutions, ethyl acetate's role is expected to expand further, potentially opening up new avenues for its application in emerging technologies and processes.
Industrial Demand Analysis
The industrial demand for ethyl acetate has been steadily increasing due to its versatile applications across various sectors. This organic compound, known for its low toxicity and pleasant fruity odor, has become a crucial component in numerous manufacturing processes.
In the coatings and paints industry, ethyl acetate has emerged as a preferred solvent due to its excellent solvency properties and rapid evaporation rate. The growing construction and automotive sectors have significantly contributed to the rising demand for high-quality paints and coatings, thereby driving the consumption of ethyl acetate. The compound's ability to dissolve a wide range of resins and polymers makes it indispensable in formulating durable and aesthetically pleasing finishes.
The packaging industry has also witnessed a surge in ethyl acetate usage, particularly in flexible packaging applications. As consumer preferences shift towards convenient and lightweight packaging solutions, the demand for ethyl acetate-based adhesives and printing inks has escalated. The food and beverage sector, in particular, relies heavily on these packaging materials, further propelling the market growth.
In the pharmaceutical industry, ethyl acetate plays a crucial role as a solvent in the production of various drugs and active pharmaceutical ingredients (APIs). The increasing global focus on healthcare and the rising prevalence of chronic diseases have led to an expansion of pharmaceutical manufacturing, consequently boosting the demand for ethyl acetate in this sector.
The electronics industry has found innovative applications for ethyl acetate in the production of printed circuit boards and semiconductor devices. Its use as a cleaning agent and in photoresist stripping processes has become essential in the manufacturing of advanced electronic components, aligning with the growing demand for smart devices and IoT technologies.
The personal care and cosmetics industry has also embraced ethyl acetate as a key ingredient in nail polish removers and as a solvent in perfumes. The compound's mild nature and pleasant scent make it an attractive option for formulators seeking alternatives to harsher chemicals.
As sustainability becomes a primary concern across industries, the biodegradability of ethyl acetate has positioned it as an environmentally friendly alternative to many traditional solvents. This aspect has further enhanced its appeal in various industrial applications, particularly in regions with stringent environmental regulations.
The global market for ethyl acetate is expected to continue its growth trajectory, driven by these diverse industrial applications and the compound's favorable properties. Manufacturers are likely to focus on expanding their production capacities to meet the rising demand, while also exploring new applications and markets to capitalize on the versatility of ethyl acetate.
In the coatings and paints industry, ethyl acetate has emerged as a preferred solvent due to its excellent solvency properties and rapid evaporation rate. The growing construction and automotive sectors have significantly contributed to the rising demand for high-quality paints and coatings, thereby driving the consumption of ethyl acetate. The compound's ability to dissolve a wide range of resins and polymers makes it indispensable in formulating durable and aesthetically pleasing finishes.
The packaging industry has also witnessed a surge in ethyl acetate usage, particularly in flexible packaging applications. As consumer preferences shift towards convenient and lightweight packaging solutions, the demand for ethyl acetate-based adhesives and printing inks has escalated. The food and beverage sector, in particular, relies heavily on these packaging materials, further propelling the market growth.
In the pharmaceutical industry, ethyl acetate plays a crucial role as a solvent in the production of various drugs and active pharmaceutical ingredients (APIs). The increasing global focus on healthcare and the rising prevalence of chronic diseases have led to an expansion of pharmaceutical manufacturing, consequently boosting the demand for ethyl acetate in this sector.
The electronics industry has found innovative applications for ethyl acetate in the production of printed circuit boards and semiconductor devices. Its use as a cleaning agent and in photoresist stripping processes has become essential in the manufacturing of advanced electronic components, aligning with the growing demand for smart devices and IoT technologies.
The personal care and cosmetics industry has also embraced ethyl acetate as a key ingredient in nail polish removers and as a solvent in perfumes. The compound's mild nature and pleasant scent make it an attractive option for formulators seeking alternatives to harsher chemicals.
