How Ethyl Acetate Aligns with Eco‑Forward Dynamics?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Evolution
Ethyl acetate has undergone a significant evolution in its production methods and applications, aligning increasingly with eco-forward dynamics. Initially synthesized in the early 19th century, ethyl acetate was primarily produced through the esterification of ethanol and acetic acid. This traditional method, while effective, was energy-intensive and relied heavily on petrochemical feedstocks.
As environmental concerns gained prominence in the late 20th century, the industry began exploring more sustainable production routes. The advent of biotechnology in the 1980s opened new avenues for ethyl acetate synthesis. Fermentation processes using renewable resources like sugarcane and corn emerged as promising alternatives. These bio-based methods not only reduced reliance on fossil fuels but also significantly lowered carbon emissions.
The turn of the millennium saw a surge in research focused on green chemistry principles. Scientists developed novel catalysts that improved reaction efficiency and selectivity, reducing waste and energy consumption in ethyl acetate production. Continuous flow reactors and process intensification techniques further enhanced sustainability by minimizing solvent use and improving heat transfer.
In recent years, the concept of circular economy has influenced ethyl acetate production. Manufacturers have begun integrating waste streams from other industries as feedstocks. For instance, carbon dioxide captured from industrial processes is now being utilized in the synthesis of ethyl acetate, effectively turning a greenhouse gas into a valuable product.
The evolution of ethyl acetate production has also been marked by improvements in purification and recovery methods. Advanced distillation techniques and membrane technologies have significantly reduced energy requirements for product separation. Moreover, the implementation of solvent recovery systems has minimized waste and improved overall process economics.
As the world moves towards a low-carbon future, the ethyl acetate industry is exploring innovative approaches to further reduce its environmental footprint. Research into electrochemical synthesis methods powered by renewable electricity shows promise for carbon-neutral production. Additionally, the development of bio-based alternatives to traditional petrochemical-derived ethyl acetate is gaining momentum, with several companies investing in pilot-scale facilities.
This ongoing evolution reflects the industry's commitment to aligning with eco-forward dynamics. By continuously improving production methods, embracing renewable resources, and integrating circular economy principles, ethyl acetate is positioning itself as a sustainable solution for various applications in the chemical industry.
As environmental concerns gained prominence in the late 20th century, the industry began exploring more sustainable production routes. The advent of biotechnology in the 1980s opened new avenues for ethyl acetate synthesis. Fermentation processes using renewable resources like sugarcane and corn emerged as promising alternatives. These bio-based methods not only reduced reliance on fossil fuels but also significantly lowered carbon emissions.
The turn of the millennium saw a surge in research focused on green chemistry principles. Scientists developed novel catalysts that improved reaction efficiency and selectivity, reducing waste and energy consumption in ethyl acetate production. Continuous flow reactors and process intensification techniques further enhanced sustainability by minimizing solvent use and improving heat transfer.
In recent years, the concept of circular economy has influenced ethyl acetate production. Manufacturers have begun integrating waste streams from other industries as feedstocks. For instance, carbon dioxide captured from industrial processes is now being utilized in the synthesis of ethyl acetate, effectively turning a greenhouse gas into a valuable product.
The evolution of ethyl acetate production has also been marked by improvements in purification and recovery methods. Advanced distillation techniques and membrane technologies have significantly reduced energy requirements for product separation. Moreover, the implementation of solvent recovery systems has minimized waste and improved overall process economics.
As the world moves towards a low-carbon future, the ethyl acetate industry is exploring innovative approaches to further reduce its environmental footprint. Research into electrochemical synthesis methods powered by renewable electricity shows promise for carbon-neutral production. Additionally, the development of bio-based alternatives to traditional petrochemical-derived ethyl acetate is gaining momentum, with several companies investing in pilot-scale facilities.
This ongoing evolution reflects the industry's commitment to aligning with eco-forward dynamics. By continuously improving production methods, embracing renewable resources, and integrating circular economy principles, ethyl acetate is positioning itself as a sustainable solution for various applications in the chemical industry.
Green Solvent Demand
The demand for green solvents has been steadily increasing in recent years, driven by growing environmental concerns and stringent regulations. Ethyl acetate, a versatile organic compound, has emerged as a promising candidate in the eco-friendly solvent market. This shift towards greener alternatives is primarily fueled by the need to reduce the environmental impact of industrial processes and consumer products.
