How Ethyl Acetate Aids in Resource Efficiency Optimization?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Background
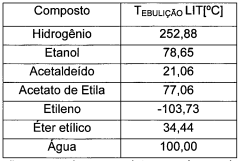
Ethyl acetate, a versatile organic compound with the chemical formula CH3COOC2H5, has been a cornerstone in various industries for decades. This colorless liquid, known for its characteristic fruity odor, is produced through the esterification of ethanol and acetic acid. Its discovery dates back to the early 19th century, with its first synthesis attributed to the French chemist Antoine Parmentier in 1807.
The compound's unique properties, including its low toxicity, high solvency power, and moderate volatility, have made it an indispensable component in numerous applications. Historically, ethyl acetate found its initial use in the production of artificial silk and leather. As industrial processes evolved, its utility expanded to encompass a wide range of sectors, from pharmaceuticals and cosmetics to food and beverage industries.
In the context of resource efficiency optimization, ethyl acetate has emerged as a key player due to its environmentally friendly profile and recyclability. Unlike many other solvents, ethyl acetate can be easily recovered and reused in industrial processes, significantly reducing waste and environmental impact. This characteristic aligns well with the growing global emphasis on sustainable practices and circular economy principles.
The compound's role in resource efficiency is further underscored by its biodegradability. Ethyl acetate naturally breaks down into harmless components, minimizing long-term environmental concerns associated with its use. This property has made it an attractive alternative to more persistent and harmful solvents, particularly in industries striving to meet stringent environmental regulations.
Over the years, advancements in production techniques have led to more efficient and cost-effective methods of synthesizing ethyl acetate. Modern industrial processes often employ continuous distillation and reactive distillation techniques, which have significantly improved yield and purity while reducing energy consumption. These innovations have not only made ethyl acetate more accessible but have also enhanced its potential for resource optimization across various applications.
The compound's versatility extends to its role as a precursor in the synthesis of other valuable chemicals. This cascading effect in chemical production chains contributes to overall resource efficiency by maximizing the utility of raw materials and minimizing waste streams. As industries continue to seek ways to optimize their resource use, ethyl acetate stands out as a model compound that demonstrates the potential for balancing industrial needs with environmental stewardship.
The compound's unique properties, including its low toxicity, high solvency power, and moderate volatility, have made it an indispensable component in numerous applications. Historically, ethyl acetate found its initial use in the production of artificial silk and leather. As industrial processes evolved, its utility expanded to encompass a wide range of sectors, from pharmaceuticals and cosmetics to food and beverage industries.
In the context of resource efficiency optimization, ethyl acetate has emerged as a key player due to its environmentally friendly profile and recyclability. Unlike many other solvents, ethyl acetate can be easily recovered and reused in industrial processes, significantly reducing waste and environmental impact. This characteristic aligns well with the growing global emphasis on sustainable practices and circular economy principles.
The compound's role in resource efficiency is further underscored by its biodegradability. Ethyl acetate naturally breaks down into harmless components, minimizing long-term environmental concerns associated with its use. This property has made it an attractive alternative to more persistent and harmful solvents, particularly in industries striving to meet stringent environmental regulations.
Over the years, advancements in production techniques have led to more efficient and cost-effective methods of synthesizing ethyl acetate. Modern industrial processes often employ continuous distillation and reactive distillation techniques, which have significantly improved yield and purity while reducing energy consumption. These innovations have not only made ethyl acetate more accessible but have also enhanced its potential for resource optimization across various applications.
The compound's versatility extends to its role as a precursor in the synthesis of other valuable chemicals. This cascading effect in chemical production chains contributes to overall resource efficiency by maximizing the utility of raw materials and minimizing waste streams. As industries continue to seek ways to optimize their resource use, ethyl acetate stands out as a model compound that demonstrates the potential for balancing industrial needs with environmental stewardship.
Market Analysis
The market for ethyl acetate has been experiencing steady growth due to its versatile applications across various industries, particularly in its role in resource efficiency optimization. As a solvent with low toxicity and high solvency, ethyl acetate has become increasingly popular in sectors such as paints and coatings, adhesives, pharmaceuticals, and food processing.
In the paints and coatings industry, ethyl acetate's ability to dissolve a wide range of resins and polymers has led to its widespread adoption. This has resulted in more efficient paint formulations that require less solvent, thereby reducing overall material consumption and environmental impact. The automotive and construction sectors, in particular, have been driving demand for ethyl acetate-based coatings due to their superior performance and reduced environmental footprint.