As sustainability becomes a primary concern across industries, the biodegradability of ethyl acetate has positioned it as an environmentally friendly alternative to many traditional solvents. This aspect has further enhanced its appeal in various industrial applications, particularly in regions with stringent environmental regulations.
The global market for ethyl acetate is expected to continue its growth trajectory, driven by these diverse industrial applications and the compound's favorable properties. Manufacturers are likely to focus on expanding their production capacities to meet the rising demand, while also exploring new applications and markets to capitalize on the versatility of ethyl acetate.
Technical Challenges
Despite the widespread use of ethyl acetate in various industries, several technical challenges persist in its production, application, and environmental impact. One of the primary concerns is the optimization of the production process to enhance yield and purity while reducing energy consumption and waste generation. Traditional methods often involve energy-intensive distillation steps, which contribute to high production costs and environmental footprint.
The synthesis of ethyl acetate typically relies on the esterification of ethanol with acetic acid or the Tishchenko reaction of acetaldehyde. Both processes face challenges in terms of reaction kinetics, equilibrium limitations, and catalyst efficiency. Developing more effective catalysts that can operate at lower temperatures and pressures while maintaining high selectivity remains an ongoing research focus.
Another significant challenge lies in the purification of ethyl acetate. The azeotropic behavior of ethyl acetate with water complicates the separation process, necessitating additional purification steps. This not only increases production costs but also impacts the overall efficiency of the manufacturing process. Innovative separation technologies, such as membrane-based systems or advanced distillation techniques, are being explored to address this issue.
The volatility of ethyl acetate poses challenges in its handling, storage, and application. Its low boiling point and high vapor pressure contribute to significant evaporative losses during use, particularly in coating and adhesive applications. This not only results in material waste but also raises environmental and health concerns due to volatile organic compound (VOC) emissions. Developing formulations with reduced volatility or improved application methods to minimize evaporation is an area of active research.
From an environmental perspective, the biodegradability and potential ecological impact of ethyl acetate and its production by-products require careful consideration. While ethyl acetate is generally considered less harmful compared to many other solvents, its large-scale use and disposal still present challenges in terms of waste management and environmental protection. Developing greener production routes, such as bio-based ethyl acetate synthesis, and improving end-of-life management strategies are crucial areas for technological advancement.
In emerging applications, such as in the electronics industry for lithium-ion battery production, ensuring the ultra-high purity of ethyl acetate is a significant technical hurdle. Trace impurities can severely impact battery performance and safety, necessitating advanced purification and quality control measures. This challenge intersects with the broader trend towards miniaturization and increased performance demands in electronic devices.
Lastly, the integration of ethyl acetate production and utilization into circular economy models presents both opportunities and challenges. Developing efficient recycling and recovery systems for ethyl acetate from various waste streams, including industrial effluents and used products, is essential for improving sustainability. This requires innovative separation technologies and process designs that can handle complex mixtures and maintain product quality across multiple use cycles.
The synthesis of ethyl acetate typically relies on the esterification of ethanol with acetic acid or the Tishchenko reaction of acetaldehyde. Both processes face challenges in terms of reaction kinetics, equilibrium limitations, and catalyst efficiency. Developing more effective catalysts that can operate at lower temperatures and pressures while maintaining high selectivity remains an ongoing research focus.
Another significant challenge lies in the purification of ethyl acetate. The azeotropic behavior of ethyl acetate with water complicates the separation process, necessitating additional purification steps. This not only increases production costs but also impacts the overall efficiency of the manufacturing process. Innovative separation technologies, such as membrane-based systems or advanced distillation techniques, are being explored to address this issue.
The volatility of ethyl acetate poses challenges in its handling, storage, and application. Its low boiling point and high vapor pressure contribute to significant evaporative losses during use, particularly in coating and adhesive applications. This not only results in material waste but also raises environmental and health concerns due to volatile organic compound (VOC) emissions. Developing formulations with reduced volatility or improved application methods to minimize evaporation is an area of active research.