In the chemical industry, ethyl acetate is gaining traction as a replacement for more harmful solvents. Its low toxicity, biodegradability, and relatively low volatility make it an attractive option for various applications, including coatings, adhesives, and pharmaceutical processes. The paint and coating sector, in particular, has shown significant interest in ethyl acetate due to its excellent solvency properties and quick evaporation rate, which contribute to improved product performance while reducing environmental footprint.
The pharmaceutical industry is another key driver of green solvent demand, with ethyl acetate finding applications in drug synthesis and purification processes. As pharmaceutical companies strive to adopt more sustainable practices, the use of ethyl acetate as a greener alternative to traditional solvents is expected to grow. This trend is further supported by regulatory bodies encouraging the use of environmentally benign solvents in drug manufacturing.
Consumer awareness of environmental issues has also played a crucial role in boosting the demand for green solvents. Products labeled as eco-friendly or containing green solvents are increasingly preferred by environmentally conscious consumers. This shift in consumer behavior has prompted manufacturers across various industries to reformulate their products using greener ingredients, including ethyl acetate.
The food and beverage industry represents another significant market for ethyl acetate as a green solvent. Its use in natural flavor extraction and as a food additive has increased due to its low toxicity and GRAS (Generally Recognized as Safe) status. The growing demand for natural and clean-label products has further accelerated the adoption of ethyl acetate in this sector.
As industries continue to prioritize sustainability and circular economy principles, the demand for green solvents like ethyl acetate is expected to grow. This trend is likely to be reinforced by ongoing research and development efforts aimed at improving the production efficiency and expanding the application range of ethyl acetate. The alignment of ethyl acetate with eco-forward dynamics positions it as a key player in the transition towards more sustainable industrial practices and consumer products.
In the chemical industry, ethyl acetate is gaining traction as a replacement for more harmful solvents. Its low toxicity, biodegradability, and relatively low volatility make it an attractive option for various applications, including coatings, adhesives, and pharmaceutical processes. The paint and coating sector, in particular, has shown significant interest in ethyl acetate due to its excellent solvency properties and quick evaporation rate, which contribute to improved product performance while reducing environmental footprint.
The pharmaceutical industry is another key driver of green solvent demand, with ethyl acetate finding applications in drug synthesis and purification processes. As pharmaceutical companies strive to adopt more sustainable practices, the use of ethyl acetate as a greener alternative to traditional solvents is expected to grow. This trend is further supported by regulatory bodies encouraging the use of environmentally benign solvents in drug manufacturing.
Consumer awareness of environmental issues has also played a crucial role in boosting the demand for green solvents. Products labeled as eco-friendly or containing green solvents are increasingly preferred by environmentally conscious consumers. This shift in consumer behavior has prompted manufacturers across various industries to reformulate their products using greener ingredients, including ethyl acetate.
The food and beverage industry represents another significant market for ethyl acetate as a green solvent. Its use in natural flavor extraction and as a food additive has increased due to its low toxicity and GRAS (Generally Recognized as Safe) status. The growing demand for natural and clean-label products has further accelerated the adoption of ethyl acetate in this sector.
As industries continue to prioritize sustainability and circular economy principles, the demand for green solvents like ethyl acetate is expected to grow. This trend is likely to be reinforced by ongoing research and development efforts aimed at improving the production efficiency and expanding the application range of ethyl acetate. The alignment of ethyl acetate with eco-forward dynamics positions it as a key player in the transition towards more sustainable industrial practices and consumer products.
Eco-Challenges in EA
The production and use of ethyl acetate (EA) present several environmental challenges that need to be addressed to align with eco-forward dynamics. One of the primary concerns is the volatile organic compound (VOC) emissions associated with EA. As a solvent, EA readily evaporates at room temperature, contributing to air pollution and potentially forming ground-level ozone when it reacts with other pollutants in the presence of sunlight. This poses risks to both human health and the environment, necessitating stringent emission control measures in industrial settings.
Another significant eco-challenge is the traditional production method of EA, which often relies on petrochemical feedstocks. This dependency on non-renewable resources contradicts sustainability goals and contributes to the carbon footprint of EA manufacturing. The production process typically involves the esterification of ethanol and acetic acid, both of which are commonly derived from fossil fuels. This not only depletes finite resources but also results in greenhouse gas emissions throughout the production chain.