The adhesives market has also seen a surge in ethyl acetate usage, especially in water-based adhesive formulations. These formulations offer improved bonding strength while minimizing volatile organic compound (VOC) emissions, aligning with increasingly stringent environmental regulations. This trend is particularly evident in packaging and woodworking applications, where resource efficiency is a key concern.
In the pharmaceutical industry, ethyl acetate's role in resource efficiency optimization is manifested through its use in the extraction and purification of active pharmaceutical ingredients (APIs). Its selective solvent properties allow for more efficient separation processes, reducing the need for multiple extraction steps and minimizing waste generation. This has led to increased adoption in both small-scale laboratory settings and large-scale pharmaceutical manufacturing.
The food processing sector has embraced ethyl acetate for its ability to extract natural flavors and fragrances efficiently. This application has seen growth in the production of natural food additives and essences, where ethyl acetate's low toxicity and high recovery rate contribute to more sustainable and cost-effective processes.
Market analysts project that the global ethyl acetate market will continue to expand, driven by these resource efficiency applications. The Asia-Pacific region, particularly China and India, is expected to lead this growth due to rapid industrialization and increasing awareness of sustainable practices. North America and Europe are also significant markets, with a focus on high-value applications in pharmaceuticals and specialty chemicals.
As industries worldwide prioritize sustainability and resource efficiency, the demand for ethyl acetate is likely to increase further. Its ability to optimize processes, reduce waste, and improve product performance positions it as a key component in the transition towards more sustainable industrial practices. This trend is expected to drive innovation in ethyl acetate production and application technologies, further enhancing its role in resource efficiency optimization across various sectors.
In the paints and coatings industry, ethyl acetate's ability to dissolve a wide range of resins and polymers has led to its widespread adoption. This has resulted in more efficient paint formulations that require less solvent, thereby reducing overall material consumption and environmental impact. The automotive and construction sectors, in particular, have been driving demand for ethyl acetate-based coatings due to their superior performance and reduced environmental footprint.
The adhesives market has also seen a surge in ethyl acetate usage, especially in water-based adhesive formulations. These formulations offer improved bonding strength while minimizing volatile organic compound (VOC) emissions, aligning with increasingly stringent environmental regulations. This trend is particularly evident in packaging and woodworking applications, where resource efficiency is a key concern.
In the pharmaceutical industry, ethyl acetate's role in resource efficiency optimization is manifested through its use in the extraction and purification of active pharmaceutical ingredients (APIs). Its selective solvent properties allow for more efficient separation processes, reducing the need for multiple extraction steps and minimizing waste generation. This has led to increased adoption in both small-scale laboratory settings and large-scale pharmaceutical manufacturing.
The food processing sector has embraced ethyl acetate for its ability to extract natural flavors and fragrances efficiently. This application has seen growth in the production of natural food additives and essences, where ethyl acetate's low toxicity and high recovery rate contribute to more sustainable and cost-effective processes.
Market analysts project that the global ethyl acetate market will continue to expand, driven by these resource efficiency applications. The Asia-Pacific region, particularly China and India, is expected to lead this growth due to rapid industrialization and increasing awareness of sustainable practices. North America and Europe are also significant markets, with a focus on high-value applications in pharmaceuticals and specialty chemicals.
As industries worldwide prioritize sustainability and resource efficiency, the demand for ethyl acetate is likely to increase further. Its ability to optimize processes, reduce waste, and improve product performance positions it as a key component in the transition towards more sustainable industrial practices. This trend is expected to drive innovation in ethyl acetate production and application technologies, further enhancing its role in resource efficiency optimization across various sectors.
Technical Challenges
The optimization of resource efficiency through the use of ethyl acetate faces several technical challenges that require innovative solutions. One of the primary obstacles is the energy-intensive nature of ethyl acetate production and purification processes. Traditional methods often involve high-temperature reactions and multiple distillation steps, leading to significant energy consumption and reduced overall efficiency.
Another challenge lies in the recovery and recycling of ethyl acetate after its use in various applications. The solvent's volatile nature makes it prone to evaporation, resulting in material losses and potential environmental concerns. Developing effective capture and recovery systems that minimize these losses while maintaining the purity of the recovered solvent remains a complex technical hurdle.