From an environmental perspective, the biodegradability and potential ecological impact of ethyl acetate and its production by-products require careful consideration. While ethyl acetate is generally considered less harmful compared to many other solvents, its large-scale use and disposal still present challenges in terms of waste management and environmental protection. Developing greener production routes, such as bio-based ethyl acetate synthesis, and improving end-of-life management strategies are crucial areas for technological advancement.
In emerging applications, such as in the electronics industry for lithium-ion battery production, ensuring the ultra-high purity of ethyl acetate is a significant technical hurdle. Trace impurities can severely impact battery performance and safety, necessitating advanced purification and quality control measures. This challenge intersects with the broader trend towards miniaturization and increased performance demands in electronic devices.
Lastly, the integration of ethyl acetate production and utilization into circular economy models presents both opportunities and challenges. Developing efficient recycling and recovery systems for ethyl acetate from various waste streams, including industrial effluents and used products, is essential for improving sustainability. This requires innovative separation technologies and process designs that can handle complex mixtures and maintain product quality across multiple use cycles.
Current Applications
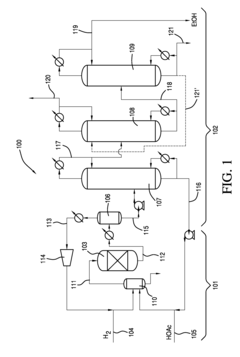
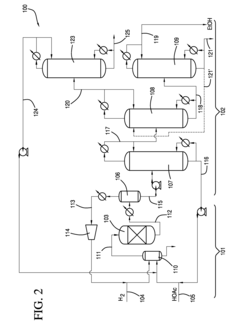
01 Production and purification of ethyl acetate
Various methods and processes for producing and purifying ethyl acetate are described. These include esterification reactions, distillation techniques, and the use of specific catalysts or reactants to improve yield and purity. Some processes focus on continuous production methods or the recovery of ethyl acetate from waste streams.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described. These include distillation processes, reactive distillation, and the use of specific catalysts to improve yield and purity. The techniques aim to enhance the efficiency of ethyl acetate production and separation from byproducts.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in diverse chemical processes, including as a solvent in reactions, extractions, and formulations. It plays a role in the production of various compounds and materials, showcasing its versatility in industrial applications.
- Ethyl acetate in pharmaceutical and cosmetic formulations: The use of ethyl acetate in pharmaceutical and cosmetic preparations is explored. It serves as a solvent or carrier for active ingredients, and its properties make it suitable for various formulations in these industries.
- Ethyl acetate in polymer and material science: Ethyl acetate finds applications in polymer synthesis and material science. It is used in the production of certain polymers, as a solvent in polymer processing, and in the development of novel materials with specific properties.
- Environmental and safety considerations for ethyl acetate: The environmental impact and safety aspects of ethyl acetate use are addressed. This includes methods for its safe handling, storage, and disposal, as well as techniques for reducing emissions and improving its eco-friendly profile in industrial processes.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes and industries. It serves as a solvent, reactant, or intermediate in the production of other chemicals, pharmaceuticals, and materials. Some applications include its use in coating formulations, as an extraction solvent, or in the synthesis of specific compounds.Expand Specific Solutions03 Ethyl acetate in polymer and material science
Ethyl acetate plays a role in polymer and material science applications. It is used in the preparation of certain polymers, as a solvent in polymer processing, or as a component in material formulations. Some inventions describe its use in creating specific material properties or in the development of novel materials.Expand Specific Solutions04 Separation and recovery of ethyl acetate
Methods for separating and recovering ethyl acetate from mixtures or waste streams are described. These include various separation techniques such as distillation, extraction, or membrane processes. Some inventions focus on improving the efficiency of separation or on recovering ethyl acetate from specific industrial processes.Expand Specific Solutions05 Environmental and safety considerations for ethyl acetate
Inventions related to the environmental impact and safety aspects of ethyl acetate use are presented. These may include methods for reducing emissions, improving handling safety, or developing more environmentally friendly processes involving ethyl acetate. Some focus on regulatory compliance or on reducing the environmental footprint of ethyl acetate production and use.Expand Specific Solutions
Key Industry Players
The ethyl acetate market is experiencing a dynamic phase of growth and innovation, driven by its expanding role in various industrial applications. The industry is in a mature stage but witnessing renewed interest due to emerging applications. Market size is substantial, with steady growth projected. Technologically, the field is advancing, with companies like Celanese International Corp., Eastman Chemical Co., and BASF Corp. leading innovation. These firms, along with others such as BP Chemicals Ltd. and China Petroleum & Chemical Corp., are investing in research and development to enhance production efficiency and explore novel applications. The competitive landscape is characterized by a mix of established chemical giants and specialized manufacturers, each contributing to the technological maturity of ethyl acetate production and utilization.