Water pollution is an additional environmental concern in EA production and usage. Effluents from manufacturing plants may contain traces of EA and other chemical byproducts, which can contaminate water bodies if not properly treated. This can lead to adverse effects on aquatic ecosystems and potentially impact drinking water sources. Moreover, the improper disposal of EA-containing products or waste can result in soil contamination, further exacerbating environmental risks.
The energy-intensive nature of EA production also presents an eco-challenge. The distillation processes required to purify EA consume significant amounts of energy, often derived from fossil fuels. This energy consumption contributes to the overall carbon footprint of EA, making it less aligned with eco-forward principles that prioritize energy efficiency and the use of renewable energy sources.
Biodegradability and end-of-life management of EA-containing products pose additional environmental challenges. While EA itself is biodegradable, its presence in complex product formulations can hinder recycling efforts and complicate waste management strategies. This is particularly problematic in industries such as coatings and adhesives, where EA is widely used and product disposal can lead to environmental contamination if not managed properly.
Addressing these eco-challenges requires a multifaceted approach that encompasses greener production methods, improved emission control technologies, and more sustainable product design. Innovations in bio-based EA production, using renewable feedstocks such as agricultural waste, offer promising alternatives to traditional petrochemical routes. Additionally, the development of closed-loop systems and advanced recycling technologies could help mitigate the environmental impact of EA throughout its lifecycle, aligning its use more closely with eco-forward dynamics.
Another significant eco-challenge is the traditional production method of EA, which often relies on petrochemical feedstocks. This dependency on non-renewable resources contradicts sustainability goals and contributes to the carbon footprint of EA manufacturing. The production process typically involves the esterification of ethanol and acetic acid, both of which are commonly derived from fossil fuels. This not only depletes finite resources but also results in greenhouse gas emissions throughout the production chain.
Water pollution is an additional environmental concern in EA production and usage. Effluents from manufacturing plants may contain traces of EA and other chemical byproducts, which can contaminate water bodies if not properly treated. This can lead to adverse effects on aquatic ecosystems and potentially impact drinking water sources. Moreover, the improper disposal of EA-containing products or waste can result in soil contamination, further exacerbating environmental risks.
The energy-intensive nature of EA production also presents an eco-challenge. The distillation processes required to purify EA consume significant amounts of energy, often derived from fossil fuels. This energy consumption contributes to the overall carbon footprint of EA, making it less aligned with eco-forward principles that prioritize energy efficiency and the use of renewable energy sources.
Biodegradability and end-of-life management of EA-containing products pose additional environmental challenges. While EA itself is biodegradable, its presence in complex product formulations can hinder recycling efforts and complicate waste management strategies. This is particularly problematic in industries such as coatings and adhesives, where EA is widely used and product disposal can lead to environmental contamination if not managed properly.
Addressing these eco-challenges requires a multifaceted approach that encompasses greener production methods, improved emission control technologies, and more sustainable product design. Innovations in bio-based EA production, using renewable feedstocks such as agricultural waste, offer promising alternatives to traditional petrochemical routes. Additionally, the development of closed-loop systems and advanced recycling technologies could help mitigate the environmental impact of EA throughout its lifecycle, aligning its use more closely with eco-forward dynamics.
Green EA Solutions
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The production methods aim to optimize the reaction conditions and separation processes to obtain high-quality ethyl acetate efficiently.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The production methods aim to optimize the synthesis of ethyl acetate from ethanol and acetic acid or other precursors.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in various chemical processes as a solvent, reactant, or intermediate. It finds applications in the production of pharmaceuticals, polymers, and other organic compounds. The versatility of ethyl acetate in different chemical reactions and its role in industrial processes are highlighted.
- Ethyl acetate in extraction and separation processes: Ethyl acetate is employed in extraction and separation processes for various substances. Its properties make it suitable for liquid-liquid extraction, azeotropic distillation, and other separation techniques. The use of ethyl acetate in these processes can improve efficiency and yield in the isolation of target compounds.
- Ethyl acetate as a green solvent: The use of ethyl acetate as an environmentally friendly solvent is explored. Its relatively low toxicity, biodegradability, and potential for recovery and recycling make it an attractive alternative to more hazardous solvents in various applications, including coatings, adhesives, and cleaning products.