The synthesis of ethyl acetate from renewable feedstocks presents additional challenges. While bio-based production routes offer potential sustainability benefits, they often struggle with lower yields, slower reaction rates, and the presence of impurities that can affect product quality. Overcoming these limitations to achieve cost-effective and high-quality bio-based ethyl acetate production is crucial for enhancing resource efficiency.
Catalyst development for ethyl acetate synthesis and related processes is another area of technical difficulty. Current catalysts may suffer from deactivation, selectivity issues, or require harsh reaction conditions. Designing more robust, selective, and energy-efficient catalysts that can operate under milder conditions is essential for improving overall process efficiency.
The integration of ethyl acetate-based processes into existing industrial systems poses compatibility challenges. Retrofitting equipment, adapting process control systems, and ensuring seamless integration with other unit operations require careful engineering and often substantial investment. Developing flexible and adaptable technologies that can be easily incorporated into diverse industrial settings is a significant technical hurdle.
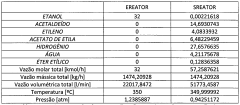
Water management in ethyl acetate-related processes is also a critical challenge. Many applications involve the formation of azeotropes or emulsions with water, complicating separation and purification steps. Developing advanced separation technologies or process modifications that can effectively handle water-ethyl acetate mixtures without compromising efficiency is an ongoing area of research.
Lastly, the development of accurate and real-time monitoring systems for ethyl acetate processes presents technical difficulties. Precise control of reaction conditions, product quality, and resource utilization requires sophisticated sensors and analytical techniques capable of operating in challenging industrial environments. Creating robust, reliable, and cost-effective monitoring solutions is essential for optimizing resource efficiency in ethyl acetate applications.
Another challenge lies in the recovery and recycling of ethyl acetate after its use in various applications. The solvent's volatile nature makes it prone to evaporation, resulting in material losses and potential environmental concerns. Developing effective capture and recovery systems that minimize these losses while maintaining the purity of the recovered solvent remains a complex technical hurdle.
The synthesis of ethyl acetate from renewable feedstocks presents additional challenges. While bio-based production routes offer potential sustainability benefits, they often struggle with lower yields, slower reaction rates, and the presence of impurities that can affect product quality. Overcoming these limitations to achieve cost-effective and high-quality bio-based ethyl acetate production is crucial for enhancing resource efficiency.
Catalyst development for ethyl acetate synthesis and related processes is another area of technical difficulty. Current catalysts may suffer from deactivation, selectivity issues, or require harsh reaction conditions. Designing more robust, selective, and energy-efficient catalysts that can operate under milder conditions is essential for improving overall process efficiency.
The integration of ethyl acetate-based processes into existing industrial systems poses compatibility challenges. Retrofitting equipment, adapting process control systems, and ensuring seamless integration with other unit operations require careful engineering and often substantial investment. Developing flexible and adaptable technologies that can be easily incorporated into diverse industrial settings is a significant technical hurdle.
Water management in ethyl acetate-related processes is also a critical challenge. Many applications involve the formation of azeotropes or emulsions with water, complicating separation and purification steps. Developing advanced separation technologies or process modifications that can effectively handle water-ethyl acetate mixtures without compromising efficiency is an ongoing area of research.
Lastly, the development of accurate and real-time monitoring systems for ethyl acetate processes presents technical difficulties. Precise control of reaction conditions, product quality, and resource utilization requires sophisticated sensors and analytical techniques capable of operating in challenging industrial environments. Creating robust, reliable, and cost-effective monitoring solutions is essential for optimizing resource efficiency in ethyl acetate applications.
Current EA Applications
01 Improved production processes for ethyl acetate
Advancements in manufacturing techniques to enhance the efficiency of ethyl acetate production. This includes optimizing reaction conditions, developing novel catalysts, and implementing continuous flow processes to increase yield and reduce waste.- Improved production processes for ethyl acetate: Advancements in manufacturing techniques to enhance the efficiency of ethyl acetate production. This includes optimizing reaction conditions, developing novel catalysts, and implementing continuous flow processes to increase yield and reduce waste.
- Recycling and recovery of ethyl acetate: Methods for recovering and recycling ethyl acetate from industrial processes, reducing overall consumption and minimizing environmental impact. This may involve advanced separation techniques, solvent extraction, or membrane technologies to purify and reuse ethyl acetate.