Celanese International Corp.
Technical Solution: Celanese has developed a proprietary VAntage® ethyl acetate technology, which utilizes a unique combination of catalysts and process conditions to achieve high selectivity and yield. The process incorporates an advanced separation system that minimizes energy consumption and reduces waste generation. Celanese's approach also includes the use of acetic acid produced from their proprietary TCX® technology, creating a more integrated and efficient production chain[3]. The company has further enhanced their process by implementing real-time monitoring and predictive maintenance systems, ensuring optimal performance and reducing downtime[4].
Strengths: High selectivity and yield, integrated production chain, and advanced process control. Weaknesses: Dependence on proprietary technologies may limit flexibility in raw material sourcing.
Eastman Chemical Co.
Technical Solution: Eastman has developed a novel process for ethyl acetate production using their proprietary Eastman™ Gasification Technology. This approach involves the gasification of coal or biomass to produce syngas, which is then converted to acetic acid and subsequently to ethyl acetate. The process offers flexibility in feedstock selection and allows for the co-production of valuable chemicals. Eastman has also implemented advanced catalytic systems that improve conversion efficiency and reduce byproduct formation[5]. Additionally, the company has invested in membrane separation technology to enhance product purification and reduce energy consumption in the distillation process[6].
Strengths: Feedstock flexibility, co-production of valuable chemicals, and improved energy efficiency. Weaknesses: Potential higher capital costs associated with gasification technology and complexity in process integration.
Innovative Formulations
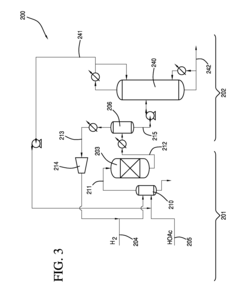
Process for producing an ethyl acetate solvent and co-production of ethanol
PatentInactiveUS20110190531A1
Innovation
- A process involving the hydrogenation of acetic acid in the presence of a catalyst, followed by a series of distillation columns to separate and recover ethanol and ethyl acetate solvent, with specific catalyst compositions and conditions to optimize ethanol and ethyl acetate production, including the use of platinum-based catalysts and modified silica supports.
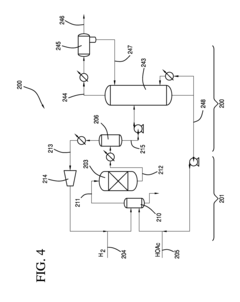
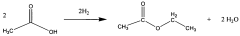
Direct and selective production of ethyl acetate from acetic acid utilizing a bimetal supported catalyst
PatentWO2010014145A2
Innovation
- A process utilizing a bimetallic catalyst supported on a suitable catalyst support, comprising metals like platinum, palladium, copper, and cobalt, which selectively hydrogenates acetic acid to ethyl acetate with high yield and selectivity, minimizing by-product formation.