- Recovery and recycling of ethyl acetate: Methods for recovering and recycling ethyl acetate from industrial processes are described. These include adsorption techniques, membrane separation, and advanced distillation processes. The recovery and recycling of ethyl acetate can improve process economics and reduce environmental impact in various industries.
02 Applications of ethyl acetate in industrial processes
Ethyl acetate finds diverse applications in industrial processes. It is used as a solvent in various industries, including pharmaceuticals, coatings, and adhesives. The compound is also employed in extraction processes, as a reaction medium, and in the production of other chemicals. Its versatility makes it a valuable component in many manufacturing processes.Expand Specific Solutions03 Ethyl acetate in polymer and material science
Ethyl acetate plays a significant role in polymer and material science. It is used in the synthesis and processing of various polymers, as well as in the development of advanced materials. The compound's properties make it suitable for applications such as surface treatments, coatings, and as a component in composite materials.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
The use of ethyl acetate in industrial processes requires careful consideration of environmental and safety aspects. This includes developing methods for reducing emissions, improving handling and storage practices, and implementing proper disposal techniques. Research focuses on minimizing the environmental impact of ethyl acetate production and use while ensuring worker safety.Expand Specific Solutions05 Novel synthesis routes and catalysts for ethyl acetate
Research into novel synthesis routes and catalysts for ethyl acetate production aims to improve efficiency and sustainability. This includes exploring new reaction pathways, developing more effective catalysts, and optimizing reaction conditions. The goal is to enhance yield, reduce energy consumption, and minimize waste in the production process.Expand Specific Solutions
Key EA Manufacturers
The ethyl acetate market is in a mature growth stage, with a global market size expected to reach $4.3 billion by 2025. The industry is characterized by established players and increasing demand from various end-use sectors. Technologically, ethyl acetate production is well-developed, with companies like BASF, Eastman Chemical, and Celanese leading in innovation and eco-friendly processes. These firms are focusing on sustainable production methods and bio-based alternatives to align with environmental trends. Emerging players, such as LanzaTech and Xyleco, are exploring novel biotechnology approaches for ethyl acetate synthesis, potentially disrupting traditional petrochemical routes. The competitive landscape is evolving as companies invest in green chemistry to meet growing demand for environmentally sustainable solutions.
BASF Corp.
Technical Solution: BASF has developed a bio-based production process for ethyl acetate using renewable feedstocks. Their approach involves fermenting sugars derived from agricultural waste to produce ethanol, which is then esterified with acetic acid to form ethyl acetate. This process reduces greenhouse gas emissions by up to 70% compared to conventional fossil-based production methods [1]. BASF has also implemented energy-efficient distillation techniques and heat integration systems to minimize energy consumption during purification, further enhancing the sustainability profile of their ethyl acetate production [2].
Strengths: Significant reduction in carbon footprint, utilization of renewable resources, and improved energy efficiency. Weaknesses: Potential higher production costs compared to traditional methods and dependence on agricultural feedstock availability.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has developed a novel catalytic process for ethyl acetate production that aligns with eco-forward dynamics. Their approach uses a heterogeneous catalyst system that enables direct formation of ethyl acetate from ethanol and oxygen, bypassing the need for acetic acid as an intermediate [3]. This one-step process significantly reduces energy consumption and waste generation. Additionally, Eastman has implemented advanced process control systems and heat recovery technologies to optimize resource utilization and minimize environmental impact [4]. The company has also explored the use of bio-ethanol as a feedstock to further enhance the sustainability of their ethyl acetate production.
Strengths: Simplified production process, reduced energy consumption, and potential for bio-based feedstock integration. Weaknesses: Possible challenges in catalyst longevity and selectivity, and initial capital investment for process conversion.
EA Eco-Innovations
Process of low energy consumption for preparing a carboxylic acid ester
PatentWO2012123279A1
Innovation
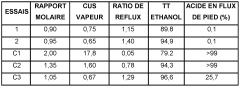
- A process involving the reaction of ethyl alcohol with acetic acid in the presence of a solid acid catalyst, using a reactive distillation system with a centrally placed reaction zone between upper and lower separation zones, optimizing the molar ratio of acetic acid to ethyl alcohol between 0.85 and 0.97, and controlling the reflux ratio between 1.0 and 1.5, significantly reduces energy costs and minimizes acetic acid at the column bottom.