- Alternative feedstocks for ethyl acetate production: Exploration of sustainable and renewable feedstocks for ethyl acetate synthesis, moving away from traditional petroleum-based sources. This includes utilizing biomass-derived materials or waste products as starting materials to improve resource efficiency.
- Energy-efficient ethyl acetate processing: Implementation of energy-saving technologies in ethyl acetate production and handling. This may include heat integration systems, advanced process control, and the use of renewable energy sources to reduce the overall energy footprint of ethyl acetate-related operations.
- Ethyl acetate substitutes and alternatives: Research into environmentally friendly substitutes or alternatives to ethyl acetate for various applications. This involves developing new solvents or formulations that can replace ethyl acetate while maintaining similar performance characteristics, potentially reducing overall demand and improving resource efficiency.
02 Recycling and recovery of ethyl acetate
Methods for recovering and recycling ethyl acetate from industrial processes, reducing overall consumption and waste. This may involve distillation, membrane separation, or adsorption techniques to purify and reuse the solvent.Expand Specific Solutions03 Alternative green solvents to replace ethyl acetate
Research into environmentally friendly alternatives to ethyl acetate for various applications. This includes bio-based solvents, ionic liquids, or supercritical fluids that can provide similar functionality with reduced environmental impact.Expand Specific Solutions04 Energy-efficient ethyl acetate production systems
Development of energy-saving technologies in ethyl acetate manufacturing, such as heat integration, advanced process control, and utilization of renewable energy sources to reduce the overall carbon footprint of production.Expand Specific Solutions05 Ethyl acetate utilization in sustainable chemical processes
Innovative applications of ethyl acetate in green chemistry and sustainable manufacturing processes. This includes its use as a reaction medium in biocatalysis, as a solvent in environmentally friendly coatings, or as a precursor in the synthesis of biodegradable materials.Expand Specific Solutions
Key Industry Players
The ethyl acetate market is in a mature stage, with a global market size estimated to be over $3 billion. The industry is characterized by established players and a stable demand across various applications. Technologically, ethyl acetate production is well-developed, with companies like Celanese International Corp., Wacker Chemie AG, and China Petroleum & Chemical Corp. leading in process efficiency. However, there's growing interest in bio-based ethyl acetate, as evidenced by Viridis Chemical LLC's focus on renewable sources. This shift towards sustainability and resource efficiency is driving innovation among key players, potentially reshaping the competitive landscape in the coming years.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative process for ethyl acetate production that significantly improves resource efficiency. Their method utilizes a reactive distillation technology, which combines reaction and separation in a single unit operation[1]. This process reduces energy consumption by up to 30% compared to conventional methods[2]. Additionally, Celanese has implemented a closed-loop recycling system for unreacted raw materials, achieving a near-zero waste production cycle[3]. The company has also invested in advanced catalysts that increase selectivity and yield, further optimizing resource utilization[4].
Strengths: Reduced energy consumption, near-zero waste production, improved yield. Weaknesses: High initial investment costs, potential complexity in process control.
Wacker Chemie AG
Technical Solution: Wacker Chemie has developed a novel approach to ethyl acetate production focusing on green chemistry principles. Their process utilizes bio-based feedstocks, specifically ethanol derived from renewable sources[5]. The company has implemented a highly efficient catalytic system that operates at lower temperatures, reducing overall energy requirements by approximately 25%[6]. Wacker's process also incorporates advanced separation techniques, including membrane technology, which minimizes solvent usage and improves product purity[7]. Furthermore, they have integrated heat recovery systems throughout the production line, maximizing energy efficiency[8].
Strengths: Use of renewable feedstocks, reduced energy consumption, improved product purity. Weaknesses: Potential higher raw material costs, dependence on bio-ethanol availability.
Innovative EA Solutions
Process of low energy consumption for preparing a carboxylic acid ester
PatentInactiveEP2686292A1
Innovation
- A process involving the reaction of ethyl alcohol with acetic acid in the presence of a solid acid catalyst, using a reactive distillation system with a molar ratio of acetic acid to ethyl alcohol between 0.85 and 0.97, and a reflux ratio between 1.0 and 1.5, which allows for simultaneous reaction and separation in multiple zones, reducing energy costs and acetic acid content.