Environmental Impact
The environmental impact of ethyl acetate's increasing use in industrial applications is a critical consideration for sustainable development. As a volatile organic compound (VOC), ethyl acetate can contribute to air pollution and the formation of ground-level ozone when released into the atmosphere. However, compared to many other solvents, ethyl acetate has a relatively low toxicity and environmental persistence, making it a more environmentally friendly option in many cases.
One of the primary environmental concerns associated with ethyl acetate is its potential to contribute to photochemical smog formation. When exposed to sunlight and nitrogen oxides, ethyl acetate can undergo photochemical reactions, leading to the creation of ground-level ozone and other secondary pollutants. This can have adverse effects on air quality, particularly in urban and industrial areas where VOC emissions are concentrated.
Despite these concerns, ethyl acetate has several environmental advantages over traditional solvents. It has a lower global warming potential and ozone depletion potential compared to many chlorinated solvents. Additionally, ethyl acetate is biodegradable and does not bioaccumulate in the environment, reducing its long-term ecological impact. These properties have led to its increased adoption as a replacement for more harmful solvents in various industrial processes.
In terms of water pollution, ethyl acetate's high solubility in water can lead to contamination of aquatic ecosystems if not properly managed. However, its rapid biodegradation in water helps mitigate long-term effects. Proper handling, storage, and disposal practices are essential to prevent accidental releases and minimize environmental contamination.
The production of ethyl acetate also has environmental implications. Traditional manufacturing methods often rely on petrochemical feedstocks, contributing to fossil fuel consumption and associated greenhouse gas emissions. However, recent advancements in bio-based production methods, using renewable resources such as ethanol derived from biomass, offer a more sustainable alternative. These bio-based production routes can significantly reduce the carbon footprint of ethyl acetate manufacturing.
As industries continue to adopt ethyl acetate in various applications, there is a growing focus on developing closed-loop systems and recycling technologies to minimize emissions and waste. Solvent recovery and reuse strategies are becoming increasingly common, reducing the overall environmental impact of ethyl acetate use in industrial processes. Furthermore, ongoing research into green chemistry principles is driving the development of even more environmentally benign alternatives and process optimizations.
One of the primary environmental concerns associated with ethyl acetate is its potential to contribute to photochemical smog formation. When exposed to sunlight and nitrogen oxides, ethyl acetate can undergo photochemical reactions, leading to the creation of ground-level ozone and other secondary pollutants. This can have adverse effects on air quality, particularly in urban and industrial areas where VOC emissions are concentrated.
Despite these concerns, ethyl acetate has several environmental advantages over traditional solvents. It has a lower global warming potential and ozone depletion potential compared to many chlorinated solvents. Additionally, ethyl acetate is biodegradable and does not bioaccumulate in the environment, reducing its long-term ecological impact. These properties have led to its increased adoption as a replacement for more harmful solvents in various industrial processes.
In terms of water pollution, ethyl acetate's high solubility in water can lead to contamination of aquatic ecosystems if not properly managed. However, its rapid biodegradation in water helps mitigate long-term effects. Proper handling, storage, and disposal practices are essential to prevent accidental releases and minimize environmental contamination.
The production of ethyl acetate also has environmental implications. Traditional manufacturing methods often rely on petrochemical feedstocks, contributing to fossil fuel consumption and associated greenhouse gas emissions. However, recent advancements in bio-based production methods, using renewable resources such as ethanol derived from biomass, offer a more sustainable alternative. These bio-based production routes can significantly reduce the carbon footprint of ethyl acetate manufacturing.
As industries continue to adopt ethyl acetate in various applications, there is a growing focus on developing closed-loop systems and recycling technologies to minimize emissions and waste. Solvent recovery and reuse strategies are becoming increasingly common, reducing the overall environmental impact of ethyl acetate use in industrial processes. Furthermore, ongoing research into green chemistry principles is driving the development of even more environmentally benign alternatives and process optimizations.