Ethyl Acetate As Fuel Or Fuel Additive
PatentInactiveUS20110296744A1
Innovation
- Using ethyl acetate as a fuel additive or blending agent in hydrocarbon-containing fuels, which is non-hygroscopic and offers desirable fuel characteristics, including higher heat of combustion and improved winter performance, thereby avoiding ethanol's drawbacks.
EA Regulatory Landscape
The regulatory landscape for ethyl acetate (EA) is evolving in response to growing environmental concerns and sustainability initiatives worldwide. As a solvent widely used in various industries, EA is subject to a complex web of regulations that aim to balance its industrial utility with ecological considerations.
In the United States, the Environmental Protection Agency (EPA) regulates EA under the Toxic Substances Control Act (TSCA). While EA is not classified as a hazardous air pollutant, it is listed as a volatile organic compound (VOC) and is subject to emission controls in many states. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for workers, reflecting the compound's potential health impacts.
The European Union's regulatory framework for EA is primarily governed by the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. Under REACH, manufacturers and importers are required to register EA and provide safety data. The EU has also implemented the Industrial Emissions Directive (IED), which sets emission limits for VOCs, including EA, in industrial processes.
In Asia, countries like China and Japan have been strengthening their chemical regulations. China's Measures for Environmental Management of New Chemical Substances requires notification and risk assessment for EA production and use. Japan's Chemical Substances Control Law (CSCL) classifies EA as a general chemical substance, subject to reporting requirements.
Global efforts to reduce VOC emissions have led to increased scrutiny of EA use. Many countries have implemented regulations to limit EA emissions from paints, coatings, and other consumer products. These regulations often set maximum VOC content limits or require the use of low-VOC alternatives.
The push towards circular economy principles is influencing EA regulations. There is a growing emphasis on recycling and recovery of solvents, including EA, to minimize waste and environmental impact. Some jurisdictions are exploring extended producer responsibility schemes that would require manufacturers to manage the entire lifecycle of EA-containing products.
As sustainability becomes a key focus in chemical policy, regulators are increasingly considering the environmental footprint of EA production and use. This includes assessing the carbon footprint, water usage, and overall lifecycle impact. Future regulations may incentivize the development and adoption of bio-based or more environmentally friendly alternatives to traditional EA production methods.
The regulatory landscape for EA is likely to continue evolving, with a trend towards stricter controls and a greater emphasis on sustainable practices. Companies operating in the EA space must stay abreast of these changes and proactively adapt their processes to align with eco-forward dynamics and regulatory expectations.
In the United States, the Environmental Protection Agency (EPA) regulates EA under the Toxic Substances Control Act (TSCA). While EA is not classified as a hazardous air pollutant, it is listed as a volatile organic compound (VOC) and is subject to emission controls in many states. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for workers, reflecting the compound's potential health impacts.
The European Union's regulatory framework for EA is primarily governed by the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. Under REACH, manufacturers and importers are required to register EA and provide safety data. The EU has also implemented the Industrial Emissions Directive (IED), which sets emission limits for VOCs, including EA, in industrial processes.
In Asia, countries like China and Japan have been strengthening their chemical regulations. China's Measures for Environmental Management of New Chemical Substances requires notification and risk assessment for EA production and use. Japan's Chemical Substances Control Law (CSCL) classifies EA as a general chemical substance, subject to reporting requirements.
Global efforts to reduce VOC emissions have led to increased scrutiny of EA use. Many countries have implemented regulations to limit EA emissions from paints, coatings, and other consumer products. These regulations often set maximum VOC content limits or require the use of low-VOC alternatives.
The push towards circular economy principles is influencing EA regulations. There is a growing emphasis on recycling and recovery of solvents, including EA, to minimize waste and environmental impact. Some jurisdictions are exploring extended producer responsibility schemes that would require manufacturers to manage the entire lifecycle of EA-containing products.
As sustainability becomes a key focus in chemical policy, regulators are increasingly considering the environmental footprint of EA production and use. This includes assessing the carbon footprint, water usage, and overall lifecycle impact. Future regulations may incentivize the development and adoption of bio-based or more environmentally friendly alternatives to traditional EA production methods.
The regulatory landscape for EA is likely to continue evolving, with a trend towards stricter controls and a greater emphasis on sustainable practices. Companies operating in the EA space must stay abreast of these changes and proactively adapt their processes to align with eco-forward dynamics and regulatory expectations.