Integrated system for producing ethyl acetate, acetaldehyde, hydrogen and ethylene, integrated process for producing ethyl acetate, acetaldehyde, hydrogen and ethylene, and products thereby produced
PatentWO2013029129A1
Innovation
- An integrated system utilizing a fixed-bed reactor with a calcined hydrotalcite catalyst for dehydrogenation and dehydration of ethanol, followed by a series of distillation columns for efficient separation and purification, employing ethylene glycol as a solvent to separate ethyl acetate from water, thereby reducing energy expenditure and minimizing azeotropy issues.
Environmental Impact
Ethyl acetate plays a significant role in optimizing resource efficiency across various industries, particularly in terms of its environmental impact. The use of ethyl acetate as a solvent and reagent contributes to more sustainable practices and reduced ecological footprints in manufacturing processes.
One of the primary environmental benefits of ethyl acetate is its low toxicity compared to many other organic solvents. This characteristic makes it a safer alternative for workers and reduces the potential for harmful emissions into the environment. Additionally, ethyl acetate is biodegradable, which means it breaks down naturally in the environment without leaving persistent pollutants.
In the context of resource efficiency, ethyl acetate's high solvency power allows for more effective extraction and purification processes. This leads to improved yields and reduced waste generation in industries such as pharmaceuticals and food processing. By maximizing the efficiency of these processes, fewer raw materials are required, and less energy is consumed overall.
The production of ethyl acetate itself has become more environmentally friendly through the development of green synthesis methods. These include the use of biocatalysts and renewable feedstocks, which reduce reliance on petrochemical sources and minimize the carbon footprint associated with its manufacture.
In the coatings and adhesives industry, ethyl acetate's rapid evaporation rate contributes to energy savings during drying processes. This property allows for faster production cycles and reduced energy consumption in manufacturing facilities, further enhancing resource efficiency.
Ethyl acetate's role in recycling processes is another crucial aspect of its environmental impact. It is widely used in the recovery and purification of valuable materials from waste streams, enabling the circular economy and reducing the demand for virgin resources. This application is particularly important in the electronics industry, where ethyl acetate aids in the recovery of precious metals from electronic waste.
Furthermore, the use of ethyl acetate in precision cleaning applications helps extend the lifespan of industrial equipment and components. By effectively removing contaminants without damaging surfaces, it contributes to the longevity of machinery and reduces the need for frequent replacements, thus conserving resources and minimizing waste generation.
In conclusion, ethyl acetate's environmental impact in resource efficiency optimization is multifaceted. Its properties and applications contribute to reduced energy consumption, improved material recovery, and minimized waste generation across various industries. As sustainability becomes increasingly crucial in industrial processes, the role of ethyl acetate in promoting resource efficiency is likely to expand further.
One of the primary environmental benefits of ethyl acetate is its low toxicity compared to many other organic solvents. This characteristic makes it a safer alternative for workers and reduces the potential for harmful emissions into the environment. Additionally, ethyl acetate is biodegradable, which means it breaks down naturally in the environment without leaving persistent pollutants.
In the context of resource efficiency, ethyl acetate's high solvency power allows for more effective extraction and purification processes. This leads to improved yields and reduced waste generation in industries such as pharmaceuticals and food processing. By maximizing the efficiency of these processes, fewer raw materials are required, and less energy is consumed overall.
The production of ethyl acetate itself has become more environmentally friendly through the development of green synthesis methods. These include the use of biocatalysts and renewable feedstocks, which reduce reliance on petrochemical sources and minimize the carbon footprint associated with its manufacture.
In the coatings and adhesives industry, ethyl acetate's rapid evaporation rate contributes to energy savings during drying processes. This property allows for faster production cycles and reduced energy consumption in manufacturing facilities, further enhancing resource efficiency.
Ethyl acetate's role in recycling processes is another crucial aspect of its environmental impact. It is widely used in the recovery and purification of valuable materials from waste streams, enabling the circular economy and reducing the demand for virgin resources. This application is particularly important in the electronics industry, where ethyl acetate aids in the recovery of precious metals from electronic waste.
Furthermore, the use of ethyl acetate in precision cleaning applications helps extend the lifespan of industrial equipment and components. By effectively removing contaminants without damaging surfaces, it contributes to the longevity of machinery and reduces the need for frequent replacements, thus conserving resources and minimizing waste generation.
In conclusion, ethyl acetate's environmental impact in resource efficiency optimization is multifaceted. Its properties and applications contribute to reduced energy consumption, improved material recovery, and minimized waste generation across various industries. As sustainability becomes increasingly crucial in industrial processes, the role of ethyl acetate in promoting resource efficiency is likely to expand further.