Regulatory Compliance
The regulatory landscape surrounding ethyl acetate's industrial applications is complex and evolving, necessitating careful attention from manufacturers and users alike. As ethyl acetate finds increasing use across various sectors, compliance with regulatory frameworks becomes paramount to ensure safe handling, environmental protection, and consumer safety.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The substance is listed on the TSCA inventory, requiring manufacturers and importers to comply with reporting, record-keeping, and testing requirements. Additionally, the Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for ethyl acetate in workplace environments, mandating proper safety measures and personal protective equipment.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation also applies to ethyl acetate. Manufacturers and importers must register the substance with the European Chemicals Agency (ECHA) and provide comprehensive safety data. The Classification, Labeling, and Packaging (CLP) Regulation further requires proper hazard communication for ethyl acetate-containing products.
In the food industry, ethyl acetate's use as a solvent and flavoring agent is subject to stringent regulations. The U.S. Food and Drug Administration (FDA) classifies it as Generally Recognized as Safe (GRAS) when used in accordance with good manufacturing practices. Similarly, the European Food Safety Authority (EFSA) has evaluated ethyl acetate and established specific purity criteria for its use in food applications.
Environmental regulations play a crucial role in ethyl acetate's industrial use. As a volatile organic compound (VOC), its emissions are regulated under various air quality laws. In the U.S., the Clean Air Act sets limits on VOC emissions from industrial processes, while the EU's Industrial Emissions Directive imposes similar restrictions. Companies must implement appropriate emission control technologies and monitoring systems to ensure compliance.
Transportation of ethyl acetate is subject to hazardous materials regulations. The U.S. Department of Transportation (DOT) classifies it as a flammable liquid, requiring specific packaging, labeling, and shipping documentation. Internationally, the transport of ethyl acetate must comply with the United Nations' Recommendations on the Transport of Dangerous Goods.
As ethyl acetate's industrial applications continue to expand, staying abreast of regulatory changes and ensuring compliance across jurisdictions becomes increasingly important. Companies must invest in robust regulatory affairs capabilities, implement comprehensive compliance programs, and actively engage with regulatory bodies to navigate the complex landscape effectively.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The substance is listed on the TSCA inventory, requiring manufacturers and importers to comply with reporting, record-keeping, and testing requirements. Additionally, the Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for ethyl acetate in workplace environments, mandating proper safety measures and personal protective equipment.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation also applies to ethyl acetate. Manufacturers and importers must register the substance with the European Chemicals Agency (ECHA) and provide comprehensive safety data. The Classification, Labeling, and Packaging (CLP) Regulation further requires proper hazard communication for ethyl acetate-containing products.
In the food industry, ethyl acetate's use as a solvent and flavoring agent is subject to stringent regulations. The U.S. Food and Drug Administration (FDA) classifies it as Generally Recognized as Safe (GRAS) when used in accordance with good manufacturing practices. Similarly, the European Food Safety Authority (EFSA) has evaluated ethyl acetate and established specific purity criteria for its use in food applications.
Environmental regulations play a crucial role in ethyl acetate's industrial use. As a volatile organic compound (VOC), its emissions are regulated under various air quality laws. In the U.S., the Clean Air Act sets limits on VOC emissions from industrial processes, while the EU's Industrial Emissions Directive imposes similar restrictions. Companies must implement appropriate emission control technologies and monitoring systems to ensure compliance.
Transportation of ethyl acetate is subject to hazardous materials regulations. The U.S. Department of Transportation (DOT) classifies it as a flammable liquid, requiring specific packaging, labeling, and shipping documentation. Internationally, the transport of ethyl acetate must comply with the United Nations' Recommendations on the Transport of Dangerous Goods.
As ethyl acetate's industrial applications continue to expand, staying abreast of regulatory changes and ensuring compliance across jurisdictions becomes increasingly important. Companies must invest in robust regulatory affairs capabilities, implement comprehensive compliance programs, and actively engage with regulatory bodies to navigate the complex landscape effectively.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!