Life Cycle Assessment
Life Cycle Assessment (LCA) plays a crucial role in evaluating the environmental impact of ethyl acetate throughout its entire lifecycle, from raw material extraction to disposal. This comprehensive analysis provides valuable insights into how ethyl acetate aligns with eco-forward dynamics.
The production of ethyl acetate primarily involves the reaction between ethanol and acetic acid. The LCA begins by examining the environmental footprint of these raw materials. Ethanol can be derived from both renewable sources (e.g., corn, sugarcane) and non-renewable sources (e.g., petroleum), while acetic acid is typically produced through methanol carbonylation or fermentation processes.
During the manufacturing phase, the LCA considers energy consumption, emissions, and waste generation. The esterification process to produce ethyl acetate is relatively energy-efficient compared to other solvents, contributing to its favorable environmental profile. However, the use of catalysts and potential byproducts must be carefully evaluated for their environmental impact.
The distribution and transportation of ethyl acetate also factor into its lifecycle assessment. As a widely used solvent, its transportation efficiency and associated carbon emissions are important considerations. The volatile nature of ethyl acetate requires proper handling and storage to prevent losses and minimize environmental risks during transit.
In the use phase, ethyl acetate's low toxicity and high volatility contribute to its eco-friendly characteristics. It evaporates quickly, reducing exposure risks and minimizing long-term environmental persistence. However, proper ventilation and handling procedures are essential to mitigate potential health and environmental hazards associated with its use.
End-of-life considerations for ethyl acetate include its potential for recycling and proper disposal methods. Due to its high volatility, much of the solvent evaporates during use, but residual amounts require appropriate treatment. Recycling and recovery processes can significantly reduce the overall environmental impact by minimizing waste and conserving resources.
The LCA also evaluates the potential for biodegradation and environmental fate of ethyl acetate. Its relatively rapid biodegradation in both aerobic and anaerobic conditions contributes to its eco-friendly profile. This characteristic reduces the risk of long-term environmental accumulation and associated ecological impacts.
Comparative LCAs between ethyl acetate and alternative solvents provide valuable insights into its relative environmental performance. These assessments often highlight ethyl acetate's lower global warming potential and reduced ozone depletion impact compared to many traditional solvents, further supporting its alignment with eco-forward dynamics.
The production of ethyl acetate primarily involves the reaction between ethanol and acetic acid. The LCA begins by examining the environmental footprint of these raw materials. Ethanol can be derived from both renewable sources (e.g., corn, sugarcane) and non-renewable sources (e.g., petroleum), while acetic acid is typically produced through methanol carbonylation or fermentation processes.
During the manufacturing phase, the LCA considers energy consumption, emissions, and waste generation. The esterification process to produce ethyl acetate is relatively energy-efficient compared to other solvents, contributing to its favorable environmental profile. However, the use of catalysts and potential byproducts must be carefully evaluated for their environmental impact.
The distribution and transportation of ethyl acetate also factor into its lifecycle assessment. As a widely used solvent, its transportation efficiency and associated carbon emissions are important considerations. The volatile nature of ethyl acetate requires proper handling and storage to prevent losses and minimize environmental risks during transit.
In the use phase, ethyl acetate's low toxicity and high volatility contribute to its eco-friendly characteristics. It evaporates quickly, reducing exposure risks and minimizing long-term environmental persistence. However, proper ventilation and handling procedures are essential to mitigate potential health and environmental hazards associated with its use.
End-of-life considerations for ethyl acetate include its potential for recycling and proper disposal methods. Due to its high volatility, much of the solvent evaporates during use, but residual amounts require appropriate treatment. Recycling and recovery processes can significantly reduce the overall environmental impact by minimizing waste and conserving resources.
The LCA also evaluates the potential for biodegradation and environmental fate of ethyl acetate. Its relatively rapid biodegradation in both aerobic and anaerobic conditions contributes to its eco-friendly profile. This characteristic reduces the risk of long-term environmental accumulation and associated ecological impacts.
Comparative LCAs between ethyl acetate and alternative solvents provide valuable insights into its relative environmental performance. These assessments often highlight ethyl acetate's lower global warming potential and reduced ozone depletion impact compared to many traditional solvents, further supporting its alignment with eco-forward dynamics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!