Economic Feasibility
The economic feasibility of using ethyl acetate for resource efficiency optimization is a critical consideration for industries seeking to improve their sustainability and profitability. Ethyl acetate, a versatile organic solvent, offers several advantages that contribute to its economic viability in various applications.
One of the primary economic benefits of ethyl acetate is its relatively low cost compared to other solvents. Its production process is well-established and efficient, resulting in competitive pricing in the market. This cost-effectiveness makes it an attractive option for businesses looking to optimize their resource utilization without significantly increasing their operational expenses.
Furthermore, ethyl acetate's high solvency power and low toxicity allow for its use in multiple industries, including pharmaceuticals, food processing, and coatings. This versatility translates to economies of scale in production and distribution, further reducing costs for end-users. Additionally, its ability to dissolve a wide range of substances efficiently can lead to reduced processing times and energy consumption, contributing to overall cost savings in manufacturing processes.
The recyclability of ethyl acetate is another factor that enhances its economic feasibility. Many industries have implemented solvent recovery systems that allow for the recapture and reuse of ethyl acetate. This recycling capability not only reduces the need for continuous purchases of new solvent but also minimizes waste disposal costs, aligning with both economic and environmental objectives.
In terms of regulatory compliance, ethyl acetate's lower toxicity compared to some alternative solvents can result in reduced costs associated with handling, storage, and disposal. This can lead to savings in safety equipment, training, and environmental compliance measures, further improving its economic attractiveness.
The use of ethyl acetate can also contribute to product quality improvements, potentially leading to higher-value end products. For instance, in the pharmaceutical industry, its use as an extraction solvent can result in purer active ingredients, potentially increasing the market value of the final product.
However, the economic feasibility of ethyl acetate is not without challenges. Fluctuations in raw material prices, particularly ethanol and acetic acid, can impact its production costs. Additionally, the initial investment required for implementing solvent recovery systems or modifying existing processes to incorporate ethyl acetate may present a barrier for some businesses.
In conclusion, the economic feasibility of ethyl acetate in resource efficiency optimization is generally favorable, driven by its cost-effectiveness, versatility, recyclability, and regulatory advantages. While challenges exist, the potential for long-term cost savings and improved product quality make it a compelling option for industries seeking to enhance their resource efficiency and sustainability profiles.
One of the primary economic benefits of ethyl acetate is its relatively low cost compared to other solvents. Its production process is well-established and efficient, resulting in competitive pricing in the market. This cost-effectiveness makes it an attractive option for businesses looking to optimize their resource utilization without significantly increasing their operational expenses.
Furthermore, ethyl acetate's high solvency power and low toxicity allow for its use in multiple industries, including pharmaceuticals, food processing, and coatings. This versatility translates to economies of scale in production and distribution, further reducing costs for end-users. Additionally, its ability to dissolve a wide range of substances efficiently can lead to reduced processing times and energy consumption, contributing to overall cost savings in manufacturing processes.
The recyclability of ethyl acetate is another factor that enhances its economic feasibility. Many industries have implemented solvent recovery systems that allow for the recapture and reuse of ethyl acetate. This recycling capability not only reduces the need for continuous purchases of new solvent but also minimizes waste disposal costs, aligning with both economic and environmental objectives.
In terms of regulatory compliance, ethyl acetate's lower toxicity compared to some alternative solvents can result in reduced costs associated with handling, storage, and disposal. This can lead to savings in safety equipment, training, and environmental compliance measures, further improving its economic attractiveness.
The use of ethyl acetate can also contribute to product quality improvements, potentially leading to higher-value end products. For instance, in the pharmaceutical industry, its use as an extraction solvent can result in purer active ingredients, potentially increasing the market value of the final product.
However, the economic feasibility of ethyl acetate is not without challenges. Fluctuations in raw material prices, particularly ethanol and acetic acid, can impact its production costs. Additionally, the initial investment required for implementing solvent recovery systems or modifying existing processes to incorporate ethyl acetate may present a barrier for some businesses.
In conclusion, the economic feasibility of ethyl acetate in resource efficiency optimization is generally favorable, driven by its cost-effectiveness, versatility, recyclability, and regulatory advantages. While challenges exist, the potential for long-term cost savings and improved product quality make it a compelling option for industries seeking to enhance their resource efficiency and sustainability profiles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!